Abstract
Objective: Stem cell transplantation is an effective method for treating sensorineural hearing loss (SNHL), but its safety needs further study. This study aimed to reveal the differentiation outcome of induced pluripotent stem cells (iPSCs) after they were transplanted into cochleae. Methods: iPSCs were labelled with CM-Dil and identified by flow cytometry. Twenty 6-8-week-old ICR mice were divided into experimental (A) and control (B) groups. Ten mice were microinjected with CM-Dil-labelled iPSC suspension (group A) or an equal volume DMEM (group B) into the left ear cochlea. The tthresholds of all mice were tested by auditory brainstem response (ABR) at 1 week pre-surgery and 4 weeks post-surgery. Differentiated cells were identified by immunohistochemical staining for neuronal cell markers (nestin, neurofilament-M), and teratoma formation was determined by HE staining. Results: The ABR thresholds in groups A and B at one week pre-surgery (24.50±5.50 vs. 26.00±6.15 dB SPL) and at 4 weeks post-surgery (70.50±4.97 vs. 68.00±5.37 dB SPL) were not significantly different; however, in both groups, the thresholds were lower at pre-surgery than at 4 weeks post-surgery. In group A, CM-Dil-labelled iPSCs were observed in the cochlear perilymph, endolymph, and modiolus, and some red fluorescence-labelled cells expressed neural cell markers. In group B, no fluorescence was observed in the cochleae, but teratomas were observed in some cochleae. A teratoma was observed in each of two cochleae after iPSCs transplantation by HE staining. Conclusion: Mouse iPSCs can differentiate into cells with neuronal cell markers 4 weeks post-cochlear transplantation, and transplanted undifferentiated iPSCs may form teratomas. However, in the short-term, hearing loss in mice caused by cell transplantation through round window pathways cannot be improved by cochlear iPSC transplantation.
Keywords: Induced pluripotent stem cells (iPSCs), inner ear transplantation, spiral ganglion neuron, cell differentiation, teratoma
Introduction
Deafness is the most frequent sensory disorder [1]. Millions of individuals worldwide suffer from disabling hearing loss. According to the World Health Organization’s latest assessment, in 2013, 600 million individuals suffered from hearing loss, of which 360 million have disabling hearing loss and account for 5% of the world’s population. Many factors including viruses, bacteria, noise, and ototoxic drugs can cause sensorineural deafness. Currently, most cases of deafness are due to sensorineural hearing loss (SNHL), which is a common otolaryngological condition. Many reports have implicated pathological changes in cochlear hair cells and a lack of spiral ganglion neurons in SNHL [2,3]. Therefore, to recover hearing after hearing impairment, a method for the repair or replacement method for inner ear hair cells and spiral ganglion neurons must be developed, which can be expected to cure SNHL.
Cell transplantation is a promising treatment for SNHL. We previously reported that newborn mouse cochleae contain sphere-forming cells capable of differentiating into cells that express hair cell markers [4]. However, it is difficult to clinically apply this technique because of the difficulty in obtaining these cells. Recently, many studies have confirmed that transplanted stem cells can be used to treat certain diseases: e.g., induced pluripotent stem cell transplantation has been used to treat Parkinson’s disease [5] and myocardial stem cell transplantation to treat SHNL [6-8] Induced pluripotent stem cells (iPSCs) have a multi-directional differentiation potential similar to that of embryonic stem cells (embryonic stem cells, ESCs), i.e., they can be induced by specific environments to differentiate into a wide variety of cells from specific lineages [9]. The present study confirmed that iPSCs can differentiate into any cell type in the body [10], including functionally mature nerve cells, hematopoietic cells, pancreatic cells, liver cells, myocardial cells, endothelial cells, and osteoblasts. Transplanting iPSCs into the inner ear to repair damaged hair cells and spiral ganglion cells is an ideal way to treat SNHL.
