Abstract
Plasma cell myeloma is a clonal proliferation of neoplastic plasma cells and typically expresses a monoclonal heavy and/or light chain immunoglobulin. Plasma cell myeloma with dual expression of kappa and lambda light chains in a single clone is extremely rare. Here we report three cases of plasma cell myeloma with a co-expression of both kappa and lambda light chains. All three cases were confirmed by comprehensive workup including IHC, ISH and flow cytometry analysis to detect light chain expression patterns at the mRNA and protein levels. We also reviewed three cases so far published in the literature. Our study suggests that plasma cell myeloma with dual light chain expression may be more likely to be light chain only myeloma. It may have a high frequency of complex cytogenetic and/or FISH abnormalities, associated with a high-risk disease.
Keywords: Dual expression of kappa and lambda, plasma cell myeloma, high risk disease
Introduction
Plasma cell myeloma is characterized by a multifocal clonal proliferation of plasma cells based in bone marrow and the presence of paraproteins in the blood and/or urine with associated end organ damage. It is the second most common hematological malignancy accounting for approximately 10-15% of hematopoietic cancers [1]. The diagnosis of plasma cell myeloma requires ≥10% clonal plasma cells in the bone marrow. Plasma cell myeloma is further classified into asymptomatic (smoldering) myeloma, symptomatic myeloma, non-secretory myeloma, and plasma cell leukemia based on a combination of clinicopathological and radiological features [1,2].
Normal plasma cells are terminally differentiated effector B cells developed from naïve marginal zone B cells and follicular B cells after an antigen encounter [3]. The immunoglobulins produced by normal plasma cells are central to the body’s adaptive immune response to foreign antigens. Immunoglobulins are either secretory or cell surface bound proteins which are composed of two heavy chains (α, γ, δ, ε or μ) and two light chains (κ or λ). Neoplastic plasma cells in the majority of plasma cell myeloma cases retain the ability to produce either complete immunoglobulins or at least a fragment of paraproteins.
Plasma cell myelomas produce or express different paraproteins. The frequency of these paraproteins is as following: IgG (60%), IgA (24%), IgD (3%), IgM (0.5%), IgE (very rare), light chain only myeloma (11%), and non-secretory myeloma (less than 1%). Up to 2% of myeloma cases are also found to secrete more than one paraprotein, in which the majority secrete two different heavy chain isotypes or subclasses. These myeloma cases are classified as biclonal plasma cell myeloma [1,4,5]. There are very few reported cases in the literature in which the myeloma cells are found to express both kappa and lambda light chains in a single clonal plasma cell population and only three cases have been studied in detail [6,7]. Here we report three cases of plasma cell myeloma with dual expression of both kappa and lambda light chains. We summarize their clinical, pathological, flow cytometric, cytogenetic, FISH, and molecular characteristics along with previously reported three cases.
Case studies
The first case is a 65-year-old male patient presenting with anemia and lytic bone lesions in 2011 (Case 1 in Table 1). Serum protein electrophoresis revealed monoclonal kappa light chain gammopathy. A quantitative serum immunoglobulin analysis showed decreased IgM (<5 mg/dL; reference range 48-271 mg/dl) and IgG (211 mg/dl; reference range 694-1618 mg/dl) levels, and an increased IgA (4481 mg/dl; reference range 81-463 mg/dl) level. A serum immunoglobulin free light chain study revealed increased kappa free light chain (1370 mg/dl; reference range 158-502 mg/dl) and reduced lambda free light chain (23 mg/dl; reference range 100-317 mg/dl) levels. The kappa/lambda ratio was increased to 59.57 (reference range: 1.35-2.95). The urine immunoelectrophoresis revealed clonal IgA kappa and a free kappa light chain. Flow cytometric analysis of the marrow aspirate detected a distinct population of abnormal plasma cells expressing CD38, CD138 (Figure 1A), IgA, and both cytoplasmic kappa and lambda light chains (Figure 1B) with negativity for CD56. A morphological evaluation revealed markedly increased plasma cells (~60-70% of total marrow cellularity, Figure 1C and 1D) in a hypercellular marrow (80-90%). Nodules of plasma cells were seen. Plasma cells demonstrated atypical features including large sizes, a high nuclear to cytoplasmic ratio, centrally located nuclei, and visible nucleoli. Immunohistochemistry (IHC) stains and mRNA in-situ hybridization (ISH) tests confirmed that these plasma cells were positive for both kappa and lambda light chains (Figure 1E-H). Plasma cells were positive for IgA and negative for IgG, IgM, IgD. This patient was diagnosed with plasma cell myeloma with a dual expression of kappa and lambda light chains. No significant pleomorphic or plasmablastic features were evident. Karyotype was normal. FISH studies (including probes for t(4;14), +5, +7, +11q, t(11;14), -13, 13q-, t(14;16) and TP53(17p-) detected monosomy 13. A molecular test by IgH PCR detected a clonal immunoglobulin heavy chain gene rearrangement with a single high peak, indicating the presence of a single clonal plasma cell population.
