Abstract
Background: Although glioma-associated oncogene homolog 1 (Gli1) is a key mediator of the Hedgehog pathway, Gli1 involvement in the maintenance of cancer stem-like cells (CSCs) in prostate cancer (PCa) is unclear. Methods: Herein, we assessed the expression of Gli1 and its relationship with cancer stemness genes, cell cycle markers, epithelial-mesenchymal transition (EMT), and signaling pathway genes in 145 paraffin-embedded PCa tissue samples using immunohistochemistry. In addition, we further confirmed the correlation between Gli1 and CSC marker in PC3 cells using immunofluorescence imaging. Results: High Gli1 expression was significantly associated with advanced primary tumor stage, positive lymph node metastasis, advanced clinical stage, and HIF-1α expression. The microvessel density was significantly higher in the Gli1 positive-cases than in the negative-cases. Furthermore, Gli1 expression was positively correlated with stemness markers CD44. Survival analysis demonstrated that Gli1 and CD44 were strongly associated with the worse clinical outcome and an independent poor prognostic factor for overall survival. The enrichment analysis revealed that Gli1 was not correlated with E-cadherin, while positively correlated with Snail and vimentin. Notably, Gli1 expression was positively associated with the expression of cell cycle regulating genes such as cyclin D1, p21 and CDK4. Additionally, Gli1 expression was positively correlated with pPI3K p85, pAkt-Ser473 and NF-κB p65 expression. Conclusions: Our results indicate that Gli1 is a potential diagnostic marker of CSCs and that Gli1 expression is strongly associated with epithelial-mesenchymal transition in PCa via PI3K/Akt/NF-κB signaling.
Keywords: Glioma-associated oncogene homolog 1, prostate cancer, prognosis, cancer stem-like cells, PI3K/Akt/NF-κB signaling
Introduction
In patients with prostate cancer (PCa), metastasis is the most serious complication and the major cause of death [1]. Most patients with PCa ultimately develop invasive and drug-resistant metastatic cancer [2-4]. A study reported that PCa is the second leading cause of cancer-related death among males in the United States [5]. Therefore, studying the progression mechanisms of PCa is crucial to improve the survival rate of patients with PCa through effective treatment.
Cancer stem-like cells (CSCs) theory provides a new direction regarding the recurrence mechanism in tumors. Various human tumors contain CSCs which are positively correlated with the initiation, progression, and recurrence of cancer [6]. Therefore, the identification of novel targets of CSCs or diagnostic markers of PCa to develop highly potent cancer molecular targets might be useful to improve the prognosis of PCa.
Glioma-associated oncogene homolog 1 (Gli1) is a transcriptional activator of Hedgehog (Hh) signaling, which is involved in regulating the growth and proliferation of glioma CSCs and endogenous brain stem cells [7-11]. Previous studies indicated that Gli1 might act as a potential stem cell marker in esophageal squamous cell carcinoma (ESCC) [12], lung squamous cell carcinoma (LSCC) [13], and ductal breast carcinoma (DBC) [14], as Gli1 upregulation promotes the growth and proliferation of these tumors.
In this study, we aimed to determine the prognostic and functional significance of Gli1 and the association of Gli1 with CSCs markers in PCa. A positive correlation between Gli1 expression and CSCs might provide a promising target to develop novel and efficient strategies to treat PCa. In addition, we explored whether Gli1 expression is associated with epithelial-mesenchymal transition (EMT) in PCa via the phosphatidylinositol-3-kinase (PI3K)/Akt/nuclear factor kappa B (NF-κB) signaling.
Materials and methods
Tissue specimens
A total of 145 cases of PCa tissue microarray paraffin-embedded samples were obtained from the Tissue Bank of the second hospital of Jilin University. The Institutional Review Board of Jilin University Medical College approved the study protocol and conducted in accordance with the 1996 Declaration of Helsinki. All patients provided written informed consent according to institutional guidelines. No patient received preoperative chemotherapy or radiotherapy. Pathologic parameters were carefully reviewed in all 145 PCa patients, including age, Gleason scores, tumor differentiation, primary tumor (pT), lymph node metastasis, and clinical stage. Follow-up survival data were collected retrospectively through medical-record analyses. The pTNM classification was applied according to guidelines from the 2010 American Joint Committee on Cancer staging manual (AJCC 7th edition) [15].
Cell lines
PC3 cells (PCa cell line), were maintained in RPMI-1640 with high glucose (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies), 100 mg/ml penicillin G, and 50 mg/ml streptomycin (Life Technologies) at 37°C in a humidified atmosphere containing 5% CO2. All cell lines were purchased from the ATCC (Manassas, USA).