Since iPSCs were discovered in 2006, little research has been conducted on iPSC differentiation in inner ear hair cells and spiral ganglion cells. We previously confirmed that mouse iPSCs can be induced to differentiate into inner ear hair cells and spiral ganglion cells in vitro [11]; however, the distribution, migration, and survival of undifferentiated iPSCs after transplantation into the inner ear remains to be studied. In this study, we microinjected CM-Dil-labelled iPSCs through the round window of normal adult cochleae of ICR mice to observe changes occurring after stem cell transplantation.
Methods and materials
Cells and animals
Mouse iPSCs (C402B-OS-1) were obtained from the Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences [12]. SPF CD-1 (ICR) mice were obtained from vitalriver (Beijing, China; certification number: SCXK (Jing) 2012-0001). This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Nanchang University (Nanchang, China).
CM-Di1-labelled iPSCs
Once the mouse iPSCs were cultured to a high confluency, the medium was removed by suction and the cells were washed twice with PBS solution. This was followed by digestion in 0.25% trypsin resulting in a single cell suspension. By performing differential adherence for 1 h, the MEFs were isolated from the iPSCs. Next, the iPSCs were counted post separation, and 1 × 106/ml cells were resuspended in knockout DMEM. Dimethyl sulfoxide (DMSO) (2 μl/ml) was added to this cell suspension followed by CM-Di1 at a concentration of 1 ml/ml. The suspension was then stored at 37°C for 1 h. After incubation, the cells were washed 3 times with PBS, and then resuspended in DMEM, observed, and eventually photographed under a fluorescence microscope.
Transplantation into the cochleae
To examine the outcome post transplantation into the mouse cochleae, CM-Di1-labelled-iPSCs were transplanted into the left cochleae of 6-8 weeks old CD1/ICR mice (n = 20). The surgery was performed under general anesthesia with 10% chloral hydrate and local anesthesia with 0.5% lidocaine. The left otic bulla of each mouse was opened to expose a round window, through which the iPSC suspension (4 × 106 cells in 1 μl knockout DMEM) was microinjected using a microsyringe pump (UMP3-1, WPI Inc., USA: injection speed of 10 nl/ms). The small hole in the round window was covered with a small piece of muscle tissue, followed by intraperitoneal injection of sodium cefoxitin saline to prevent infection, once a day for 3 consecutive days.
Testing auditory function
The auditory thresholds of all mice were tested by auditory brainstem response (ABR) at one week before surgery and 4 weeks after surgery. The bilateral external auditory meatus cerumen was cleared before the test under general anesthesia with 10% chloral hydrate. We used the ABR detection system, with stimulus for a click, for a duration of 0.1 ms, a repetition rate of 11.1 times/s, a superposition of 1024, and sweeps period of 10 ms. In ABR, III wave was used as a standard to determine the ABR threshold.
Immunohistochemistry
At 4 weeks post-transplantation, all mice were sacrificed under anesthesia, and the cochleae were fixed with 4% paraformaldehyde. Cryostat sections (8 mm in thickness) were immunostained for neurofilament-M and nestin. At the end of immunostaining, the nuclei were stained with 4’,6-diamino-2-phenyl-indole (DAPI). Primary antibodies used were as follows: anti-neurofilament-M rabbit polyclonal antibody (1:500; Santa Cruz, USA), anti-nestin mouse polyclonal antibody (1:200; Santa Cruz, USA). The secondary antibodies used were FITC-labelled anti-mouse or rabbit antibodies (1:100). Specimens were viewed using a ZEISS confocal laser-scanning microscope (ZEISS Inc. LSM 700, Germany).
Data processing and statistical analyses
SPSS 18.0 was used to process the data. All data in the figures are presented as the mean ± standard error of the mean (SEM). The ABR thresholds for the two groups were analyzed by the unpaired Student’s t-test. A value of P < 0.05 was considered statistically significant.
Results
In total, 96.42% of the mouse iPSCs were successfully labelled by the fluorescent dye (CM-Di1) (Figure 1A-D).
Figure 1.
