Abstract
Bcl2-associated athanogene 3 (BAG3) belongs to the BAG family and regulates many biologic behaviors of tumors. When tumor cells are in a hypoxic condition, BAG3 protein expression level increases, as does HIF-1α which is an important transcription factor induced by hypoxia. Glioblastoma is one of the typical solid tumors existing in a hypoxic microenvironment that can activate expression of BAG3 and HIF-1α. This research aimed to reveal the relationship between BAG3 and HIF-1α and their effects in glioblastoma multiforme. We found that down-regulated BAG3 inhibited proliferation and promoted apoptosis of glioblastoma multiforme U87 and U251 cell lines by decreasing HIF-1α expression level. The mechanism of BAG3 regulating HIF-1α is mainly through increasing degradation of HIF-1α by HSP70. When HIF-1α was up-regulated, induced by HIF-1α plasmid transfection on the basis of down-regulation of BAG3, the proliferation inhibition and apoptosis promotion were partially reversed. This novel result showed, for the first time, that down-regulation of BAG3 resulted in a low expression of HIF-1α under both normoxic or hypoxic conditions and finally caused inhibited proliferation and promoted apoptosis in glioblastoma. The mechanism of down-regulated BAG3 decreased HIF-1α protein expression through enhancing formation of HSP70-HIF-1α complex and promoting degradation of HIF-1α by HSP70.
Keywords: Bcl2-associated athanogene 3 (BAG3), hypoxia inducible factor 1 alpha (HIF-1α), heat shock protein 70 (HSP70), proliferation, apoptosis, glioblastoma multiforme
Introduction
Glioma is the most common central nervous system tumor, comprising about 80% of malignant brain tumors. Despite improvements in diagnosis and treatment, the prognosis of glioma remains dismal with a 5-year overall survival rate of less than 30% [1]. The tumor cells often invade adjacent brain tissue, which is largely responsible for the poor outcome of the disease [2]. Glioblastoma is the highest grade glioma variant, and is associated with high morbidity, mortality, and recurrence. Like other solid tumors such as hepatocellular carcinoma, prostatic carcinoma, and cervical cancer, rapid proliferation of tumor cells and relative slow growth of tumor microvessels usually form a regional hypoxia microenvironment.
In order to adapt this hypoxic microenvironment, tumor cells express many kinds of cytokines. Hypoxia inducible factor 1 alpha (HIF-1α) is one of the most active cytokines under hypoxic conditions. HIF-1α can serve as a transcription factor promoting transcription of many genes including vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF) and matrix metalloproteinase 2 (MMP2) [3-5]. Under normoxic circumstances, HIF-1α protein is expressed at a very low level because of rapid degradation. However, the degradation of HIF-1α protein was significantly inhibited in hypoxia tumors. The reason for inhibited degradation of HIF-1α protein was unclear until now.
Bcl2-associated athanogene 3 (BAG3) belongs to the BAG family of co-chaperones-encoding genes which was found by Takayama et al. in 1999 [6]. BAG3 is expressed in many primary tumors, such as leukemia, thyroid, neuroblastoma, prostate carcinoma, pancreatic cancer, ovarian cancer, and glioblastoma [7-12]. It has been reported that BAG3 may regulate diverse biologic processes including apoptosis, proliferation, cytoskeleton organization, invasion, metastasis, and autophagy [7,13,14]. BAG3 regulates a cancer related signaling network usually by interacting with heat shock protein 70 (HSP70) which can form a co-chaperone with BAG3 [6]. Immunohistochemical detection of BAG3 protein in different grades of glioma showed that its expression level significantly increased according to the tumor grade [15]. Down-regulation of BAG3 promotes apoptosis by enhancing the expression of Bax, p21, p53, and many other apoptosis-associated proteins [13,15,16].
The literature reported that BAG3 and HIF-1α co-expression was detected in hepatocellular carcinoma after liver transplantation by immunohistochemical technique. A significant association between high BAG3/HIF-1α levels and shorter overall survival was detected [17]. In hepatocellular carcinoma, BAG3 knockdown decreased HIF-1α protein expression level [18], but the mechanism was unclear.
In glioma, little was known about the relationship of BAG3 and HIF-1α. Thus, we hypothesized that: (1) High expression level of BAG3 was co-expressed with HIF-1α in glioblastoma mutiforme; (2) BAG3 can regulate HIF-1α in glioblastoma mutiforme; (3) Down-regulation of BAG3 inhibits proliferation and promotes apoptosis of glioblastoma mutiforme by BAG3/HSP70/HIF-1α proteasome pathway. To verify our hypotheses, we performed this research. U251and U87 cell lines were used as research models, because U251 and U87 are the most frequent human primary brain tumors and represent the most malignant stage of astrocytoma progression.
Materials and methods
Main reagents
HA-HIF-1alpha-pcDNA3 plasmid was purchased from Kaelin lab (Boston, USA). HA-pcDNA3 plasmid was from our lab. EGFP-shBAG3-GV248 and EGFP-GV248 plasmids were purchased from Genechem (Shanghai, China). HIF-1alpha and BAG3 primary antibodies were purchased from Abcam Company. HSP70 primary antibody was purchased from Enzolife Company. Fluorescent secondary antibodies were purchased from Licor Biosciences Company (Nebraska, USA). Transfection reagent was purchased from QIAGEN Company.
Specimens and patients
Specimens were collected at Renmin Hospital of Wuhan University (Hubei, China) from 20 cases of glioma in patients who received surgical resection from January 2014 to February 2015. The average age of glioma patients was 45 years old (range 19-75). Tumor samples were harvested from the tumors at the time of surgery and were subsequently snap frozen and stored at -80°C in 30 minutes. Our research was approved by the Ethics Committee of the Faculty of Medicine of Renmin Hospital, Wuhan University. Informed consent was obtained from the patients and/or guardians.
Cell culture and plasmid transfection
The U251 and U87 glioblastoma cell lines were from in our lab. Cells were grown in DMEM medium supplemented with 10% FBS in a humidified incubator at 37°C/5% CO2. When the cells were full, washed with PBS buffer, digested by trypsin (without EDTA) and seeded into either 6-well plates (Costar, USA) with 20 thousand cells per well or 96-well plates (Costar, USA) with 8 thousand cells per well. When cells covered 80-90% proportion of plates, HA-HIF-1alpha-pcDNA3, HA-pcDNA3, EGFP-shBAG3-GV248 and EGFP-GV248 plasmids were transfected into U251 and U87 glioblastoma cell lines using Attactene transfection reagent according to the manufacturer’s instructions. To screen out the transfected cells, 0.5% puromicin was added into the medium after tranfection.