Table 1.
Characteristics of plasma cell myeloma with dual expression of kappa and lambda light chains
| CASE# | Age (year)/sex | Pathology diagnosis | Serum immunoglobulin analysis | Urine protein analysis | Flow cytometry | IHC test of plasma cells | ISH test of plasma cells | Karyotype and/or FISH |
|---|---|---|---|---|---|---|---|---|
| CASE 1 | 65/M | Plasma cell myeloma | Increased IgA kappa and free kappa chain | Increased IgA kappa and free kappa chain | Kappa+, Lambda+ | Kappa+, lambda+, IgA+ | Kappa+, Lambda+ | Normal karyotype FISH: -13 |
| CASE 2 | 58/M | Plasma cell myeloma | Increased free lambda light chain, no increased Ig | Increased free lambda chain | Kappa+, Lambda+ | Kappa+, Lambda+, No heavy chains | Kappa+, Lambda+ | Complex karyotype |
| CASE 3 | 76/F | Plasma cell myeloma | Increased free kappa chain, no increased Ig | NA | Kappa+, Lambda+ | Kappa+, Lambda+, No heavy chains | Kappa+, Lambda+ | Normal karyotype FISH: 1q+, +5 and 13q- |
| CASE 4 [6] | 68/F | Plasma cell myeloma and plasmacell leukemia | Increased IgG kappa and free lambda | Increased both free kappa and lambda chains | Kappa+, Lambda+ | Kappa-, Lambda+ | Kappa+, Lambda+ | Complex karyotype |
| CASE 5 [7] | 52/M | Plasma cell myeloma | Increased free lambda chain, no increased Ig | NA | Kappa+, Lambda+ | Kappa-, Lambda+, Heavy chains: not done | Kappa+, Lambda+ | Complex karyotype |
| CASE 6 [7] | 58/F | Plasma cell myeloma | Increased IgG lambda and free lambda | Increased free lambda chain | Kappa+, Lambda+ | Kappa+, lambda+, IgG+ | Kappa+, Lambda+ | Complex karyotype |
Figure 1.