Immunohistochemical (IHC) staining procedure
Serial 4 μm tissue sections were deparaffinized with xylene, hydrated using a diluted alcohol series, and immersed in 3% H2O2 in methanol to quench endogenous peroxidase activity. Then sections were heated with TE buffer (1 mM EDTA and 10 mM Tris, pH 9.2) at 98°C for 30 minutes. To reduce non-specific staining, each section was blocked with 4% bovine serum albumin in PBS for 30 min. The sections were incubated with anti-Gli1 (1:100, Abcam, Cambridge, UK), anti-CD44 (1:100, Abcam, Cambridge, UK), anti-Sox2 (1:100, R&D, Minneapolis, MN, USA), anti-LSD1 (1:250, Sigma, St. Louis, MO, USA), anti-Sox9 (1:100, Abnova, Walnut, CA, USA), anti-HIF-1α (1:80, Proteintech, Chicago, IL, USA), anti-CD68 (1:100, Abcam, Cambridge, UK), anti-cyclin D1 (1:100, Abcam, Cambridge, UK), anti-p21 (1:100, Abcam, Cambridge, UK), anti-CDK4 (1:100, Abcam, Cambridge, UK), anti-p27 (1:100, Abcam, Cambridge, UK), anti-p16 (1:100, Abcam, Cambridge, UK), anti-E-cadherin (1:250, Abcam, Cambridge, UK), anti-Snail (1:100, Abcam, Cambridge, UK), anti-vimentin (1:80, Millipore, Bedford, MA, USA), anti-pPI3K p85 (1:30, Abcam, Cambridge, UK), anti-pAkt-Thr308 (1:80, Millipore, Bedford, MA, USA), anti-pAkt-Ser473 (1:80, Millipore, Bedford, MA, USA) and anti-NF-κB p65 (1:100, CST, Danvers, MA, USA), antibodies in PBST containing 3 mg/ml goat globulin (Sigma, St. Louis, MO, USA) for 2 hours at room temperature, followed by three washes with PBST buffer. Then sections were incubated with an anti-mouse/rabbit antibody (Envision plus, Dako, Denmark) for 30 minutes at room temperature. Next, the sections were immersed in chromogenic reagent 3, 3’-diaminobenzidine (Dako, Denmark) and then counterstaining with Meyer’s hematoxylin.
The double immunostaining procedure of Gli1/CD105 in PCa tissues were the same as before. First, for the Gli1 protocols, except that the chromogen with the 3, 3’-diaminobenzidine (Dako) for 10 minutes, all steps were the same as before. Then, the subsequent staining of the same section was performed after incubating the samples with an antibody to CD105 by ImmPACT AEC Peroxidase Substrate for 20 minutes [14].
Evaluation of the IHC analysis
Two pathologists evaluated the IHC results with no prior knowledge of clinical data results, and discussed any discrepancies in scores until a consensus was reached. CD105 positive individual microvessel counts were performed on 100× fields and three area micro-vessel numbers were averaged as the micro-vessel density (MVD). In case of discrepancy, a final score was established by reassessment by both pathologists on a double-headed microscope. IHC scores were according to the staining intensity of tissue sections, and scored as 0, 1, 2, 3. [IHC score 0], negative or no staining; [IHC score 1], weak staining in <50% or moderate staining in <20% of cells; [IHC score 2], weak staining in ≥50%, moderate staining in 20-50% or strong staining in <20%; [IHC score 3], moderate staining in ≥50% or strong staining in ≥20%. Staining scores of 2 or 3 were considered as positive for each protein expression; the staining results were semi-quantitatively scored as negative and positive [16].
Immunofluorescence (IF) analysis
PC3 cells were plated (50,000/well) on coverslips in 6-well plates. We fixed the cells with 4% paraformaldehyde in PBS for 10 min at room temperature and permeabilized with 0.2% Triton X-100 in PBS for 5 min at room temperature. To reduce non-specific staining, each section was blocked with 2% FBS in PBS for 30 min. The sections were then incubated with primary antibodies against anti-Gli1 and anti-CD44 (1:50), overnight at 4°C. The next day, cells were incubated with Alexa Fluor 568 goat anti-mouse IgG (Invitrogen, A12380) and Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen, A11008) secondary antibodies (1:150 dilution) for 1 hour. Nuclei were stained with DAPI and sections were mounted with vector shield mounting medium with DAPI for fluorescence detection (vector lab, H-1200). Fluorescence detection was performed with the Axiovert200II (Carl-Zeiss) and the intensity of IF cells was measured using Meta-Morph software.
Statistical analysis
Statistical analysis was performed by IBM SPSS17.0 statistical software (SPSS Inc, Chicago, IL, USA). Correlations were assessed using Pearson’s chi-square test as appropriate. Overall survival (OS) rates were determined using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate survival analyses were performed on all characteristics using the Cox proportional hazards model. Hazard ratios (HRs) and 95% confidence intervals (CI) were assessed for each factor. All tests were two sided, and P<0.05 was considered significant.