RNA extraction and real time PCR
Total RNA was extracted using the Total RNA Isolation System from Promega according to the manufacturer’s instructions. Reverse transcription and PCR amplification were done according to Takara Reverse transcription kit manufacturer’s instructions by the real time PCR System supplied by Promega. Based on the information of BAG3, HIF-1α, GAPDH, β-actin supplied on PubMed, four pairs of gene-specific primers were designed: BAG3 sense 5’-CCCATGACCCATCGAGAAACTGC-3’ and anti-sense 5’-GCTGGGAGGACAAGGAACTG-3’; HIF-1α sense 5’-AAGCCCTAACGTGTTATCTGTCG-3’ and anti-sense 5’-ATGTAGTAGCTGCATGATCGTCT-3’; GAPDH sense 5’-CGGAGTCAACGGATTTGGTCGTAT-3’ and anti-sense 5’-AGCCTTCTCCATGGTGGTGAAGAC-3’; β-actin sense 5’-CCCATGACCCATCGAGAAACTGC-3’ and anti-sense 5’-GCTGGGAGGACAAGGAACTG-3’. Reverse transcription was carried out in a reaction mixture containing 1 μl RNA, 1 μl oligo dT primer, 2 μl reaction buffer (5 ×), 1 μl reverse transcriptase, 1 μl reverse transcriptase inhibitor, 10 μl ddH2O. Real time PCR was carried out in a reaction mixture containing 2 μl cDNA, 10 μl SYBR Green PCR mix, 1 μl forward primer (5 pmol/ml), 1 μl reverse primer (5 pmol/ml) and 6 μl ddH2O. Quantitative PCR was carried out in a reaction mixture containing 2.5 μl of cDNA, 12.5 ml of SYBR Green qPCR mix, 2.5 μl of plus solution, 2 μl of primers (5 pmol/ml), 5.5 μl of ddH2O. Furthermore, PCR was performed on an ABI Prism 7500 Sequence Detection System (Applied Biosystems) using SYBR Green qPCR kit (Toyobo Biologics, Japan). The cycling conditions were as follows: a denaturation step at 95°C for 10 min, followed by 35 cycles at 95°C for 30 s, 57°C for 30 s, 72°C for 45 s, and a final extension step at 72°C for 10 min. Results were normalized relative to the amount of GADPH or β-actin mRNA and were plotted by the amount relative to the reference sample.
Western blotting
After treatments or transfections, total protein extracts were prepared by incubation of cells in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100 5 mM EDTA) for 30 min at 0°C. Samples were normalized for protein content using BCA kit according to its manufacturer’s instructions. 30 mg of lysates was separated on 12% polyacrylamide gels and electro-transferred onto PVDF membranes for 2 hours (BAG3, HIF-1α, HSP70) or 1.5 hours (GAPDH, cleaved-caspase 3, NF-κBp65, BAD, p53, Bcl2, Bax). Membranes were blocked in 5% BSA in TBS-Tween, incubated with primary antibody (1:2000 for HIF-1α; 1:1000 for BAG3, HSP70, GAPDH, cleaved-caspase 3, NF-κBp65, BAD, p53, Bcl2, Bax), followed by incubation with secondary antibody (Licor, 1:10000). The signals were visualized using Odyssey infrared imaging system (Odyssey, USA).
Immunofluorescence
Cells were planted into 6-well plates which laid a glass slide in each well. A wash of the cells three times using PBS (1 ×) was done when glioblastoma cells covered 80%-90% percent square of glass slides. Liquid was aspirated, and then cells were covered with 2 ml 4% formaldehyde to fix cells for 15 min at room temperature. Fixative was aspirated, and cells were rinsed three times in 1 × PBS. Specimens were blocked in blocking buffer (1 × PBS/5% BSA/0.3% Triton X-100) for 60 min. Blocking solution was aspirated and primary antibodies were added which were diluted to 5 mg/ml in antibody dilution buffer (1 × PBS/1% BSA/0.3% Triton X-100). One slide in each group had nuclear staining by DAPI. Primary antibodies were incubated overnight at 4°C. Cells were rinsed three times and incubated in fluorochrome-conjugated secondary antibody for 60 min at room temperature away from light. Slides were coverslipped using antifade mounting medium and photographed under fluorescent microscopy (OYMPUS BX51, Japan).
Immunoprecipitation
For immunoprecipitation, U251 glioblastoma cells were lysed by cold RIPA lysis buffer (50 mM Tris-HCl; 1% Nonidet P-40; 0.25% Deoxycholate Na; 120 mM NaCl; 1 mM EDTA; 1 mM PMSF; 1 μg/ml Leupeptin; 1 μg/ml Aprotinin; 1 μg/ml Pepstatin; 1 mM Na3VO4; 1 mM NaF). 500 μg total protein were incubated with 5 μg anti-BAG3 rabbit monoclonal antibody, anti-HIF-1α rabbit monoclonal antibody, anti-HSP70 rabbit monoclonal antibody, or control rabbit IgG at 4°C with gentle agitation. Following addition of 60 ml protein G-agarose and incubation for 3 hours at 4°C, the immunocomplexes were pelleted; the immunocomplexes were washed with cold RIPA lysis buffer for three times at 4°C, and then subjected to SDS-PAGE and probed by western blotting for BAG3, HIF-1α and HSP70.
Evaluation of proliferation by CCK8 cell viability and 5-ethynyl-2’-deoxyuridine (EdU) incorporation assay
Cells were harvested at 48 hours post-transfection and cultured in 96-well plates (8000 cells per well). Cell viability was detected at 12, 24, 48 and 72 hours post-cultured using Cell Counting Kit-8 (Dojindo, Japan) according to the manufacturer’s protocol. Absorbance values at the wavelength of 450 nm were measured by enzyme standard instrument (PERKIN ELMER VICTOR 1420, USA). Proliferating cells were detected using the Cell-Light EdU imaging detecting kit (RiboBio, Guangzhou, China) according to the manufacturer’s instructions. Cells were incubated with 10 μM EdU for 24 hours, and then fixed with 4% formaldehyde for 30 min at room temperature. After washing, cells were incubated with staining reaction (EdU Apollo) for 30 min. Subsequently, cell nuclei were stained with DAPI (5 μg/ml) for 30 min and visualized under fluorescence microscope.
Evaluation of apoptosis by annexin-V-APC assay
48 hours after transfection, cells were harvested and washed by PBS buffer (1 ×) three times, and resuspended in 500 μl binding buffer (10 mM Hepes/NaOH, 140 mM NaCl, 2.5 mM CaCl2). 5 μl Annexin-V-APC was added into Annexin-V binding buffer, and then added 10 μl propidium iodide (PI, 20 μg/ml). Reaction mixture was mixed well lightly and incubated for 10 min at room temperature away from light. Cells were analyzed by flow cytometry using a Becton Dickinson FACScan flow cytometer with Cell Quest software (Becton Dickinson, Mountain View, CA).
