Abstract
MicroRNAs (miRNAs) play important roles in the development of head and neck squamous cell carcinoma (HNSCC). However, their potential clinical value as biomarkers remains poorly known. The aim of this study was to assess the association between tissue/serum miR-31 expression levels and prognosis of HNSCC. In this clinical study, tumor samples were obtained from 118 patients with HNSCC and 48 patients with oral epithelial dysplasia, and blood samples were collected from all the HNSCC cases and 60 normal controls. The expression levels of tissue/serum miR-31 were measured by real-time PCR. Chi-square test was used to evaluate the correlation between tissue/serum miR-31 and clinical parameters of HNSCC. Survival curves were constructed using the Kaplan-Meier method and log-rank test. Multivariate Cox regression analyses were used to estimate independent predictors of survival for HNSCC. Our findings showed that tissue miR-31 levels in HNSCC tumor specimens exhibited higher than that in oral epithelial dysplasia samples and normal tissues. Oral epithelial dysplasia with higher expression of miR-31 was more prone to progress into HNSCC. Likewise, serum miR-31 expression in HNSCC patients was markedly increased in compared to normal controls. Moreover, serum miR-31 performed well to distinguish HNSCC subjects from controls. In addition, increased tissue/serum miR-31 expression was positively correlated with poor clinical variables and dismal prognosis. Finally, tissue miR-31 was confirmed to be an independent prognostic factor for HNSCC. Taken together, miR-31 had strong potential as a promising biomarker in HNSCC detection.
Keywords: Head and neck squamous cell carcinoma, oral epithelial dysplasia, prognostic biomarker, miR-31
Introduction
Head and neck squamous cell carcinoma (HNSCC), which commonly occurs in the oral cavity, pharynx, oropharynx and larynx, is the sixth most common cause ofcancer death around the world [1-3]. Although there are multiple treatment modalities for HNSCC including surgery, radiotherapy and chemotherapy, the prognosis of patients with HNSCC has remained unfavorable over the past 30 years [4-6]. Therefore, it is urgent to identify novel biomarkers highly specific to HNSCC for its early detection and prognosis.
MicroRNAs (miRNAs) are a subset of small highly conserved non-coding RNAs of 19-25 nucleotides in length and negatively regulate gene expression by interacting with 3’ untranslated regions (UTR) of target mRNAs [7,8]. Aberrant miRNAs expression occurs in many types of malignant cancers and they have been shown to play important roles in the initiation and progression of human cancers including HNSCC. For instance, Li et al revealed that miR-93 expression was greatly upregulated in HNSCC tissue specimens and cell lines. High miR-93 expression was closely correlated with poor prognosis in HNSCC patients, suggesting that miR-93 played an oncogenic role in HNSCC [9]. In contrast, Li and colleagues found that miR-512-5p was markedly decreased in HNSCC cell lines. Moreover, inhibition of miR-512-5p promoted cancer cell proliferation and migration through targeting human telomerase reverse transcriptase, indicating that miR-512-5p acted as a tumor suppressor in HNSCC [10].
In terms of miR-31, growing evidence have shown that abnormal expression of miR-31 involved in the progression of various types of cancers, including gastric cancer [11], rectal carcinoma [12], lung cancer [13], triple negative breast cancer [14] and gallbladder cancer [15]. However, whether the aberrantly expressed miR-31 in HNSCC has any clinical significance remained unclear. In the current study, we examine miR-31 expression levels in the tissue and serum samples from patients with HNSCC, and analyze its potential prognostic value.
Patients and methods
HNSCC patients and tissue and plasma samples
A total of 118 HNSCC patients and 48 patients with oral epithelial dysplasia were enrolled and none of the patients had received any treatment before surgery. All the tissue samples were collected and snap-frozen immediately after surgical resection. Moreover, sixty healthy volunteers were recruited as controls. Up to 5 mL of fasting venous blood was obtained from the HNSCC patients and normal controls. Blood samples were separated by centrifugation (10 min, 2800×g), and the supernatant fluids were divided into aliquots and stored at -80°C for further analysis.
Smokers were defined as those patients who smoked > 100 cigarettes during their lifetime and alcoholics were defined as those who had four or more drinks per week. Detailed clinical characteristics of the HNSCC patients were presented in Table 1. This study was performed with approval from the Ethics Committee of Baotou Cancer Hospital and prior informed consent was obtained from the patients for collection of specimens. All specimens were handled and made anonymous according to ethical and legal standards.
Table 1.