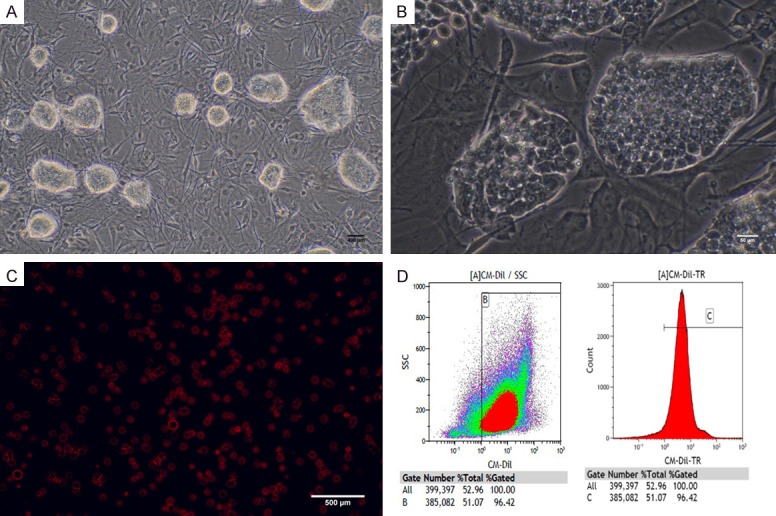
A. Mouse induced pluripotent cells (miPSCs) (Scale bar = 500 um). B. miPSCs that form cell groups with a strong edge refraction were undifferentiated (Scale bar = 50 um). C. Cells display a strong red fluorescence after CM-Dil labelling (Scale bar = 500 um). D. Labelling efficiency of CM-Di1 indicated by results of flow cytometry.
One week before the transplantation, the ABR response threshold in group A and B was 24.50±5.50 dB SPL and 26.00±6.15 dB SPL, respectively; The difference in these ABR thresholds between group A and B was not statistically significant (P = 0.572 > 0.05). Additionally, the ABR response threshold at 4 weeks post-surgery was 70.50±4.97 dB SPL in group A and 68.00±5.37 dB SPL in group B with no statistically significant difference (P = 0.295 > 0.05). However, the difference between the values before and after transplantation was statistically significant (P < 0.05) (Figure 2A, 2B and Table 1).
Figure 2.
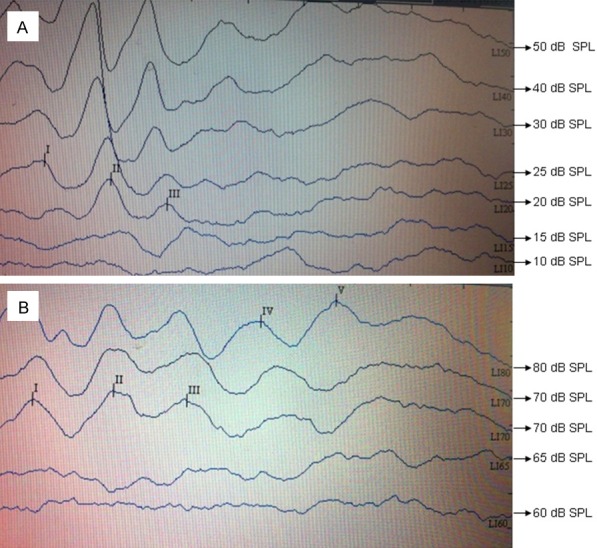
ABR hearing threshold was (A) 20 dB SPL at one week pre-surgery and (B) 70 dB SPL before the mice were sacrificed at four weeks post-surgery.
Table 1.
ABR (click) test results (x ± u dB SPL)
| Groups | 1 week before transplantation | After 4 weeks transplantation | T value | P value |
|---|---|---|---|---|
| group A | 24.50±5.50 | 70.50±4.97 | 31.659 | P < 0.001 |
| group B | 26.00±6.15 | 68.00±5.37 | 24.711 | P < 0.001 |
| T value | 0.575 | 1.080 | ||
| P value | P = 0.572 | P = 0.295 |
Data were compared using the t-test. Δt = 1.789, P = 0.090 > 0.05.
In group A, CM-Dil-labelled iPSCs were seen inside and outside the lymph and modiolus of the cochlea. Some red fluorescence-labelled cells expressed neural cell markers (nestin, neurofilament-M). No red fluorescence was observed in the cochlea of group B (Figures 3 and 4).