Statistical analysis
Data Are presented as mean ± SD. Statistical differences were calculated using the Student’s t-test. Statistics were performed using one-factor ANOVA test. The significance level was set to P < 0.05. All experiments were repeated three times.
Results
Correlation between BAG3 and HIF-1α mRNA expression level in glioma
Samples from 20 patients with glioma receiving glioma resection in our hospital (Renmin Hospital of Wuhan University, Hubei, China) between 2012 and 2014 were collected for this study. Detail information of the 20 glioma patients was provided in Table 1. Giving priority to pathological examination, the rest of glioma resection tissues were collected to extract RNA. BAG3 and HIF-1α mRNA expression level were evaluated by qPCR, and data showed that BAG3 expression was significantly positively correlated with HIF-1α expression (Pearson, R2 = 0.804, P < 0.001) in human glioma samples (Figure 1).
Table 1.
Detailed information of the 20 glioma patients
| Patient number | Sex | Age | Pathologic diagnosis | Tumor grade (WHO) | HIF-1α mRNA level* | BAG3 mRNA level* |
|---|---|---|---|---|---|---|
| 1 | Female | 40 | Astrocytoma | I | 5.452 | 5.173 |
| 2 | Male | 19 | Astrocytoma | I | 2.701 | 4.441 |
| 3 | Male | 43 | Astrocytoma | II | 0.875 | 0.211 |
| 4 | Female | 24 | Astrocytoma | II | 5.103 | 3.629 |
| 5 | Female | 58 | Astrocytoma | II | 0.913 | 0.347 |
| 6 | Female | 58 | Astrocytoma | II | 5.184 | 4.405 |
| 7 | Male | 53 | Astrocytoma | II | 2.377 | 1.383 |
| 8 | Male | 58 | Astrocytoma | II | 7.120 | 5.041 |
| 9 | Male | 61 | Astrocytoma | II | 0.346 | 0.352 |
| 10 | Male | 73 | Astrocytoma | II | 2.511 | 3.704 |
| 11 | Male | 52 | Glioblastoma | IV | 1.000 | 1.000 |
| 12 | Male | 39 | Glioblastoma | IV | 1.646 | 3.106 |
| 13 | Male | 20 | Glioblastoma | IV | 1.384 | 1.901 |
| 14 | Female | 39 | Glioblastoma | IV | 0.485 | 1.049 |
| 15 | Male | 59 | Glioblastoma | IV | 2.379 | 3.619 |
| 16 | Female | 44 | Glioblastoma | IV | 1.957 | 2.210 |
| 17 | Female | 75 | Glioblastoma | IV | 1.161 | 0.262 |
| 18 | Male | 30 | Glioblastoma | IV | 10.966 | 7.867 |
| 19 | Female | 50 | Glioblastoma | IV | 1.292 | 0.661 |
| 20 | Female | 73 | Glioblastoma | IV | 3.760 | 2.682 |
HIF-1α and BAG3 mRNA expression levels were relative to β-actin.
Figure 1.
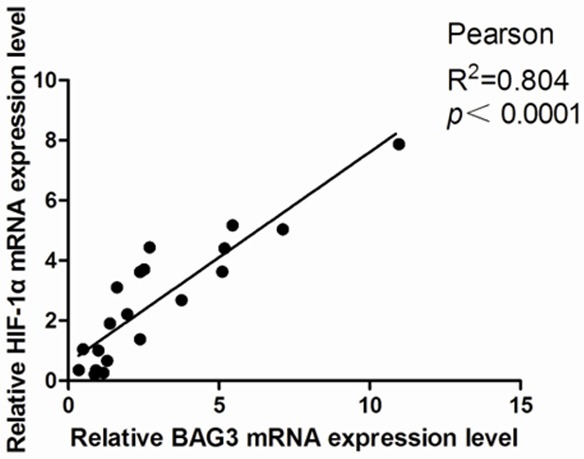
Correlation between BAG3 and HIF-1α mRNA expression level in 20 glioma tissues. Results were normalized relative to the amount of β-actin mRNA. Statistical analysis was performed with Pearson’s test.
HIF-1α cannot regulate BAG3 expression in gliomblastoma
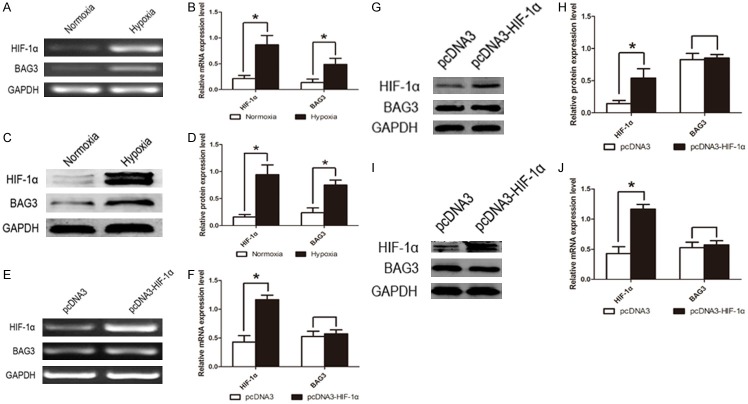
After we realized that BAG3 expression level was positive correlated to HIF-1α, we needed to do more research to explain the relationship between BAG3 and HIF-1α. When a hypoxic condition induced by cobalt dichloride (CoCl2, 200 μM) was provided to U87 glioblastoma cells, we found that both the HIF-1α and BAG3 mRNA expression level were significantly increased (Figure 2A, 2B), as well as HIF-1α and BAG3 protein (Figure 2C, 2D). So we tried to reveal whether HIF-1α could regulate the expression of BAG3, or the contrary. To verify our consideration, U87 and U251 glioblastoma multiforme cell lines were transfected with pcDNA3-HIF-1α and negative control pcDNA3 plasmids. After 48 hours of transfection, U87 and U251 glioblastoma multiforme cells were harvested to extract mRNA and protein. Real-time PCR and western blot showed that up-regulation of HIF-1α did not affect BAG3 expression level (Figure 2E-H). Similarly, U87 and U251 cell lines were transfected with pEGFP-shBAG3 plasmids to down-regulate BAG3 expression level, and HIF-1α mRNA expression was not increased or decreased (Figure 3C-F).
Figure 2.