Relationship between tissue/serum miR-31 expression and clinical data
| Clinical data | Patients (N=118) | Tissue miR-31 expression | P | Serum miR-31 expression | P | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Low (n=57) | High (n=61) | Low (n=45) | High (n=73) | ||||
| Gender | 0.5530 | 0.6443 | |||||
| Men | 65 | 33 | 32 | 26 | 39 | ||
| Women | 53 | 24 | 29 | 19 | 34 | ||
| Age | 0.2109 | 0.1743 | |||||
| <56 | 51 | 28 | 23 | 23 | 28 | ||
| ≥56 | 67 | 29 | 38 | 22 | 45 | ||
| Smoking | 0.5101 | 0.6972 | |||||
| No | 42 | 22 | 20 | 17 | 25 | ||
| Yes | 76 | 35 | 41 | 28 | 48 | ||
| Alcohol consumption | 0.3875 | 0.2337 | |||||
| No | 47 | 25 | 22 | 21 | 26 | ||
| Yes | 71 | 32 | 39 | 24 | 47 | ||
| Distant metastasis | 0.3426 | 0.1101 | |||||
| M0 | 114 | 56 | 58 | 45 | 69 | ||
| M1 | 4 | 1 | 3 | 0 | 4 | ||
| T stage | 0.1046 | 0.3466 | |||||
| T1/T2 | 41 | 24 | 17 | 18 | 23 | ||
| T3/T4 | 77 | 33 | 44 | 27 | 50 | ||
| Node stage | 0.0160 | 0.0296 | |||||
| N0 | 61 | 36 | 25 | 29 | 32 | ||
| N+ | 57 | 21 | 36 | 16 | 41 | ||
| TNM stage | 0.0002 | <0.0001 | |||||
| I/II | 33 | 25 | 8 | 22 | 11 | ||
| III/IV | 85 | 32 | 53 | 23 | 62 | ||
Isolation of total RNA and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from tissue or serum samples by using a mirVana PARIS isolation kit (Ambion, Austin, TX, USA) in accordance with the manufacturer’s instructions. The reverse transcription reaction was carried out using the miScript Reverse Transcription (Biosystems, Shanghai, China). Quantitative PCR was performed using TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, USA) on the Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, USA). The relative expression levels of miR-31 were normalized to the small nucleolar RNA RNU6b. Moreover, the relative quantification value of miR-31 expression was calculated using the 2-ΔΔCt method. Each experiment was run in triplicate.
Statistical analysis
Statistical analyses were carried out using GraphPad Prism 5.01 (GraphPad Software Inc., San Diego, California, USA) and SigmaPlot 13.0 (Systat, Chicago, IL, USA) software. Kruskal-Wallis test or Mann-Whitney U test was performed to compare the difference in miR-31 expression levels among groups. The Pearson Chi-square test was conducted to assess the correlation between tissue/serum miR-31 expression and clinical parameters. Receiver operating characteristic (ROC) curve was constructed, and the area under the ROC curve (AUC) was measured to evaluate the diagnostic value of miR-31. Survival curves were plotted by the Kaplan-Meier method, and comparisons were made using the log-rank tests. Cox proportional hazards model was used to estimate independent prognostic factors for HNSCC. A difference was defined statistically significant when P<0.05.
Results
Tissue miR-31 levels were increased in HNSCC samples
Tissue miR-31 expression levels in normal mucosa, oral epithelial dysplasia, and HNSCC were detected by qRT-PCR. The results showed that tissue miR-31 levels progressively increased from normal mucosa to oral epithelial dysplasia to HNSCC (P<0.01, Figure 1).
Figure 1.
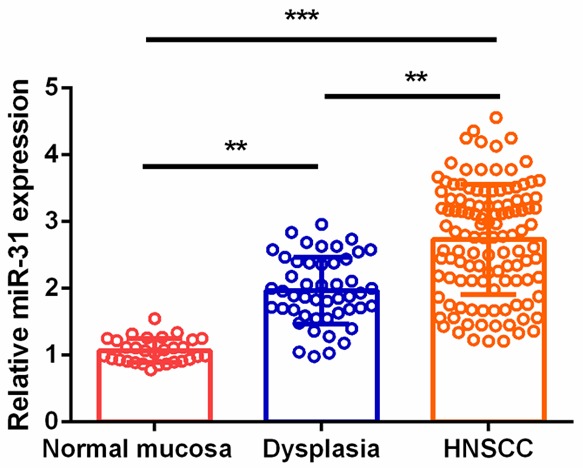
Tissue miR-31 levels in the normal mucosa, oral epithelial dysplasia, and HNSCC.