Results
Expression level of Gli1 and its association with clinicopathologic characteristics in PCa
Gli1 expression in PCa was significantly associated with advanced pT (P = 0.005), positive lymph node metastasis (P = 0.023), and advanced clinical stage (P = 0.003), as these manifestations were highly common in patients with PCa. Additionally, we found that Gli1 expression (P = 0.002) was significantly higher in the HIF-1α-positive group (57.6%, 68/118) than in the HIF-1α-negative group (25.9%, 7/27). Moreover, IHC staining revealed that Gli1 co-localized with HIF-1α in the serial sections of tissue samples from patients with PCa (Figure 1A, 1B). In the results of double staining using Gli1 and CD105 antibodies, MVD (P<0.05) was significantly higher in the Gli1-positive cases (79.82±32.119) than in the Gli1-negative cases (62.17±34.777) (Figure 2C, 2D). However, significant association was not observed between Gli1 expression and clinicopathologic features, such as age, Gleason score, tumor differentiation, and CD68 expression (Table 1).
Figure 1.
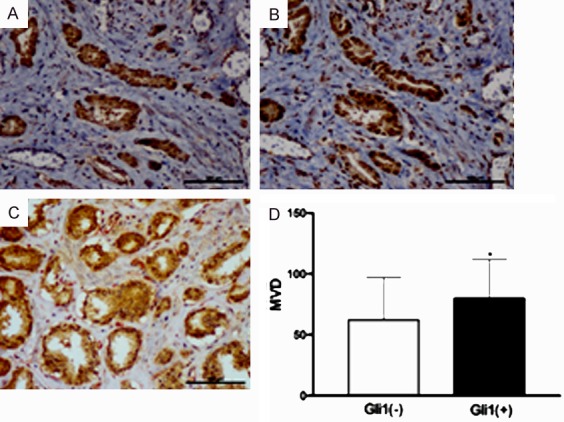
IHC staining of Gli1 (A) and HIF-1α (B) expression in the serial section of PCa tissues. (C) IHC double staining showed that Gli1 (brown reaction product) in the cancer cell and CD105 (red reaction product) indicated the micro-vessel, and MVD was significantly higher in Gli1 positive cases than in the negative cases (D). *P<0.05. (original magnification ×200).
Figure 2.
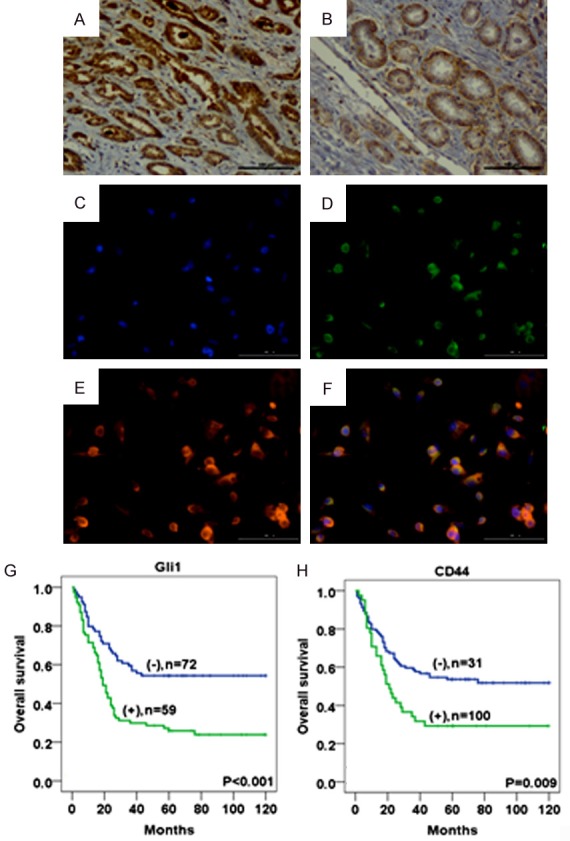
IHC staining revealed Gli1 (A) and CD44 (B) in PCa tissues. IF staining for Gli1 and CD44 in the PCa cells (C-F). Blue for DAP1; green for Gli1; red for CD44; double labeling for Merge. Kaplan-Meier analysis of OS that the positive expression of Gli1 (G) and CD44 (H) in PCa was significantly associated with a shortened OS compared to the negative groups. (original magnification ×200).
Table 1.