HIF-1α and BAG3 were up-regulated under hypoxic condition and HIF-1α cannot regulate BAG3 expression. A. HIF-1α and BAG3 mRNA expression level in U87 glioblastoma cell line under hypoxic and normoxic conditions. Hypoxic group was induced by CoCl2 (200 μM) and normoxic group was not treated with CoCl2. B. Relative expression level of HIF-1α and BAG3 mRNA in U87 glioblastoma cell line under hypoxic condition. C. HIF-1α and BAG3 protein expression level in U87 glioblastoma cell line under hypoxic and normoxic condition. D. Relative expression level of HIF-1α and BAG3 protein in U87 glioblastoma cell line under hypoxic condition. E. BAG3 mRNA cannot be regulated by HIF-1α in U87 glioblastoma cell line. Cells were transfected with cDNA3-HIF-1α plasmid or cDNA3 plasmid for control. F. Relative expression level of BAG3 mRNA in U87 glioblastoma cell line. G. BAG3 protein cannot be regulated by HIF-1α in U87 glioblastoma cell line. H. Relative expression level of BAG3 protein in U87 glioblastoma cell line. I. BAG3 protein cannot be regulated by HIF-1α in U251 glioblastoma cell line. J. Relative expression level of HIF-1α and BAG3 protein in U251 glioblastoma cell line. Protein expression level was tested by western blot and mRNA expression level was tested by RT-PCR. Each experiment was repeated three times. Protein and mRNA expression level were normalized relative to the amount of GAPDH. Data were showed as mean ± SD. *P < 0.05 represented statistical significance.
Figure 3.
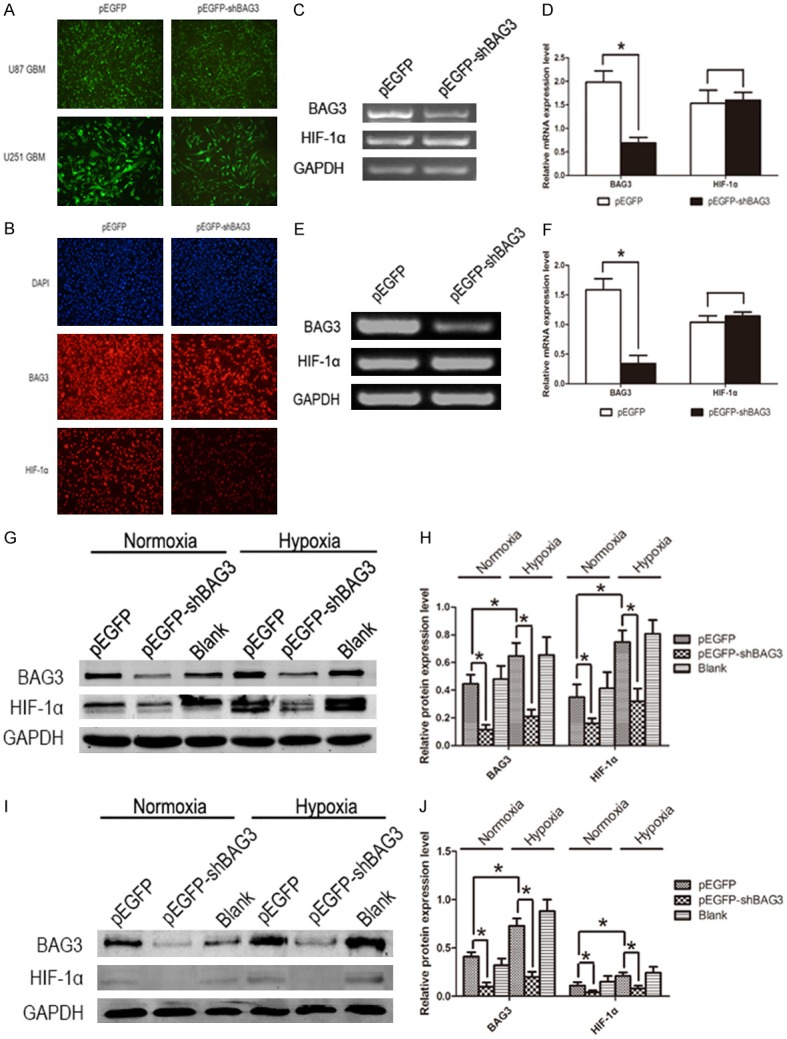
Down-regulation of BAG3 decreased HIF-1α protein expression level in glioblastoma. A. Fluorescence micrographs showing U87 and U251cells transfected with EGFP and EGFP-shBAG3 plasmid (original magnification, 100 ×). B. Immunofluorescent micrographs of U87 glioblastoma cell line (original magnification, 100 ×). C. HIF-1α mRNA expression level in U87 glioblastoma cell lines transfected with EGFP-shBAG3 or EGFP plasmid. D. Relative expression level of HIF-1α mRNA in U87 glioblastoma cell line with down-regulation of BAG3. E. HIF-1α mRNA expression level in U251 glioblastoma cell lines transfected with EGFP-shBAG3 or EGFP plasmid. F. Relative expression level of HIF-1α mRNA in U251 glioblastoma cell line with down-regulation of BAG3. G. Down-regulation of BAG3 decreased HIF-1α protein expression level in U87 glioblastoma cell line both under normoxic and hypoxic conditions. H. Relative expression level of HIF-1α protein in U87 glioblastoma cell line with down-regulation of BAG3 under normoxic and hypoxic conditions. I. Down-regulation of BAG3 decreased HIF-1α protein expression level in U251 glioblastoma cell line both under normoxic and hypoxic conditions. J. Relative expression level of HIF-1α protein in U251 glioblastoma cell line with down-regulation of BAG3 under normoxic and hypoxic conditions. Protein expression level was tested by western blot and mRNA expression level was tested by RT-PCR. Each experiment was repeated three times. Protein and mRNA expression level were normalized relative to the amount of GAPDH. Data were showed as means ± SD. *P < 0.05 represented statistical significance.
Down-regulation of BAG3 decreased HIF-1α protein expression level in glioblastoma
To explore whether BAG3 could regulate HIF-1α protein expression, we constructed U87 and U251 glioblastoma cell lines with stably low expression level of BAG3 by EGFP-shBAG3 plasmid transfection. Transfection rate was observed by fluorescence micrographs (Figure 3A). Fluorescence demonstrated that the percentage of transfection was higher than 90%. Immunofluorescent micrographs of U87 glioblastoma cell line were performed to detect the expression of HIF-1α protein after EGFP-shBAG3 plasmid transfection. Both the EGFP and EGFP-shBAG3 transfected groups were treated with BAG3 and HIF-1α primary antibodies overnight and then stained with CY5 secondary antibody. Immunofluorescence was photographed and data showed that HIF-1α protein was significantly decreased induced by down-regulated BAG3 (Figure 3B). In addition, BAG3 and HIF-1α protein expression level were detected by western blot and we also found that HIF-1α protein expression level decreased when BAG3 was down-regulated in U87 and U251 glioblastoma cell lines both under hypoxic and normoxic condition (Figure 3G-J). Thus, we found that BAG3 could regulate HIF-1α post-transcriptionally.