The median value of miR-31 was used to categorize patients with oral epithelial dysplasia into two groups. Oral epithelial dysplasia patients in the high tissue miR-31 group had a significantly higher risk of progression to HNSCC (8/24) compared to those in the low tissue miR-31 group (1/24) (Figure 2).
Figure 2.
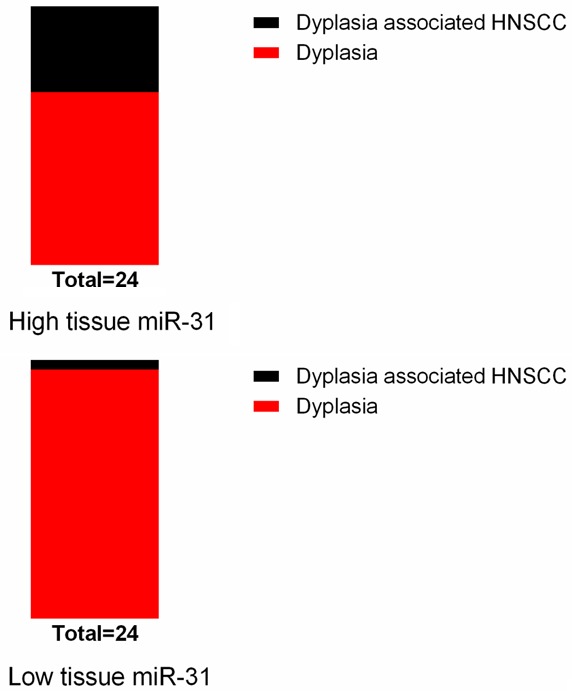
Oral epithelial dysplasia with higher tissue miR-31 level was more prone to progress to HNSCC.
Serum miR-31 levels were increased in HNSCC samples and its diagnostic significance
Serum miR-31 levels in all the HNSCC cases and normal controls were measured, and serum miR-31 expression levels in HNSCC patients were significantly higher than in healthy volunteers (P<0.01, Figure 3). In addition, ROC curve analysis was carried out and the results showed that serum miR-31 could easily distinguish HNSCC patients from healthy controls with an AUC value of 0.831, and with a specificity of 78.3% and a sensitivity of 79.7% (Figure 4).
Figure 3.
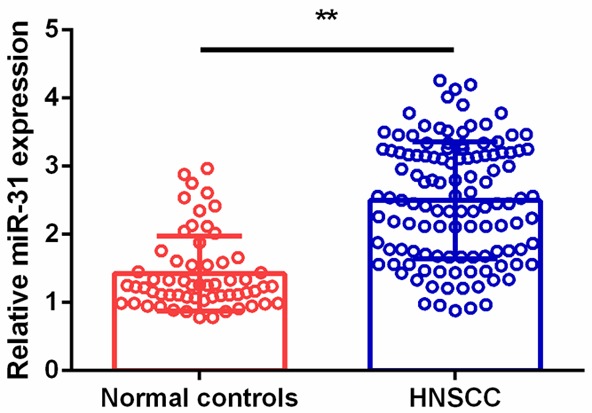
Serum miR-31 levels in normal controls and HNSCC.
Figure 4.
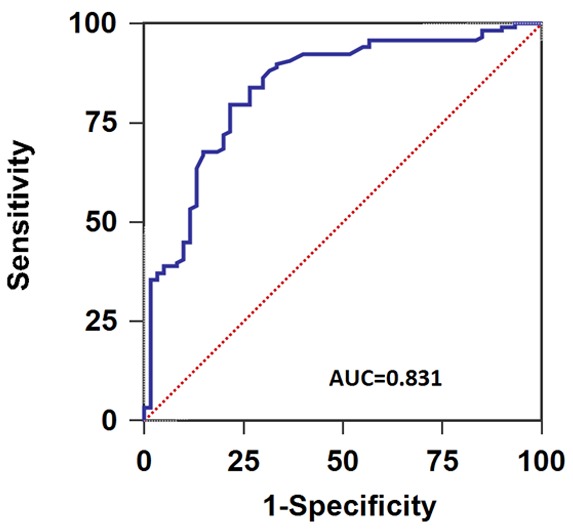
ROC curve of serum miR-31 for the diagnosis of HNSCC.