Comparison of clinicopathological characteristics according to Gli1 expression in prostate cancer
| Variable | N | Gli1 (-) n (%) | Gli1 (+) n (%) | χ2 | R | P-value |
|---|---|---|---|---|---|---|
| Age (years) | 0.442 | 0.056 | 0.506 | |||
| ≤65 | 51 | 27 (52.9) | 24 (47.1) | |||
| >65 | 94 | 43 (45.7) | 51 (54.3) | |||
| Gleason scores | 1.025 | 0.024 | 0.599 | |||
| 4-7 | 9 | 3 (33.3) | 6 (66.7) | |||
| 8-9 | 114 | 58 (50.9) | 56 (49.1) | |||
| 10 | 22 | 9 (40.9) | 13 (59.1) | |||
| Differentiation | 0.113 | 0.029 | 0.945 | |||
| Well | 8 | 4 (50.0) | 4 (50.0) | |||
| Moderately | 83 | 41 (49.4) | 42 (50.6) | |||
| Poorly | 54 | 25 (46.3) | 29 (53.7) | |||
| pT | 8.020 | 0.248 | 0.005* | |||
| 1-2 | 89 | 51 (57.3) | 38 (42.7) | |||
| 3-4 | 56 | 19 (33.9) | 37 (66.1) | |||
| Lymph node metastasis | 5.187 | 0.200 | 0.023* | |||
| Negative | 135 | 69 (51.1) | 66 (48.9) | |||
| Positive | 10 | 1 (10.0) | 9 (90.0) | |||
| Clinical stage | 8.748 | 0.259 | 0.003* | |||
| I+II | 86 | 51 (59.3) | 35 (40.7) | |||
| III+IV | 59 | 19 (32.2) | 40 (67.8) | |||
| CD68 | 0.424 | 0.055 | 0.515 | |||
| Negative | 65 | 35 (53.8) | 30 (46.2) | |||
| Positive | 80 | 35 (43.8) | 45 (56.2) | |||
| HIF-1α | 9.778 | 0.258 | 0.002* | |||
| Negative | 27 | 20 (74.1) | 7 (25.9) | |||
| Positive | 118 | 50 (42.4) | 68 (57.6) |
Statistically significant.
Gli1 is a potential diagnostic marker of prostate CSCs
IHC staining revealed that Gli1 (Figure 2A) expression positively correlated with CD44 (P<0.001) (Figure 2B) expression in PCa tissues (Table 2). However, Gli1 expression did not correlate with the expression of other cancer stemness-related factors such as Sox2, Sox9, and LSD1. In addition, double IF staining also revealed that the Gli1 positive cell population mostly has a high overlap with CD44 positive cell population in PC3 cells (Figure 2C-F).
Table 2.
Correlation of Gli1 expression with cancer stem cell maker expression in prostate cancer
| Variable | N | Gli1 (-) n (%) | Gli1 (+) n (%) | χ2 | R | P-value |
|---|---|---|---|---|---|---|
| Sox2 | 0.000 | 0.001 | 0.989 | |||
| Negative | 43 | 20 (46.5) | 23 (53.5) | |||
| Positive | 102 | 50 (49.0) | 52 (51.0) | |||
| Sox9 | 1.721 | 0.111 | 0.190 | |||
| Negative | 8 | 2 (25.0) | 6 (75.0) | |||
| Positive | 137 | 68 (49.6) | 69 (50.4) | |||
| LSD1 | 0.002 | 0.004 | 0.968 | |||
| Negative | 21 | 10 (47.6) | 11 (52.4) | |||
| Positive | 124 | 60 (48.4) | 64 (51.6) | |||
| CD44 | 17.363 | 0.356 | <0.001* | |||
| Negative | 34 | 27 (79.4) | 7 (20.6) | |||
| Positive | 111 | 43 (38.7) | 68 (61.3) |
Statistically significant.
Kaplan-Meier survival analysis revealed that the positive expression of Gli1 (P<0.001) and CD44 (P = 0.009) in patients with PCa was associated with a shortened OS rate compared with that of the Gli1- and CD44-negative patients (Figure 2G, 2H). Furthermore, in the univariate as well as multivariate Cox regression analyses, the Gli1 (Univariate: HR = 2.191, P<0.001; Multivariate: HR = 1.963, P = 0.002) and CD44 (Univariate: HR = 1.83, P = 0.01; Multivariate: HR = 1.654, P = 0.034) expression levels in addition to the status of lymph node metastasis (Univariate: HR = 2.13, P = 0.002; Multivariate: HR = 1.687, P = 0.041) were an adverse independent prognostic factor of the OS rate in patients with PCa (Table 3).
Table 3.