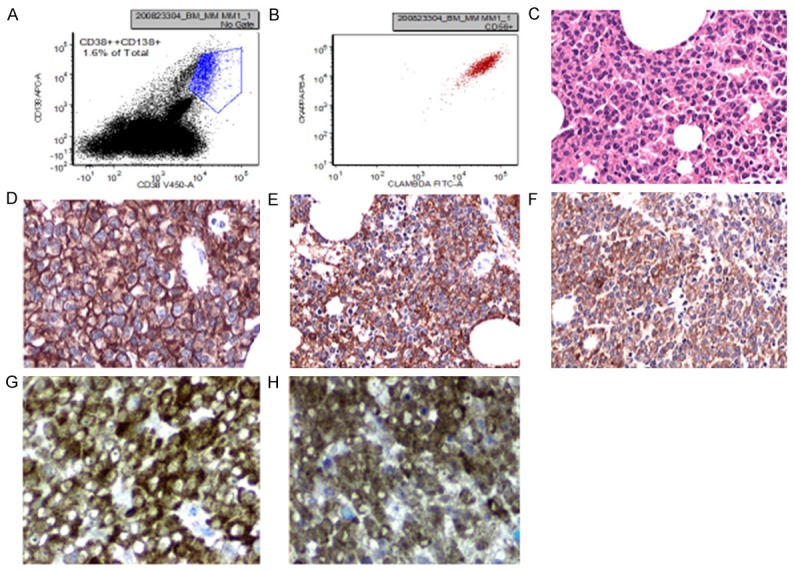
Plasma cell myeloma with dual expression of kappa and lambda light chains (case 1 in Table 1). A and B: Flow cytometric analysis revealing CD138 positive plasma cells with dual expression of kappa and lambda light chains. C: Hematoxylin and eosin staining showing diffuse infiltration of bone marrow by myeloma cells in a core biopsy. D: Immunohistochemical staining of bone marrow core biopsy showing diffuse positive staining for CD138. E and F: Immunohistochemical stains of bone marrow core biopsy showing diffuse positive staining for both kappa and lambda. G and H: In-situ hybridization detecting kappa and lambda light chain mRNA in bone marrow core biopsy.
The second case is a 58-year-old male patient with a history of stage IIIB IgG lambda multiple myeloma diagnosed in October 2003 (case 2 in Table 1). At that time serum protein electrophoresis and immunofixation revealed a monoclonal lambda light chain. Quantitative serum immunoglobulin analysis showed decreased IgM (6 mg/dL; reference range 40-230 mg/dl) and IgG (491 mg/dl; reference range 700-1600 mg/dl) levels and normal IgA (111 mg/dl; reference range 70-400 mg/dl) level. The urine immunoelectrophoresis revealed a monoclonal free lambda light chain. The monoclonal free lambda chain was at 20.6% of the total urine protein. This patient was in remission after treatment with Revlimid and Dexamethasone followed by an autologous stem cell transplant. The patient then presented with lytic bone lesions and anemia in November 2011. Flow cytometry detected clonal plasma cells expressing CD56 and both cytoplasmic kappa and lambda light chains in bone marrow aspirate. A core biopsy revealed large aggregates of atypical plasma cells, representing around 20-40% of total marrow cellularity. Plasma cells strongly expressed both kappa and lambda light chains by IHC stains and ISH studies. Immunostains for IgA, IgD, IgM, and IgG heavy chains were negative. The findings are consistent with recurrent plasma cell myeloma with dual expression of kappa and lambda light chains. Cytogenetic analysis showed a complex karyotype with several bizarre structural and numerical changes: 44~46, del(X)(q24), -Y, del(1)(p13p22), t(2;8)(p12;q11.2), der(2)(2;8)(p12;q11.2), add(3)(p14), add(5)(p15.1), del(7)(q22), -8, i(12)(q10;q10), -14, -15, -18, add(22)(q13), +1-4mar[cp4]/46, XY [16].
The third case is 76-year-old female patient presenting with eosinophilia and increased LDH level in November 2017 (case 3 in Table 1). Quantitative serum immunoglobulin analysis showed normal IgM (61 mg/dL; reference range 48-271 mg/dl), decreased IgG (688 mg/dl; reference range 694-1618 mg/dl) and normal IgA (98 mg/dl; reference range 81-463 mg/dl) levels. A serum immunoglobulin free light chain study revealed increased kappa free light chain (294.9 mg/l; reference range 3.3-19.4 mg/l) and normal lambda free light chain (20.8 mg/l; reference range 5.7-26.3 mg/l) levels. The Kappa/lambda ratio was increased at 14.18 (reference range 0.26-1.65). Bone lytic lesions were not detected. A bone marrow biopsy was performed. Flow cytometry detected abnormal plasma cells expressing CD56 and both kappa and lambda light chains. The core biopsy revealed ~15% atypical plasma cells with no detectable IgA, IgD, IgM, and IgG heavy chains. The plasma cells strongly expressed both kappa and lambda by IHC and ISH studies. The findings are consistent with a plasma cell myeloma with a dual expression of kappa and lambda light chains. The IgH PCR test for clonality was negative and compatible with negative IHC stains for heavy chains (negative for IgA, IgG, IgM, IgD). Her karyotype was normal. However, the FISH analysis with probes for 1p/1q, 5p/5q, 13q14/13q34, IGH rearrangement, and TP53 detected 1q+, +5 and 13q-.