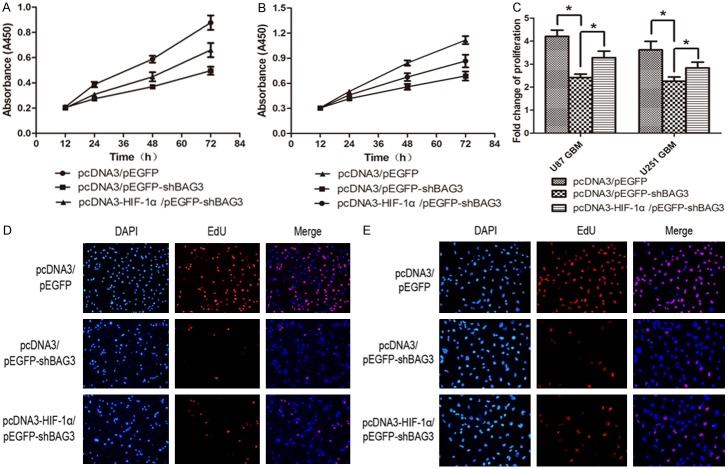
Down-regulation of BAG3 inhibits proliferation and promotes apoptosis of glioblastoma cells
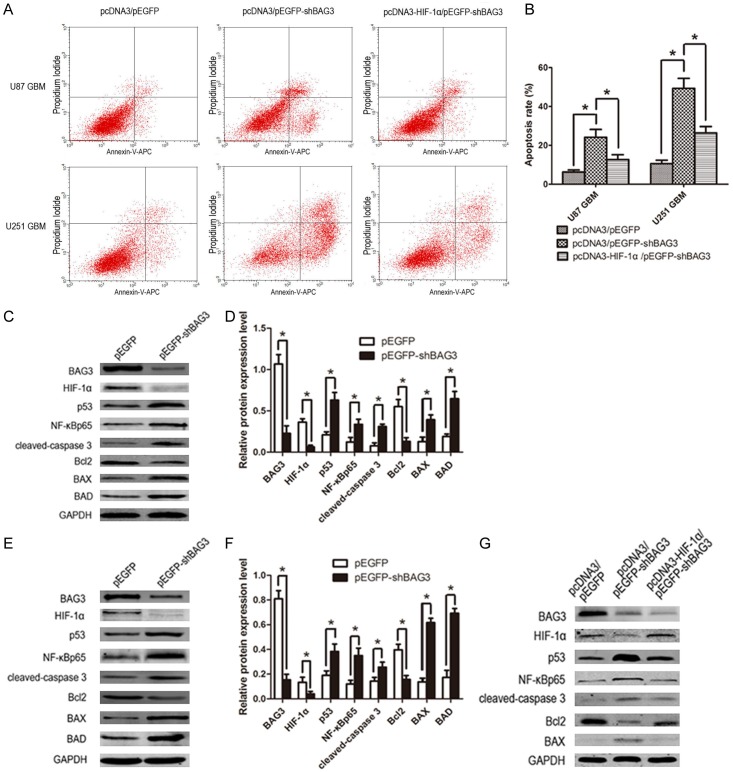
To investigate the influence of down-regulation of BAG3 on glioblastoma, we tested proliferation and apoptosis rate of U87 and U251 glioblastoma cell lines transfected with pEGFP-shBAG3 or pEGFP plasmid. Data of CCK8 assay showed that the proliferation rate of U87 and U251 glioblastoma cell lines significantly declined (Figure 4A, 4B); especially 72 hours after plating into a 96-well plate (Figure 4C). EdU assay also showed that down-regulation of BAG3 decreased proliferation rate of glioblastoma cells (Figure 4D, 4E). Anexin V-APC assay was performed to detect the apoptosis rate, and data showed increased apoptosis of glioblastoma cells induced by down-regulation of BAG3 (Figure 5A, 5B). Western blot data showed that apoptosis-associated proteins including p53, NF-κB, cleaved-caspase 3, Bax, and BAD expression level significantly increased and anti-apoptosis protein Bcl2 decreased in the pEGFP-shBAG3 transfected group (Figure 5C-F).
Figure 4.
Down-regulation of BAG3 inhibits proliferation of glioblastoma. A. CCK8 results of U87 glioblastoma cell line. U87 cells were double-transfected with pcDNA3/pEGFP, pcDNA3/pEGFP-shBAG3 or pcDNA3-HIF-1α/pEGFP-shBAG3. Absorbance values (A450) were detected at 12, 24, 48 and 72 hours after plating into 96-well plate. Data were showed as mean ± SD. B. CCK8 results of U251 glioblastoma cell line. C. Proliferation fold change of U87 and U251 glioblastoma cell lines at 72 hours after plating into 96-well plate. D. EdU assay result of U87 glioblastoma cell line. Double-transfected U87 cells were stained by DAPI or EdU 24 hours after plating into 6-well plate. EdU-positive cells were stain as red. Representative images were shown (original magnification, 200 ×). E. EdU assay result of U251 glioblastoma cell line. Double transfected U251 cells were stained by DAPI or EdU 24 hours after planted into 6-well plate. Representative images were shown (original magnification, 200 ×). Data were showed as means ± SD. *P < 0.05 represented statistical significance.
Figure 5.
Down-regulation of BAG3 promotes apoptosis of glioblastoma. A. Flow cytometry results of U87 and U251 glioblastoma cell lines. Cells were double-transfected and planted into a 6-well plate and apoptosis was detected after 48 hours of culture. B. Percentage rate of apoptosis. C. The expression of apoptosis-associated proteins in BAG3 down-regulated U87 glioblastoma cells. Cells in experimental group were transfected with EGFP-shBAG3 plasmid, and control group was transfected with EGFP plasmid. D. Relative expression level of apoptosis-associated proteins in U87 glioblastoma cells. E. The expression of apoptosis-associated proteins in BAG3 down-regulated U251 glioblastoma cells. F. Relative expression level of apoptosis-associated proteins in U251 glioblastoma cells. G. Reverse experiment of apoptosis-associated protein expression of U251 glioblastoma cells. Cells were double-transfected with plasmids. Protein expression level was tested by western blot. Each experiment was repeated three times. Protein and mRNA expression level were normalized relative to the amount of GAPDH. Data were showed as means ± SD. *P < 0.05 represented statistical significance.