Univariate and multivariate analyses for prognostic variables of overall survival in prostate cancer patients using Cox proportional-hazards regression
| Characteristic | Univariate analyses | Multivariate analyses | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (years) | 0.097 | 0.205 | ||||
| ≤65 | 1.00 | 1.00 | ||||
| >65 | 1.493 | 0.931-2.397 | 1.382 | 0.838-2.278 | ||
| pT | 0.002* | 0.054 | ||||
| 1-2 | 1.00 | 1.00 | ||||
| 3-4 | 5.933 | 1.879-18.733 | 3.177 | 0.979-10.307 | ||
| Lymph node metastasis | 0.002* | 0.041* | ||||
| Negative | 1.00 | 1.00 | ||||
| Positive | 2.13 | 1.326-3.422 | 1.687 | 1.021-2.788 | ||
| CD44 | 0.01* | 0.034* | ||||
| Negative | 1.00 | 1.00 | ||||
| Positive | 1.83 | 1.153-2.904 | 1.654 | 1.038-2.635 | ||
| Gli1 | <0.001* | 0.002* | ||||
| Negative | 1.00 | 1.00 | ||||
| Positive | 2.191 | 1.442-3.329 | 1.963 | 1.289-2.991 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval.
Statistically significant.
Gli1 regulates EMT and enhances the expression of cell cycle regulating factors via PI3K/Akt/NF-κB signaling in PCa
We estimated the variations in the levels of EMT markers in tissue samples by using IHC. Snail was localized in the cytoplasm and nuclei, and E-cadherin was present in the cytoplasm of cancer cells, whereas vimentin was detected in the cytoplasm of stromal fibroblasts (Figure 3A-C). Furthermore, IHC double staining for Gli1 and vimentin were performed (Figure 3A). The results demonstrated that Gli1 was negatively correlated with E-cadherin (P = 0.016), while positively correlated with Snail (P = 0.001) and vimentin (P = 0.033) (Table 4).
Figure 3.
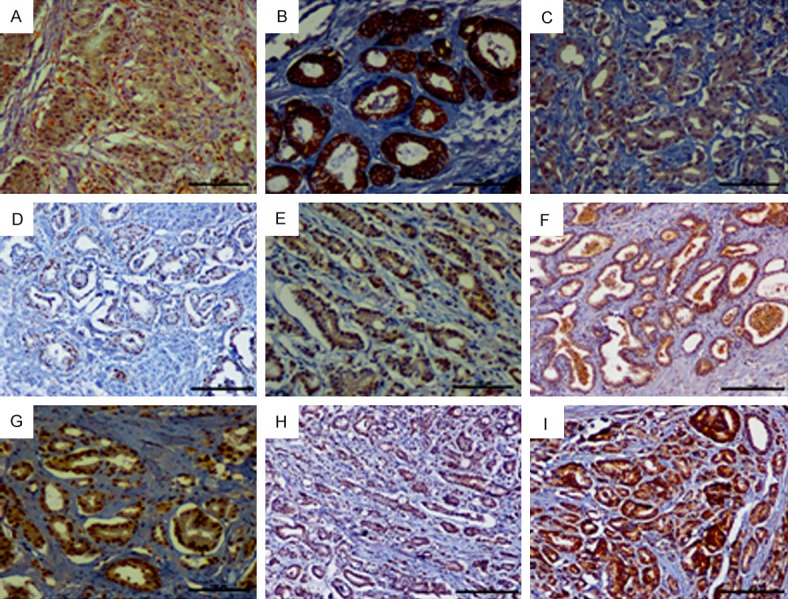
(A) IHC double staining showed that Gli1 (brown reaction product) in cancer cells and vimentin (red reaction product) in the stromal fibroblasts; IHC staining of E-cadherin (B), Snail (C), cyclinD1 (D), CDK4 (E), p21 (F), pPI3K p85 (G), pAkt-Ser473 (H) and NF-κB p65 (I) in prostate cancer tissues. (original magnification ×200).
Table 4.
Correlation of Gli1 expression with epithelial-mesenchymal transition maker expression in prostate cancer
| Variable | N | Gli1 (-) n (%) | Gli1 (+) n (%) | χ2 | R | P-value |
|---|---|---|---|---|---|---|
| E-cadherin | 5.842 | 0.201 | 0.016* | |||
| Negative | 36 | 11 (30.6) | 25 (69.4) | |||
| Positive | 109 | 59 (54.1) | 50 (45.9) | |||
| vimentin | 4.544 | 0.176 | 0.033* | |||
| Negative | 44 | 27 (61.4) | 17 (38.6) | |||
| Positive | 101 | 43 (42.6) | 58 (57.4) | |||
| Snail | 11.261 | 0.283 | 0.001* | |||
| Negative | 117 | 65 (55.6) | 52 (44.4) | |||
| Positive | 28 | 5 (17.9) | 23 (82.1) |
Statistically significant.
IHC staining of PCa patient tissues revealed that Gli1 expression was positively associated with the cell cycle regulating factors such as cyclin D1 (P<0.001), p21 (P<0.001), and CDK4 (P = 0.014) expression (Table 5). Positive expression signals were localized mainly in the nuclei of cancer cells (Figure 3D-F). However, Gli1 expression did not correlate with p16 and p27 expression (Table 5). In addition, Gli1 was positively correlated with the pPI3K (P<0.001) and pAkt-Ser473 (P = 0.001) as well as NF-κB p65 (P<0.001) expression (Table 6; Figure 3G-I).