The association between miR-31 expression and clinical characteristics of HNSCC patients
To analyze the association between tissue/serum miR-31 expression levels and the clinical features of HNSCC, the median expression level of tissue/serum miR-31 was used as a cut-off point to divide 118 HNSCC patients into low tissue miR-31 level (n=57) and high tissue miR-31 level (n=61) groups, and low serum miR-31 level (n=45) and high serum miR-31 level (n=73) groups, respectively. As shown in Table 1, tissue miR-31 expression was significantly correlated with TNM stage (P=0.0002) and node stage (P=0.0160). Likewise, serum miR-31 expression was closely associated with TNM stage (P<0.0001) and node stage (P=0.0296). However, no significant correlation was detected between tissue/serum miR-31 expression and gender, age, alcohol consumption, smoking, T stage, or distant metastasis (all P > 0.05).
Up-regulation of miR-31 correlates with poor prognosis in HNSCC patients
Using a Kaplan-Meier survival analysis plus log-rank test, we found that the HNSCC patients in low tissue miR-31 expression group had longer overall survival (OS) (P=0.019, Figure 5A) and disease-free survival (DFS) than those in high expression group (P=0.006, Figure 5B). Similarly, the HNSCC cases in high serum miR-31 expression group had shorter OS (P=0.016, Figure 6A) and DFS (P=0.004, Figure 6B) in comparison with those in low expression group.
Figure 5.
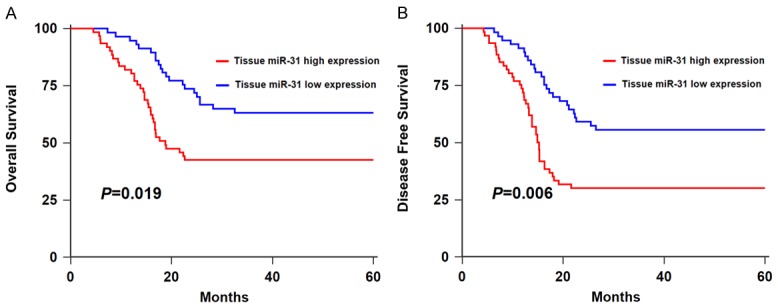
A. Kaplan-Meier curve of OS of HNSCC patients stratified by tissue miR-31 level.B. Kaplan-Meier curve of DFS of HNSCC patients stratified by tissue miR-31 level.
Figure 6.
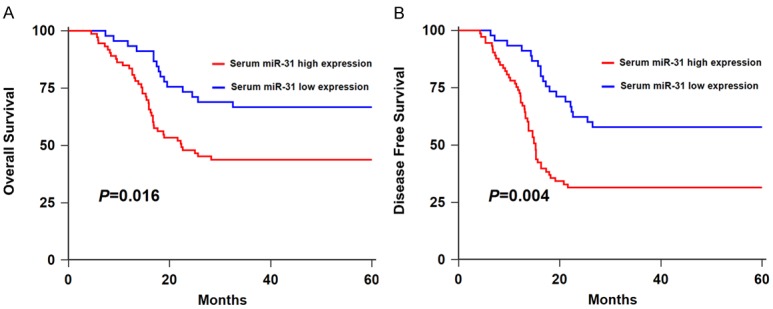
A. Kaplan-Meier curve of OS of HNSCC patients stratified by serum miR-31 level. B. Kaplan-Meier curve of DFS of HNSCC patients stratified by serum miR-31 level.
Next, multivariate Cox regression analysis was performed to determine the influence of serum miR-31 expression and clinicopathological features. As showed in Table 2, we demonstrated that TNM stage (OS: HR=4.36, 95% CI=1.56-7.23, P=0.008; DFS: HR=4.57, 95% CI=1.71-7.54, P=0.001), node stage (OS: HR=3.17, 95% CI=1.33-4.90, P=0.023; DFS: HR=3.52, 95% CI=1.42-5.69, P=0.017) and tissue miR-31 expression (OS: HR=3.31, 95% CI=1.42-5.36, P=0.015; DFS: HR=3.86, 95% CI=1.53-6.05, P=0.009) were independent prognostic factors of overall survival and tumor recurrence in HNSCC patients.
Table 2.