Up-regulation of HIF-1α can partially reverse proliferation inhibition and apoptosis promotion induced by down-regulation of BAG3
To confirm that the proliferation inhibition and apoptosis promotion induced by decreased BAG3 were obtained through down-regulation of HIF-1α, a reversal experiment was performed with secondary transfection of pcDNA3-HIF-1α on the basis of transfection of pEGFP-shBAG3 both in U87 and U251 glioblastoma cell lines. Proliferation and apoptosis rate was tested by EdU assay and Annexin V-APC assay, and apoptosis associated proteins were tested by western blot. Data showed that up-regulation of HIF-1α on the basis of down-regulation of BAG3 can partially reverse proliferation inhibition and apoptosis promotion compared to down-regulation of BAG3 alone (Figures 4A-E, 5A, 5B). Apoptosis proteins, such as cleaved-caspase 3, Bax and p53 expression level were decreased after secondary transfection of pcDNA3-HIF-1α, and anti-apoptosis protein Bcl2 expression was increased relatively (Figure 5G).
Down-regulated BAG3 decreased HIF-1α protein expression through enhancing more formation of HSP70-HIF-1α complex and promoting degradation of HIF-1α by HSP70
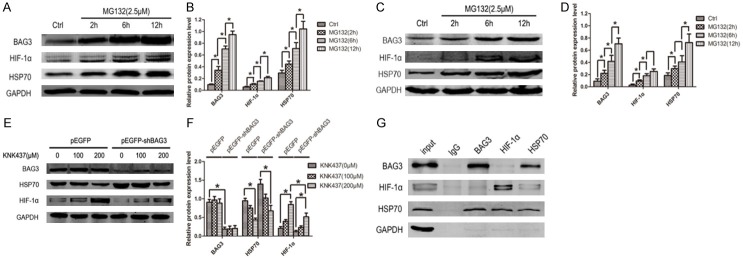
Having demonstrated that BAG3 can modulate HIF-1α protein expression, we explored the mechanism. As we know, BAG3 can just regulate HIF-1α protein expression but not mRNA, so protein-protein interaction must be taken into consideration. We used a proteasome inhibitor (MG132) to treat U87 and U251 glioblastoma cells. After treatment of MG132 (2.5 μM), both BAG3 and HIF-1α protein expression level increased. With the extension of treatment time of MG132, BAG3 and HIF-1α protein expression significantly increased both in U87 and U251 cell lines (Figure 6A-D). HSP70 serving as co-chaperon with BAG3 was up-regulated by MG132. Thus, the relationship between BAG3, HIF-1α and HSP70 had to be clarified. To accomplish this, co-immunoprecipitation of BAG3, HIF-1α and HSP70 was done. Data showed that both of BAG3 and HIF-1α could bind to HSP70. However, BAG3 antibody could not precipitate HIF-1α protein and vice versa (Figure 6G). So we infer that BAG3 in the regulation of HIF-1α may be reached through degradation effect of HSP70 to HIF-1α. To confirm our inference, HSP70 inhibitor (KNK437) was used to treat U251 glioblastoma cell transfected with pEGFP or pEGFP-shBAG3 plasmid. When HSP70 expression was inhibited by KNK437, HIF-1α expression level was significantly increased both in pEGFP and pEGFP-shBAG3 group (Figure 6E, 6F). These data suggest that down-regulated BAG3 can decrease HIF-1α which was reached by enhancing the degradation effect of HSP70 to HIF-1α.
Figure 6.
Down-regulated BAG3 decreased HIF-1α protein expression through enhancing more formation of HSP70-HIF-1α complex and promoting degradation of HIF-1α by HSP70. A. Proteasome inhibitor increased the expression of BAG3, HIF-1α, and HSP70 in U87 glioblastoma cell line. With the treatment duration of proteasome inhibitor MG132 (2.5 μM) extended, the expression of BAG3, HIF-1α and HSP70 increased. B. Relative expression level of BAG3, HIF-1α, and HSP70 in U87 glioblastoma cell line. C. MG132 increased the expression of BAG3, HIF-1α, and HSP70 in U251 glioblastoma cell line. With the treatment duration of MG132 (2.5 μM) extended, the expression of BAG3, HIF-1α and HSP70 increased. D. Relative expression level of BAG3, HIF-1α, and HSP70 in U251 glioblastoma cell line. E. HSP70 inhibitor increased the expression of HIF-1α. With the concentration HSP70 inhibitor KNK437 increased, HIF-1α expression increased. F. Relative expression level of BAG3, HIF-1α, and HSP70 after a treatment of KNK437. G. Immunoprecipitation of U251 glioblastoma cell line. Whole protein was immunoprecipitated using BAG3, HIF-1α, HSP70 and IgG antibody, and immunoprecipitates were analyzed by western blot to detect BAG3, HIF-1α, and HSP70. Protein expression level was tested by western blot. Each experiment was repeated three times. Protein and mRNA expression level were normalized relative to the amount of GAPDH. Data were showed as means ± SD. *P < 0.05 represented statistical significance.
Discussion
Similar to many other tumors such as hepatocellular carcinoma, prostatic carcinoma, and cervical cancer, glioblastoma usually grows with boundaries and forms a solid tumor. Tumor cells grow rapidly and microvessels grow relatively slowly, which leads to a regional hypoxic microenvironment. Previous studies showed that BAG3 and HIF-1α expression were enhanced under hypoxic condition [19]. HIF-1α is a regulatory subunit of the hypoxia inducible factor 1 (HIF1), which serves as a key transcription factor in response to hypoxic stress by regulating genes involved in maintaining oxygen homeostasis, as well as many other genes including VEGF, EPO, PDGF, and apoptosis-associated genes. HIF-1α mRNA and protein expression level was increased and degradation of HIF-1α protein was inhibited under hypoxic conditions [20,21]. BAG3 is a stress-responsive gene which can be activated or up-regulated under stress such as hyperthermia, ischemia, oxidation or hypoxia [22]. The reason that BAG3 is highly expressed under hypoxic stress may attribute to heat shock transcription factor 1 (HSF-1) which is a transcription factor of BAG3 [23]. Kawabe et al. reported that HSF-1 mRNA was significantly up-regulated during hypoxia, and its expression level was positive relative to hypoxic time [24]. Thus, we infer that up-regulation of BAG3 under hypoxia was induced by HSF-1. Although eleven putative hypoxic response elements which are hypoxia-inducible factor 1 (HIF-1) binding sequences were found located in the HSF1 promoter region in oysters, no direct evidence was proposed to prove this in humans. Our research results draw an opposite conclusion that BAG3 could not be modulated by HIF-1α in human glioblastoma multifome.