Table 5.
Correlation of Gli1 expression with cell-cycle related gene expression in prostate cancer
| Variable | N | Gli1 (-) n (%) | Gli1 (+) n (%) | χ2 | R | P-value |
|---|---|---|---|---|---|---|
| Cyclin D1 | 16.165 | 0.337 | <0.001* | |||
| Negative | 35 | 27 (77.1) | 8 (22.9) | |||
| Positive | 110 | 43 (39.1) | 67 (60.9) | |||
| P16 | 0.095 | 0.026 | 0.757 | |||
| Negative | 15 | 6 (40.0) | 9 (60.0) | |||
| Positive | 130 | 64 (49.2) | 66 (50.8) | |||
| CDK4 | 6.021 | 0.210 | 0.014* | |||
| Negative | 111 | 60 (54.1) | 51 (45.9) | |||
| Positive | 34 | 10 (29.4) | 24 (70.6) | |||
| P21 | 21.753 | 0.387 | <0.001* | |||
| Negative | 55 | 40 (72.7) | 15 (27.3) | |||
| Positive | 90 | 30 (33.3) | 60 (66.7) | |||
| p27 | 0.039 | 0.017 | 0.843 | |||
| Negative | 8 | 4 (50.0) | 4 (50.0) | |||
| Positive | 137 | 66 (48.2) | 71 (51.8) |
Statistically significant.
Table 6.
Correlation of Gli1 expression with PI3K/Akt/NF-ΚB signaling in prostate cancer
| Variable | N | Gli1 (-) n (%) | Gli1 (+) n (%) | χ2 | R | P-value |
|---|---|---|---|---|---|---|
| pPI3K p85 | 13.720 | 0.306 | <0.001* | |||
| Negative | 71 | 46 (64.8) | 25 (35.2) | |||
| Positive | 74 | 24 (32.4) | 50 (67.6) | |||
| pAkt-Ser473 | 11.319 | 0.288 | 0.001* | |||
| Negative | 31 | 23 (74.2) | 8 (25.8) | |||
| Positive | 114 | 47 (41.2) | 67 (58.8) | |||
| pAkt-Thr308 | 0.693 | 0.071 | 0.405 | |||
| Negative | 10 | 3 (30.0) | 7 (70.0) | |||
| Positive | 135 | 67 (49.6) | 68 (50.4) | |||
| NF-ΚB p65 | 24.204 | 0.409 | <0.001* | |||
| Negative | 54 | 40 (74.1) | 14 (25.9) | |||
| Positive | 91 | 30 (33.0) | 61 (67.0) |
Statistically significant.
Discussion
CSCs are a sub-population among the various types of tumor cells. CSCs are positively correlated with the initiation, progression, and recurrence of cancer [6]. The novel finding in our study was that Gli1 might be a potential diagnostic marker of prostate CSCs and strongly associated with EMT through PI3K/Akt/NF-κB signaling in PCa.
Increasing evidence proves that Gli1 expression is positively correlated with the degree of malignancy and poor survival rate in several cancers including ESCC, LSCC, and DBC [12-14]. Consistent with our previous studies, Gli1 expression was very high in aggressive cases of PCa with advanced pT, positive lymph node metastasis and advanced clinical stage, as Gli1 promotes tumor progression in PCa. In this study, survival analysis demonstrated that Gli1 was strongly associated with worse clinical outcome and independent poor prognostic factors of OS rate in PCa. As the formation of new blood vessels is necessary for the proliferation and metastasis of PCa cells, we assessed MVD and found that it was significantly higher in the Gli1-positive cases than that in the Gli1-negative cases. This result suggested that the role of Gli1 as a prognostic factor is associated with the induction of angiogenesis. The statistical significance indicated that Gli1 is an important predictive factor to detect tumor aggressiveness and prognosis in patients with PCa.
The Hh-Gli1 signaling is involved in the regulation of the growth and proliferation of glioma CSCs and endogenous brain stem cells [7-11]. CD44 is considered a marker of prostate CSCs and plays an important role in the development and progression of PCa [17,18]. Moreover, Gli1 expression was positively correlated with CD44 expression in PCa. In the current study, Gli1 expression was significantly higher in the HIF-1α-positive group than that in the HIF-1α-negative group. This observation suggested that hypoxia promotes Gli1 expression in PCa. In tumors, hypoxic microenvironments are important stem cell niches and promote the persistence of CSCs [19]. These results indicated that the up-regulation of Gli1 might be a potential diagnostic marker of prostate CSCs. However, the mechanism of Gli1-mediated processes in CSCs need to be studied further to establish the aforementioned hypothesis.