Figure 3.
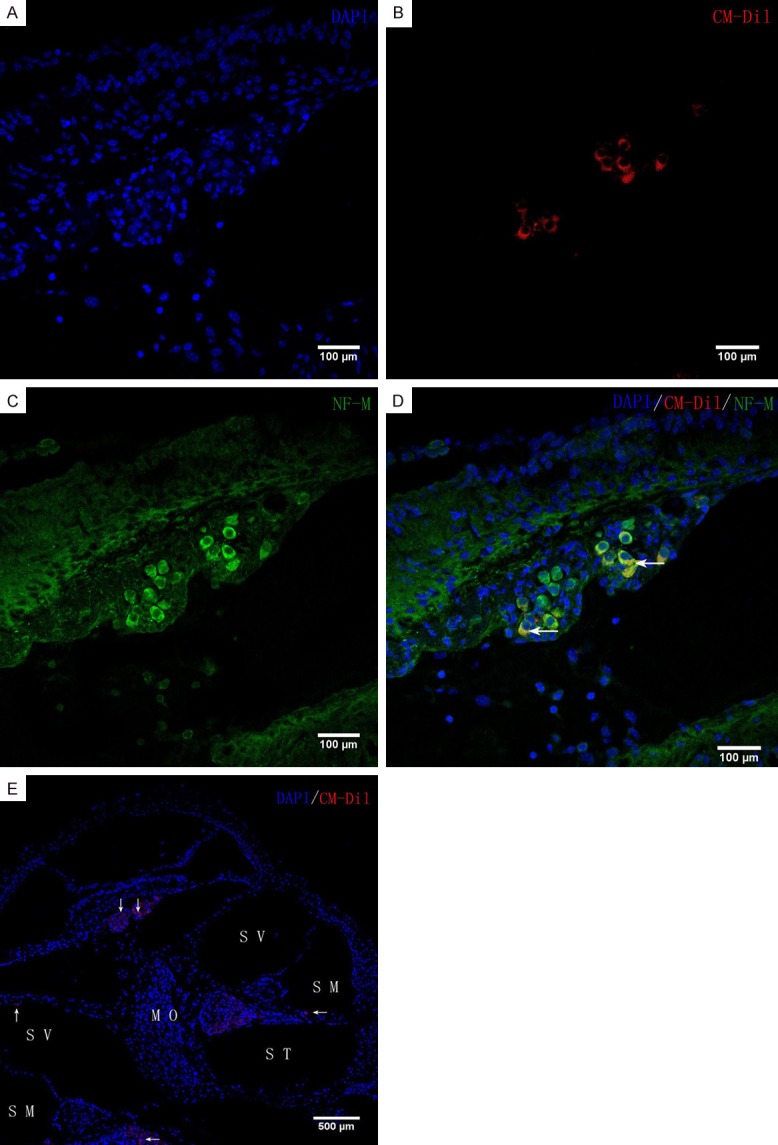
In vivo iPSC differentiation. CM-Di1-positive iPSCs were found in the scala tympani, scala media, modiolus, and scala vestibule (arrowheads in E). Blue fluorescence shows nuclear labelling with 4’,6-diamino-2-phenyl-indole (DAPI). Inside the modiolus, red fluorescence-labelled iPS cells express neuronal markers, neurofilament-M. Scale bars = 100 μm (A-D), 500 μm (E). ST, scala tympani; SM, scala media; SV, scala vestibuli; MO, modiolus.
Figure 4.