Discussion
There are few reported cases of plasma cell myeloma with a dual expression of kappa and lambda light chains in the literature. Table 1 summarizes the clinical, pathological, flow cytometric, cytogenetic, and FISH characteristics of these three cases published in the literature [6,7] and three new cases in our current report. The final diagnoses as plasma cell myeloma with a dual expression of kappa and lambda light chains in all six cases were confirmed by comprehensive workups including ISH, IHC, and flow cytometric analysis to detect light chain expression patterns at the mRNA and protein levels. A single clonal population of plasma cells was confirmed by IgH PCR test for heavy chain rearrangements when heavy chain was expressed (Table 1, case 1).
Myeloma usually occurs around the age of 60th and is more common in men than women. IgG and IgA are more frequent [1]. Similarly, the median age is 61.5 years old (range from 52 to 76) and IgG and IgA myeloma are common in these six plasma cell myeloma cases. However, no male predominance is noted. 3 cases are men and 3 cases are women (Table 1). Interestingly all six plasma cell myeloma cases with dual expression of kappa and lambda light chains harbor either complex karyotype(s) or FISH evidence of chromosome abnormalities including monosomy 13 and +1q (Table 1), which have been reported to be associated with a high risk disease [8-11].
Approximately 0.5-2% of normal B cells express dual kappa and lambda light chains [12,13]. These normal B cells may be the neoplastic counterparts leading to the formation of B cell lymphoma with dual surface immunoglobulin expression [14-16]. It is not clear whether myeloma with dual expression of kappa and lambda light chains is the result of neoplastic transformation of the dual light chain expressing B-cells.
The mechanism by which B or plasma cells prevent the production of multiple heavy chain classes or light chain classes is defined generally as allelic exclusion, but the actual molecular mechanisms are still poorly understood. The locus encoding for the heavy chain portion undergoes rearrangement first, and if successful, it is followed by a rearrangement of the kappa light chain locus. Only if the kappa light chain rearrangement for both alleles is ineffective does lambda light chain rearrangement occur. Interestingly, half of the cases (3 out 6) described in our study express dual light chains with no detectable heavy chains (Table 1), which is significantly higher than it is in the general myeloma population (~11%) [1]. The lack of heavy chains in myeloma can be due to a variety of reasons, including illegitimate switch recombination and/or deletion of the rearranged IGH locus. The phenomenon raises a possibility that clonal plasma cells with unsuccessful heavy light chain rearrangements may more likely have failed allelic exclusion machinery, leading to further kappa and lambda light chain gene rearrangements.
Our above observation reveals some interesting features of plasma cell myeloma with dual expression of kappa and lambda light chains. A high frequency of complex cytogenetic and/or FISH abnormalities associated with an unfavorable prognosis may exist in this group of patients. However, due to the limited number of cases reported, further investigation is warranted to shed more light on this condition.
Disclosure of conflict of interest
None.