EMT is a process that stimulates cancer cell invasion and metastasis [20]. It is essential to study cancer metastasis to establish the molecular mechanisms of EMT, for the development of novel therapeutic strategies [21]. Gli1 enhances invasion and EMT by promoting CSCs-like properties in the ER-positive breast cancers [22]. The high Gli1 expression promotes EMT by reducing E-cadherin expression and enhancing the Snail and vimentin expression in ESCC [23]. To identify Gli1 as a regulator of EMT process in PCa, we examined the correlation between Gli1 and EMT-related markers by IHC. Consistent with the aforementioned studies, our study demonstrated that Gli1 expression was strongly associated with EMT as it was negatively correlated with E-cadherin expression, while positively correlated with Snail and vimentin expression in PCa. These results suggested that Gli1 might be a master regulator of EMT and in turn regulates cancer cell invasion and metastasis in PCa.
Cell proliferation and cell cycle progression lead to the clonal expansion of tumor cells during tumor development [24]. Sun et al. indicated that Gli1 regulates the cyclin D1 expression and in turn modulates the cell cycle or proliferation via the Hh signal pathway [25]. Further, Cui et al. revealed that Gli1 co-localizes with cyclinD1, CDK4, and p21 in LSCC tissue samples by using IHC multi-staining [13]. Sun et al. reported that the inhibition of Gli1 suppresses cell growth and cell cycle progression [26]. Consistent with these results, we found that Gli1 expression was significantly correlated with cyclinD1, p21, and CDK4 expression in PCa. Our results indicated that the up-regulation of Gli1 was associated with the regulation of tumor cell proliferation which induces cell cycle progression in PCa.
PI3K/Akt/mTOR/NF-κB signaling is involved in cell survival, growth, migration, differentiation, and maintenance of self-renewal in the tumor cells [27,28]. Sonic Hh-Gli1 signals promote the EMT process by modulating PI3K/Akt pathway in ovarian cancer [29]. Colavito et al. reported evidence of crosstalk between Gli1 signaling and NF-κB pathway in EMT [30]. In ESCC, Gli1 interacts with NF-κB signaling and regulates the cell cycle [12]. Various studies have reported that activation of the PI3K/Akt pathway is involved in NF-κB activation [31]. Our results show that Gli1 goes along with pPI3K p85, pAkt-Ser473 and NF-κB p65 expression. Moreover, Gli1 plays a critical role in regulating the EMT and cell cycle process in PCa. This suggested that Gli1 regulates EMT process to promote tumor proliferation and metastasis probably via the activation of PI3K/Akt/NF-κB signaling and exhibits the potential as a promising target for PCa CSCs therapy. However, further studies are necessary to determine the specific regulatory mechanism through Gli1 in PCa.
Conclusions
In conclusion, the over-expression of Gli1 might act as a potential prostate CSCs marker and effectively predict poor prognosis in PCa. Moreover, Gli1 might be used as a promising target for therapeutic strategies to prevent the progression of EMT via PI3K/Akt/NF-κB signaling.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81460390, 81760531, 81560400).
Disclosure of conflict of interest
None.
References
- 1.Liu Q, Jernigan D, Zhang Y, Fatatis A. Implication of platelet-derived growth factor receptor alpha in prostate cancer skeletal metastasis. Chin J Cancer. 2011;30:612–619. doi: 10.5732/cjc.011.10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+CD24-prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Lippitt JM, Guzmán-Ramírez N, Hamdy FC, Eaton CL, Thalmann GN, Cecchini MG, Pelger RC, van der Pluijm G. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010;70:5163–5173. doi: 10.1158/0008-5472.CAN-09-3806. [DOI] [PubMed] [Google Scholar]
- 4.Dubrovska A, Elliott J, Salamone RJ, Kim S, Aimone LJ, Walker JR, Watson J, Sauveur-Michel M, Garcia-Echeverria C, Cho CY, Reddy VA, Schultz PG. Combination therapy targeting both tumor-initiating and differentiated cell populations in prostate carcinoma. Clin Cancer Res. 2010;16:5692–5702. doi: 10.1158/1078-0432.CCR-10-1601. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;60:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 6.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 7.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palma V, Ruiz i Altaba A. Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development. 2004;131:337–345. doi: 10.1242/dev.00930. [DOI] [PubMed] [Google Scholar]