High expression level of BAG3 protects tumor cells from apoptosis and inhibits proliferation usually by interacting with HSP70. In melanoma, BAG3 sustains cell survival by interfering with the binding of HSP70 to the IKK-γ subunit of the NF-κB and favoring IKK complex formation and preventing the proteasomal degradation of IKK-γ and finally enhancing NF-κB activation [25]. BAG3 promotes the binding of HSP70 to Bcl2 family member Bax, preventing its translocation to mitochondria and protecting glioblastoma cells from apoptosis [15]. HIF-1α protects tumor cells from apoptosis by modulating Bcl2, Bax, p53, cleaved caspase 3, caspase-8, caspase-9, survivin and cytochrome C [26-29]. Recently, Xiao et al. performed a research in human hepatocellular carcinoma about the correlation between BAG3 and HIF-1α, and the conclusion showed a positive correlation between BAG3 and HIF-1α [17]. However, in human glioblastoma, this conclusion and its mechanism were unclear.
In the present study, we found that BAG3 and HIF-1α mRNA expression level was significantly correlated in human glioma tissue. However, in U87 and U251 glioblastoma cell lines, up-regulation of HIF-1α cannot modulate BAG3 at transcription level and vice versa. The reason of correlation between BAG3 and HIF-1α mRNA in human glioma tissue may attribute to hypoxia microenvironment, which was consistent with cell experiment in vitro during hypoxia condition [19,30]. With more research, we found that down-regulation of BAG3 by shRNA plasmid decreased HIF-1α protein expression level in glioblastoma multiforme U87 and U251 cell lines. Proteasome inhibition and co-immunoprecipitation test showed that both BAG3 and HIF-1α protein could bind to HSP70, but we failed to confirm a direct binding relationship between BAG3 and HIF-1α protein. When HSP70 was inhibited by HSP70 inhibitor, HIF-1α expression level significantly increased. The data indicated that the mechanism of down-regulated BAG3 decreased HIF-1α protein expression was through enhancing more formation of HSP70-HIF-1α complex and promoting degradation of HIF-1α by HSP70.
When BAG3 expression was inhibited, inhibited proliferation and promoted apoptosis were observed in glioblastoma multiforme U87 and U251 cell lines. Several apoptosis-associated protein expression levels were changed including increased expression of p53, NF-κB, cleaved-caspase 3, Bax and Bad, and decreased expression of Bcl2. With the reversal of low expression level of HIF-1α protein induced by down-regulation of BAG3 through transfecting HIF-1α plasmid on the basis of shBAG3 plasmid, the proliferation inhibition and apoptosis promotion were partially reversed. Western blot data showed that apoptosis protein such as p53, NF-κB, cleaved-caspase 3, Bax and Bcl2 were reversed. This evidence demonstrated that proliferation inhibition and apoptosis promotion of glioblastoma induced by down-regulation of BAG3 was realized by enhancing degradation of HIF-1α protein, at least in part.
In summary, we have demonstrated that down-regulation of BAG3 resulted in a low expression of HIF-1α both under normoxic or hypoxic condition and finally caused inhibited proliferation and promoted apoptosis. The mechanism of down-regulated BAG3 inhibited HIF-1α protein expression was through promoting degradation of HIF-1α by HSP70 by the BAG3/HSP70/HIF-1α proteasome pathway.
Acknowledgements
We thank Kaelin for HA-pcDNA3-HIF-1α plasmid. We thank all our colleagues working in the Department of Neurosurgery, Wuhan No. 1 Hospital, Wuhan, People’s Republic of China. This work was supported by The National Natural Science Foundation of China (81372683).
Disclosure of conflict of interest
None.
References
- 1.Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il’yasova D, Kruchko C, Mc-Carthy BJ, Rajaraman P, Schwartzbaum JA, Sadetzki S, Schlehofer B, Tihan T, Wiemels JL, Wrensch M, Buffler PA Brain Tumor Epidemiology Consortium. Brain tumor epidemiology: consensus from the brain tumor epidemiology consortium. Cancer. 2008;113:1953–1968. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butowski NA, Sneed PK, Chang SM. Diagnosis and treatment of recurrent high-grade astrocytoma. J. Clin. Oncol. 2006;24:1273–1280. doi: 10.1200/JCO.2005.04.7522. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre JA, Kung TS, White LA, Cooper KR. Manipulation of the HIF-Vegf pathway rescues methyl tert-butyl ether (MTBE)-induced vascular lesions. Toxicol Appl Pharmacol. 2013;273:623–634. doi: 10.1016/j.taap.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roos TU, Heiss EH, Schwaiberger AV, Schachner D, Sroka IM, Oberan T, Vollmar AM, Dirsch VM. Caffeic acid phenethyl ester inhibits PDGF-induced proliferation of vascular smooth muscle cells via activation of p38 MAPK, HIF-1alpha, and heme oxygenase-1. J Nat Prod. 2011;74:352–356. doi: 10.1021/np100724f. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Tang Z, Xue R, Singh GK, Shi K, Lv Y, Yang L. Combined effects of TNF-alpha, IL-1beta, and HIF-1alpha on MMP-2 production in ACL fibroblasts under mechanical stretch: an in vitro study. J Orthop Res. 2011;29:1008–1014. doi: 10.1002/jor.21349. [DOI] [PubMed] [Google Scholar]
- 6.Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- 7.Felzen V, Hiebel C, Koziollek-Drechsler I, Reissig S, Wolfrum U, Kogel D, Brandts C, Behl C, Morawe T. Estrogen receptor alpha regulates non-canonical autophagy that provides stress resistance to neuroblastoma and breast cancer cells and involves BAG3 function. Cell Death Dis. 2015;6:e1812. doi: 10.1038/cddis.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Zhang HY, Wang T, Meng X, Zong ZH, Kong DH, Wang HQ, Du ZX. BAG3 promoted starvation-induced apoptosis of thyroid cancer cells via attenuation of autophagy. J Clin Endocrinol Metab. 2014;99:E2298–2307. doi: 10.1210/jc.2014-1779. [DOI] [PubMed] [Google Scholar]
- 9.Liao Q, Ozawa F, Friess H, Zimmermann A, Takayama S, Reed JC, Kleeff J, Buchler MW. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett. 2001;503:151–157. doi: 10.1016/s0014-5793(01)02728-4. [DOI] [PubMed] [Google Scholar]