References
- 1.McKenna RW, Kyle RA, Kuehl WM, Harris NL, Coupland RW, Fend F. Plasma cell neoplasms. In: Swerdlow SH, Campo E, Harris E, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon (France): IARC; 2017. pp. 241–258. [Google Scholar]
- 2.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 4.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J. International staging system for multiple myeloma. J. Clin. Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 5.García-García P, Enciso-Alvarez K, Diaz-Espada F, Vargas-Nuñez JA, Moraru M, Yebra-Bango M. Biclonal gammopathies: retro. spective study of 47 patients. Rev Clin Esp. 2015;215:18–24. doi: 10.1016/j.rce.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Gentry M, Pettenati M, Pang CS. Biclonal light chain gammopathy with aberrant CD33 expression in secondary plasma cell leukemia. Int J Clin Exp Pathol. 2013;6:2224–2229. [PMC free article] [PubMed] [Google Scholar]
- 7.Jiwani S, Bornhost J, Alapat D. Biphenotypic plasma cell myeloma: two cases of plasma cell neoplasm with a coexpression of kappa and lambda light chains. Int J Clin Exp Pathol. 2015;8:8536–8544. [PMC free article] [PubMed] [Google Scholar]
- 8.Shaughnessy JD Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, Stewart JP, Kordsmeier B, Randolph C, Williams DR, Xiao Y, Xu H, Epstein J, Anaissie E, Krishna SG, Cottler-Fox M, Hollmig K, Mohiuddin A, Pineda-Roman M, Tricot G, van Rhee F, Sawyer J, Alsayed Y, Walker R, Zangari M, Crowley J, Barlogie B. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 9.Nemec P, Zemanova Z, Kuglik P, Michalova K, Tajtlova J, Kaisarova P, Oltova A, Filkova H, Holzerova M, Balcarkova J, Jarosova M, Rabasova J, Hruba M, Spicka I, Gregora E, Adam Z, Scudla V, Maisnar V, Schutzova M, Hajek R Czech Myeloma Group. Complex karyotype and translocation t(4;14) define patients with high-risk newly diagnosed multiple myeloma: results of CMG2002 trial. Leuk Lymphoma. 2012;53:920–927. doi: 10.3109/10428194.2011.634042. [DOI] [PubMed] [Google Scholar]
- 10.Binder M, Rajkumar SV, Ketterling RP, Greipp PT, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Hayman SR, Hwa YL, Zeldenrust SR, Lust JA, Russell SJ, Leung N, Kapoor P, Go RS, Gonsalves WI, Kyle RA, Kumar SK. Prognostic implications of abnormalities of chromosome 13 and the presence of multiple cytogenetic high-risk abnormalities in newly diagnosed multiple myeloma. Blood Cancer J. 2017;7:e600. doi: 10.1038/bcj.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chesi M, Bergsagel PL. Molecular pathogenesis of multiple myeloma: basic and clinical updates. Int J Hematol. 2013;97:313–323. doi: 10.1007/s12185-013-1291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaw L, Siwarski D, DuBois W, Jones G, Huppi K. Double producers of kappa and lambda define a subset of B cells in mouse plasmacytomas. Mol Immunol. 2000;37:775–781. doi: 10.1016/s0161-5890(00)00100-0. [DOI] [PubMed] [Google Scholar]
- 13.Burtin P, Buffe D. Synthesis of human immunoglobulins in germinal centers of lymphoid organs. J Immunol. 1967;98:536. [PubMed] [Google Scholar]
- 14.Xu D. Dual surface immunoglobulin light-chain expression in B-cell lymphoproliferative disorders. Arch Pathol Lab Med. 2006;130:853–856. doi: 10.5858/2006-130-853-DSILEI. [DOI] [PubMed] [Google Scholar]
- 15.González D, van der Burg M, García-Sanz R, Fenton JA, Langerak AW, González M, van Dongen JJ, San Miguel JF, Morgan GJ. Immu-noglobulin gene rearrangements and the pathogenesis of multiple myeloma. Blood. 2007;110:3112–312. doi: 10.1182/blood-2007-02-069625. [DOI] [PubMed] [Google Scholar]
- 16.Pelanda R. Dual immunoglobulin light chain B cells: trojan horses of autoimmunity? Curr Opin Immunol. 2014;27:53–9. doi: 10.1016/j.coi.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