- 9.Palma V, Lim DA, Dahmane N, Sánchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 11.Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z, Cui Y, Ni W, Kim S, Xuan Y. Gli1, a potential regulator of esophageal cancer stem cell, is identified as an independent adverse prognostic factor in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143:243–254. doi: 10.1007/s00432-016-2273-6. [DOI] [PubMed] [Google Scholar]
- 13.Cui Y, Cui CA, Yang ZT, Ni WD, Jin Y, Xuan YH. Gli1 expression in cancer stem-like cells predicts poor prognosis in patients with lung squamous cell carcinoma. Exp Mol Pathol. 2017;102:347–353. doi: 10.1016/j.yexmp.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Ni WD, Yang ZT, Qi WB. Gli1 is a potential cancer stem cell marker and predicts poor prognosis in ductal breast carcinoma. Hum Pathol. 2017;69:38–45. doi: 10.1016/j.humpath.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th edition. New York, NY: Springer; 2010. pp. 103–111. [Google Scholar]
- 16.Yang ZT, Yeo SY, Yin YX, Lin ZH, Lee HM, Xuan YH, Cui Y, Kim SH. Tenascin-C, a prognostic determinant of esophageal squamous cell carcinoma. PLoS One. 2016;11:e0145807. doi: 10.1371/journal.pone.0145807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaworska D, Szliszka E. Targeting apoptotic activity against prostate cancer stem cells. Int J Mol Sci. 2017;18:1648–1669. doi: 10.3390/ijms18081648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korski K, Malicka-Durczak A, Bręborowicz J. Expression of stem cell marker CD44 in prostate cancer biopsies predicts cancer grade in radical prostatectomy specimens. Pol J Pathol. 2015;65:291–295. doi: 10.5114/pjp.2014.48190. [DOI] [PubMed] [Google Scholar]
- 19.Carnero A, Lleonart M. The hypoxic microenvironment: a determinant of cancer stem cell evolution. Bioessays. 2016;38(Suppl 1):S65–74. doi: 10.1002/bies.201670911. [DOI] [PubMed] [Google Scholar]
- 20.Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Lin Z, Sun L, Fan S, Huang Z, Zhang D, Yang Z, Li J, Chen W. Akt/Ezrin Tyr353/NF-κB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. Br J Cancer. 2014;110:695–705. doi: 10.1038/bjc.2013.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Wang Y, Fan C, Gao P, Wang X, Wei G, Wei J. Estrogen promotes stemness and invasiveness of ER-positive breast cancer cells through Gli1 activation. Mol Cancer. 2014;13:137–153. doi: 10.1186/1476-4598-13-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Min S, Xiaoyan X, Fanghui P, Yamei W, Xiaoli Y, Feng W. The glioma-associated oncogene homolog 1 promotes epithelial--mesenchymal transition in human esophageal squamous cell cancer by inhibiting E-cadherin via snail. Cancer Gene Ther. 2013;20:379–85. doi: 10.1038/cgt.2013.36. [DOI] [PubMed] [Google Scholar]
- 24.Zhong ZQ, Song MM, He Y, Cheng S, Yuan HS. Knockdown of ezrin by RNA interference reverses malignant behavior of human pancreatic cancer cells in vitro. Asian Pac J Cancer Prev. 2012;13:3781–3789. doi: 10.7314/apjcp.2012.13.8.3781. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Wang D, Li X, Yan J, Yuan X, Wang W. Targeting of miR-150 on Gli1 gene to inhibit proliferation and cell cycle of esophageal carcinoma EC9706. Cancer Biomark. 2017;21:203–210. doi: 10.3233/CBM-170658. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Guo W, Ren T, Liang W, Zhou W, Lu Q, Jiao G, Yan T. Gli1 inhibition suppressed cell growth and cell cycle progression and induced apoptosis as well as autophagy depending on ERK1/2 activity in human chondrosarcoma cells. Cell Death Dis. 2014;5:e979–990. doi: 10.1038/cddis.2013.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCubrey JA, Abrams SL, Stadelman K, Chappell WH, Lahair M, Ferland RA, Steelman LS. Targeting signal transduction pathways to eliminate chemotherapeutic drug resistance and cancer stem cells. Adv Enzyme Regul. 2010;50:285–307. doi: 10.1016/j.advenzreg.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitting RL, Armstrong AJ. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr Relat Cancer. 2013;20:R83–99. doi: 10.1530/ERC-12-0394. [DOI] [PubMed] [Google Scholar]
- 29.Ke Z, Caiping S, Qing Z, Xiaojing W. Sonic hedgehog-Gli1 signals promote epithelial-mesenchymal transition in ovarian cancer by mediating PI3K/AKT pathway. Med Oncol. 2015;32:368–376. doi: 10.1007/s12032-014-0368-y. [DOI] [PubMed] [Google Scholar]
- 30.Colavito SA, Zou MR, Yan Q, Nguyen DX, Stern DF. Significance of glioma-associated oncogene homolog 1 (GLI1) expression in claudinlow breast cancer and crosstalk with the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway. Breast Cancer Res. 2014;16:444–462. doi: 10.1186/s13058-014-0444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-κB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]