Prognostic factors for OS and DFS by multivariate analysis
| Clinical data | HR (95% CI) | P value |
|---|---|---|
| Overall survival (OS) | ||
| Node stage (N+ vs. N0) | 3.17 (1.33-4.90) | 0.023 |
| TNM stage (III/IV vs. I/II) | 4.36 (1.56-7.23) | 0.008 |
| Tissue miR-31 (high vs. low) | 3.31 (1.42-5.36) | 0.015 |
| Disease-free survival (DFS) | ||
| Node stage (N+ vs. N0) | 3.52 (1.42-5.69) | 0.017 |
| TNM stage (III/IV vs. I/II) | 4.57 (1.71-7.54) | 0.001 |
| Tissue miR-31 (high vs. low) | 3.86 (1.53-6.05) | 0.009 |
Discussion
Due to the limitation of current diagnostic methods, patients with HNSCC are often diagnosed at late stages and has poor clinical outcome [16,17]. Hence, it is crucial to identify new indicators with higher accuracy for prognostic prediction of HNSCC.
In the current study, our results showed that tissue miR-31 expression was remarkably increased in HNSCC tumor samples. In addition, oral epithelial dysplasia with higher tissue miR-31 levels was more prone to progress to HNSCC. Likewise, serum miR-31 expression in HNSCC patients was greatly elevated compared to normal controls. Moreover, serum miR-31 could discriminate HNSCC subjects from controls with high accuracy. In HNSCC, up-regulation of tissue/serum miR-31 was closely correlated with poor clinical outcome and unfavorable prognosis, indicating that miR-31 might play an oncogenic role in HNSCC. In line with our findings, Lu et al found that HNSCC patients with lower miR-31 expression had better survival. Moreover, miR-31 functioned asan oncogene in HNSCC by downregulating ARID1A [18]. Liu et al showed that ectopic stable miR-31 expression suppressed the tumorigenicity of HNSCC cells under normoxic conditions by inversely regulating FIH, which was a regulatory factor of hypoxia-inducible factor [19].
Besides HNSCC, miR-31 was also reported to act as an oncogene in multiple types of cancer. Caramés and colleagues demonstrated that enhanced miR-31 expression markedly promoted the proliferation and invasion of colorectal cancer cells by targeting the autophagy-related genes, including Beclin-1, ATG, DRAM and LC3. Furthermore, increased miR-31 expression predicted unfavorable clinical outcome for patients with locally advanced rectal cancer [12]. Similarly, it was validated that loss of miR-31 inhibited lung cancer cell proliferation, invasion, progression and induced cell apoptosis by negatively regulating RAS/MAPK signaling [13] or BRCA1-associated protein-1 [20].
MiR-31 has also been shown to play a tumor suppressor role in carcinogenesis. Luo and colleagues confirmed that overexpression of miR-31 inhibited migration and invasion of triple negative breast cancer cells by degrading the special AT-rich sequence-binding protein-2 [14]. In addition, ectopic miR-31 expression repressed cell viability and migration of gallbladder cancer cells by suppressing the expression of Src [15]. In gastric cancer, Wang et al showed that miR-31 expression was especially decreased in cancerous tissues. Moreover, the knockdown of miR-31 enhanced cancer cell viability and inhibited cell apoptosis in vitro and promoted tumor growth in vivo [21]. The underlying mechanisms accounting for the conflicting role of miR-31 in different types of cancer need further exploration. The number of participants in our study is relatively small, thus the clinical significance of miR-31 in HNSCC progression may require further validation with larger clinical samples.
Conclusions
In summary, this study demonstrated that tissue/serum miR-31 levels were remarkably increased in HNSCC patients and closely associated with aggressive clinical variables. In addition, high tissue/serum miR-31 expression was positively correlated with shorter OS and DFS. Moreover, tissue miR-31 was proved to be an independent predictor for both OS and DFS of HNSCC. Therefore, miR-31 might serve as a prognostic indicator for HNSCC.
Disclosure of conflict of interest
None.