- 10.Staibano S, Mascolo M, Di Benedetto M, Vecchione ML, Ilardi G, Di Lorenzo G, Autorino R, Salerno V, Morena A, Rocco A, Turco MC, Morelli E. BAG3 protein delocalisation in prostate carcinoma. Tumour Biol. 2010;31:461–469. doi: 10.1007/s13277-010-0055-3. [DOI] [PubMed] [Google Scholar]
- 11.Sugio A, Iwasaki M, Habata S, Mariya T, Suzuki M, Osogami H, Tamate M, Tanaka R, Saito T. BAG3 upregulates Mcl-1 through downregulation of miR-29b to induce anticancer drug resistance in ovarian cancer. Gynecol Oncol. 2014;134:615–623. doi: 10.1016/j.ygyno.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Zhu H, Wu W, Fu Y, Shen W, Miao K, Hong M, Xu W, Young KH, Liu P, Li J. Overexpressed BAG3 is a potential therapeutic target in chronic lymphocytic leukemia. Ann Hematol. 2014;93:425–435. doi: 10.1007/s00277-013-1883-1. [DOI] [PubMed] [Google Scholar]
- 13.Cotugno R, Basile A, Romano E, Gallotta D, Belisario MA. BAG3 down-modulation sensitizes HPV18(+) HeLa cells to PEITC-induced apoptosis and restores p53. Cancer Lett. 2014;354:263–271. doi: 10.1016/j.canlet.2014.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosati A, Graziano V, De Laurenzi V, Pascale M, Turco MC. BAG3: a multifaceted protein that regulates major cell pathways. Cell Death Dis. 2011;2:e141. doi: 10.1038/cddis.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Festa M, Del Valle L, Khalili K, Franco R, Scognamiglio G, Graziano V, De Laurenzi V, Turco MC, Rosati A. BAG3 protein is overexpressed in human glioblastoma and is a potential target for therapy. Am J Pathol. 2011;178:2504–2512. doi: 10.1016/j.ajpath.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colvin TA, Gabai VL, Gong J, Calderwood SK, Li H, Gummuluru S, Matchuk ON, Smirnova SG, Orlova NV, Zamulaeva IA, Garcia-Marcos M, Li X, Young ZT, Rauch JN, Gestwicki JE, Takayama S, Sherman MY. Hsp70-Bag3 interactions regulate cancer-related signaling networks. Cancer Res. 2014;74:4731–4740. doi: 10.1158/0008-5472.CAN-14-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao H, Tong R, Cheng S, Lv Z, Ding C, Du C, Xie H, Zhou L, Wu J, Zheng S. BAG3 and HIF-1 alpha coexpression detected by immunohistochemistry correlated with prognosis in hepatocellular carcinoma after liver transplantation. Biomed Res Int. 2014;2014:516518. doi: 10.1155/2014/516518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao H, Cheng S, Tong R, Lv Z, Ding C, Du C, Xie H, Zhou L, Wu J, Zheng S. BAG3 regulates epithelial-mesenchymal transition and angiogenesis in human hepatocellular carcinoma. Lab Invest. 2014;94:252–261. doi: 10.1038/labinvest.2013.151. [DOI] [PubMed] [Google Scholar]
- 19.Cho KO, Lee KE, Youn DY, Jeong KH, Kim JY, Yoon HH, Lee JH, Kim SY. Decreased vulnerability of hippocampal neurons after neonatal hypoxia-ischemia in bis-deficient mice. Glia. 2012;60:1915–1929. doi: 10.1002/glia.22407. [DOI] [PubMed] [Google Scholar]
- 20.Bai R, Zhao AQ, Zhao ZQ, Liu WL, Jian DM. MicroRNA-195 induced apoptosis in hypoxic chondrocytes by targeting hypoxia-inducible factor 1 alpha. Eur Rev Med Pharmacol Sci. 2015;19:545–551. [PubMed] [Google Scholar]
- 21.Rishi MT, Selvaraju V, Thirunavukkarasu M, Shaikh IA, Takeda K, Fong GH, Palesty JA, Sanchez JA, Maulik N. Deletion of prolyl hydroxylase domain proteins (PHD1, PHD3) stabilizes hypoxia inducible factor-1 alpha, promotes neovascularization, and improves perfusion in a murine model of hind-limb ischemia. Microvasc Res. 2015;97:181–188. doi: 10.1016/j.mvr.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Yunoki T, Tabuchi Y, Hayashi A, Kondo T. BAG3 protects against hyperthermic stress by modulating NF-kappaB and ERK activities in human retinoblastoma cells. Graefes Arch Clin Exp Ophthalmol. 2015;253:399–407. doi: 10.1007/s00417-014-2874-1. [DOI] [PubMed] [Google Scholar]
- 23.Franceschelli S, Rosati A, Lerose R, De Nicola S, Turco MC, Pascale M. Bag3 gene expression is regulated by heat shock factor 1. J Cell Physiol. 2008;215:575–577. doi: 10.1002/jcp.21397. [DOI] [PubMed] [Google Scholar]
- 24.Kawabe S, Yokoyama Y. Novel isoforms of heat shock transcription factor 1 are induced by hypoxia in the Pacific oyster crassostrea gigas. J Exp Zool A Ecol Genet Physiol. 2011;315:394–407. doi: 10.1002/jez.685. [DOI] [PubMed] [Google Scholar]
- 25.Ammirante M, Rosati A, Arra C, Basile A, Falco A, Festa M, Pascale M, d’Avenia M, Marzullo L, Belisario MA, De Marco M, Barbieri A, Giudice A, Chiappetta G, Vuttariello E, Monaco M, Bonelli P, Salvatore G, Di Benedetto M, Deshmane SL, Khalili K, Turco MC, Leone A. IKK{gamma} protein is a target of BAG3 regulatory activity in human tumor growth. Proc Natl Acad Sci U S A. 2010;107:7497–7502. doi: 10.1073/pnas.0907696107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Z, Chen D, Cheng H, Wang F. Hypoxiainducible factor-1alpha protects cervical carcinoma cells from apoptosis induced by radiation via modulation of vascular endothelial growth factor and p53 under hypoxia. Med Sci Monit. 2015;21:318–325. doi: 10.12659/MSM.893265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin MR, Lee HJ, Kang SK, Auh QS, Lee YM, Kim YC, Kim EC. Isocudraxanthone K induces gowth inhibition and apoptosis in oral cancer cells via hypoxia inducible factor-1alpha. Biomed Res Int. 2014;2014:934691. doi: 10.1155/2014/934691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Chang M, Shi Y, Jiang L, Zhao J, Hai L, Sharen G, Du H. Down-regulation of hypoxia-inducible factor-1 suppresses malignant biological behavior of triple-negative breast cancer cells. Int J Clin Exp Med. 2014;7:3933–3940. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B, Yin CP, Zhao Q, Yue SW. Upregulation of HIF-1alpha by hypoxia protect neuroblastoma cells from apoptosis by promoting survivin expression. Asian Pac J Cancer Prev. 2014;15:8251–8257. doi: 10.7314/apjcp.2014.15.19.8251. [DOI] [PubMed] [Google Scholar]
- 30.Ardyanto TD, Osaki M, Tokuyasu N, Nagahama Y, Ito H. CoCl2-induced HIF-1alpha expression correlates with proliferation and apoptosis in MKN-1 cells: a possible role for the PI3K/Akt pathway. Int J Oncol. 2006;29:549–555. [PubMed] [Google Scholar]