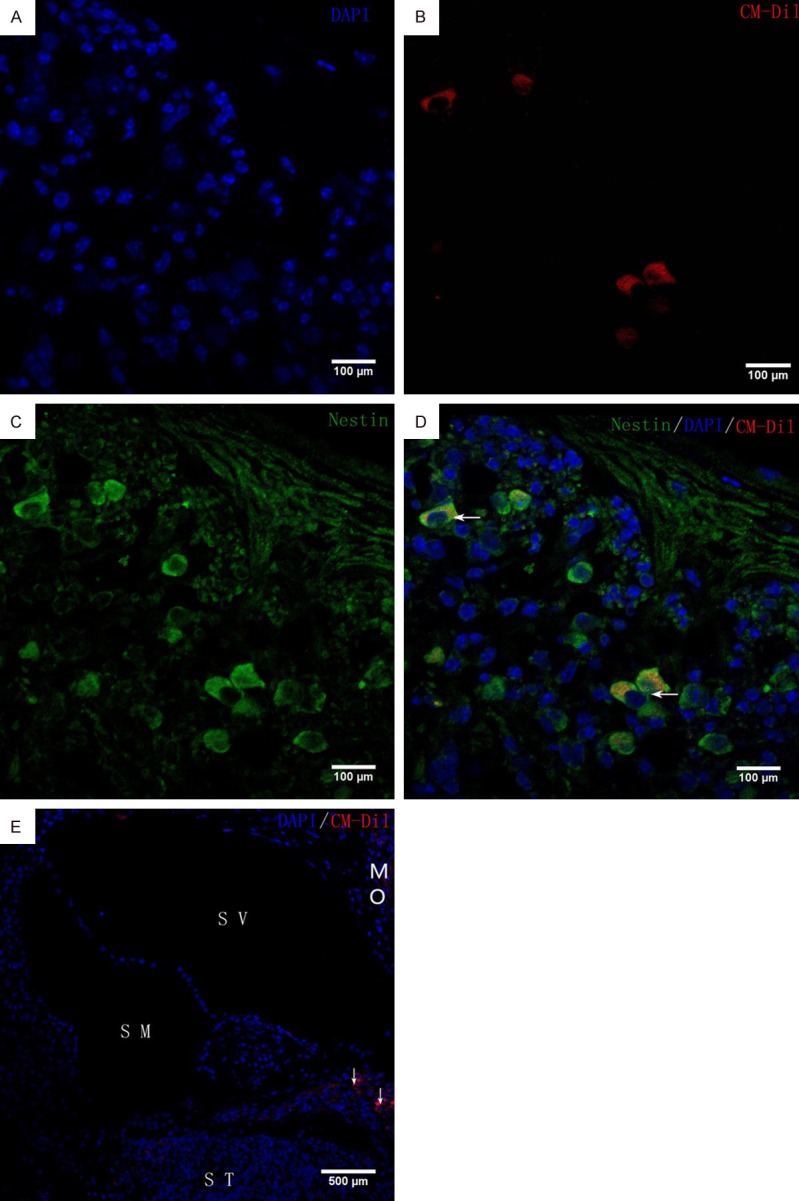
In vivo iPSC differentiation. Immunohistochemical analysis of neuronal markers (nestin) in ICR mouse cochleae at 4 weeks after transplantation. CM-Di1-positive iPSCs were found in the modiolus (arrowheads in E). Blue fluorescence shows nuclear labelling with 4’,6-diamino-2-phenyl-indole (DAPI). Inside the modiolus, red fluorescence-labelled iPSCs expressed neuronal markers, nestin. Scale bars = 100 μm (A-D), 500 μm (E). ST, scala tympani; SM, scala media; SV, scala vestibuli; MO, modiolus.
A teratoma was observed in two cochleae transplanted with miPSCs at four weeks. The tumour contained both undifferentiated cells and differentiated cells such as mesenchymal cells and duct-forming epithelial cells (Figure 5).
Figure 5.