References
- 1.Jones A. The molecular cell biology of head and neck cancer with clinical applications. Section 1: fundamental biology and the basis of cancer. Clin Otolaryngol Allied Sci. 2004;29:475–491. doi: 10.1111/j.1365-2273.2004.00834.x. [DOI] [PubMed] [Google Scholar]
- 2.Tu HF, Lin SC, Chang KW. MicroRNA aberrances in head and neck cancer: pathogenetic and clinical significance. Curr Opin Otolaryngol Head Neck Surg. 2013;21:104–111. doi: 10.1097/MOO.0b013e32835e1d6e. [DOI] [PubMed] [Google Scholar]
- 3.Cui L, Cheng S, Liu X, Messadi D, Yang Y, Hu S. Syntenin-1 is a promoter and prognostic marker of head and neck squamous cell carcinoma invasion and metastasis. Oncotarget. 2016;7:82634–82647. doi: 10.18632/oncotarget.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran N, Rose BR, O’Brien CJ. Role of human papillomavirus in the etiology of head and neck cancer. Head Neck. 2007;29:64–70. doi: 10.1002/hed.20460. [DOI] [PubMed] [Google Scholar]
- 5.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahiya K, Dhankhar R. Updated overview of current biomarkers in head and neck carcinoma. World J Methodol. 2016;6:77–86. doi: 10.5662/wjm.v6.i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Li G, Ren S, Su Z, Liu C, Deng T, Huang D, Tian Y, Qiu Y, Liu Y. Increased expression of miR-93 is associated with poor prognosis in head and neck squamous cell carcinoma. Tumour Biol. 2015;36:3949–3956. doi: 10.1007/s13277-015-3038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Lei H, Xu Y, Tao ZZ. MiR-512-5p suppresses tumor growth by targeting hTERT in telomerase positive head and neck squamous cell carcinoma in vitro and in vivo. PLoS One. 2015;10:e0135265. doi: 10.1371/journal.pone.0135265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Liu S, Xia Y, Wu K. MiR-31 regulates rho-associated kinase-myosin light chain (ROCK-MLC) pathway and inhibits gastric cancer invasion: roles of rhoA. Med Sci Monit. 2016;22:4679–4691. doi: 10.12659/MSM.898399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caramés C, Cristobal I, Moreno V, Marín JP, González-Alonso P, Torrejón B, Minguez P, Leon A, Martín JI, Hernández R, Pedregal M, Martín MJ, Cortés D, García-Olmo D, Fernández MJ, Rojo F, García-Foncillas J. MicroRNA-31 emerges as a predictive biomarker of pathological response and outcome in locally advanced rectal cancer. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17060878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmonds MD, Boyd KL, Moyo T, Mitra R, Duszynski R, Arrate MP, Chen X, Zhao Z, Blackwell TS, Andl T, Eischen CM. MicroRNA-31 initiates lung tumorigenesis and promotes mutant KRAS-driven lung cancer. J Clin Invest. 2016;126:349–364. doi: 10.1172/JCI82720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo LJ, Yang F, Ding JJ, Yan DL, Wang DD, Yang SJ, Ding L, Li J, Chen D, Ma R, Wu JZ, Tang JH. MiR-31 inhibits migration and invasion by targeting SATB2 in triple negative breast cancer. Gene. 2016;594:47–58. doi: 10.1016/j.gene.2016.08.057. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Chen W, Zhang H, Zhang Y, Ke F, Wu X, Zhang Y, Weng M, Liu Y, Gong W. MiR-31 regulates the cisplatin resistance by targeting Src in gallbladder cancer. Oncotarget. 2016;7:83060–83070. doi: 10.18632/oncotarget.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz S, Ang KK, Vermorken J, Haddad R, Suarez C, Wolf GT, Hamoir M, Machiels JP. Targeted therapies for squamous cell carcinoma of the head and neck: current knowledge and future directions. Cancer Treat Rev. 2014;40:390–404. doi: 10.1016/j.ctrv.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 18.Lu WC, Liu CJ, Tu HF, Chung YT, Yang CC, Kao SY, Chang KW, Lin SC. MiR-31 targets ARID1A and enhances the oncogenicity and stemness of head and neck squamous cell carcinoma. Oncotarget. 2016;7:57254–57267. doi: 10.18632/oncotarget.11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CJ, Tsai MM, Hung PS, Kao SY, Liu TY, Wu KJ, Chiou SH, Lin SC, Chang KW. MiR-31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res. 2010;70:1635–1644. doi: 10.1158/0008-5472.CAN-09-2291. [DOI] [PubMed] [Google Scholar]
- 20.Yu M, Liang H, Fu Z, Wang X, Liao Z, Zhou Y, Liu Y, Wang Y, Hong Y, Zhou X, Yan X, Yu M, Ma M, Zhang W, Guo B, Zhang J, Zen K, Zhang CY, Wang T, Zhang Q, Chen X. BAP1 suppresses lung cancer progression and is inhibited by miR-31. Oncotarget. 2016;7:13742–13753. doi: 10.18632/oncotarget.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Zhang X, Liu Y, Ni Z, Lin Y, Duan Z, Shi Y, Wang G, Li F. Downregulated miR-31 level associates with poor prognosis of gastric cancer and its restoration suppresses tumor cell malignant phenotypes by inhibiting E2F2. Oncotarget. 2016;7:36577–36589. doi: 10.18632/oncotarget.9288. [DOI] [PMC free article] [PubMed] [Google Scholar]