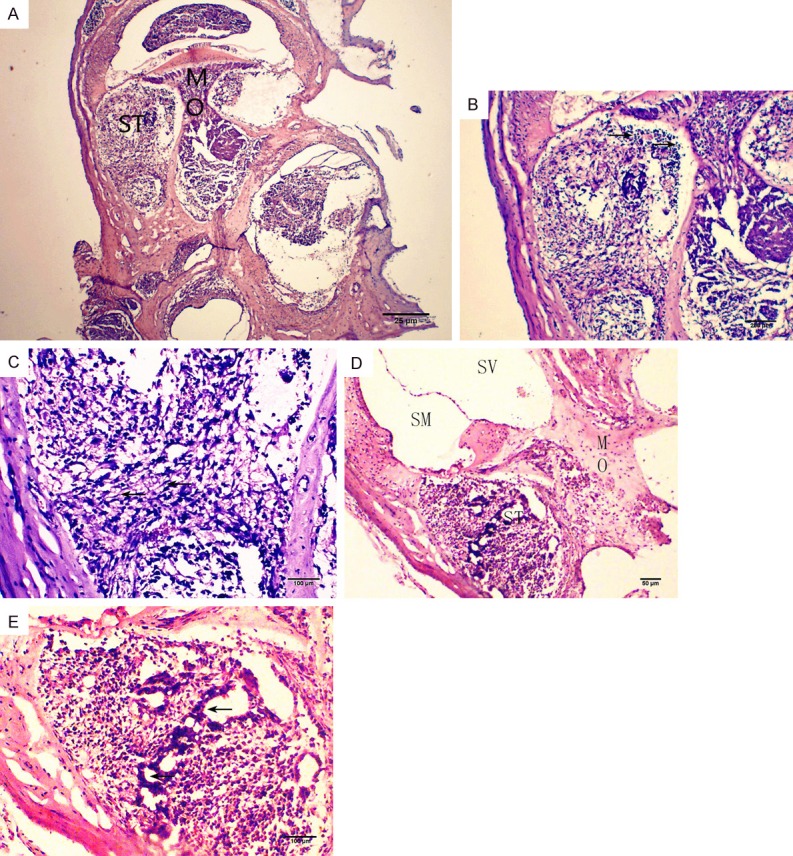
Teratoma formation at 4 weeks post transplantation. The specimen was fixed with 4% paraformaldehyde (PFA), embedded with OCT compound, sectioned, and stained with hematoxylin and eosin (HE). (A, D) A midmodiolar section of the cochlea. (B, C) Higher magnification of (A). (E) Higher magnification of (D). Arrows in B, C, and E indicate undifferentiated cells, mesenchymal cells, and ducts, respectively. Scale bars = 25 μm (A), 50 μm (D), 100 μm (B, C, E). ST, scala tympani; SM, scala media; SV, scala vestibuli; MO, modiolus.
Discussion
There are many methods available for cell transplantation into the mouse inner ear, such as injection of the transplants through the round window [13], the lateral semicircular canal and the cochlear lateral wall [14,15], and Rosenthal’s canal and the translabyrinthine surgical approach [15,16]. The cochleae of the ICR mice are small and brittle. Moreover, below the round window niche is the stapes artery that can be easily nicked during surgery, resulting in transplantation failure. Hence, we chose a relatively simple pathway through the round window [15,17].
Preoperative ABR hearing thresholds ranged between 15 dB SPL to 35 dB SPL, and postoperative ABR hearing thresholds ranged between 55 dB SPL to 75 dB SPL (Figure 2). The ABR response thresholds before surgery were significantly lower than they were at 4 weeks post-surgery in the two groups, and the difference was statistically significant (P < 0.001) (Table 1). This shows that the round window pathway for surgical access to the cochlea could cause hearing loss in mice, which is similar to that observed by Bogaerts et al. [17]. In our study, the low amount of microinjected cell suspension (1 µl cell suspension) and the use of the micropipette pump in the experiments reduced the migration of the vestibular membrane damage and retained the sound conduction portion of the cochlea.
We also investigated the proliferative and migration activity of the cells after cochlear transplantation by immunostaining for nestin and neurofilament-M. At four weeks after transplantation, transplanted cells with proliferation activity were present in the cochleae of all experimental groups, including the cochlear duct and modiolus. Cells also fluoresced red and green. This indicates that mouse iPSCs could differentiate into cells with neural cell markers at 4 weeks after transplantation into the cochlea of mice (Figures 3 and 4). The results indicate that transplanting undifferentiated iPSCs can repair the cochlear SGNs. However, the specific mechanism of how induced pluripotent stem cells migrate to the cochlear axis and differentiate into the spiral neuronal cells remains to be further studied. This conclusion is similar to Nishimura’s report [18].
We observed teratomas in two cochleae at 4 weeks after transplantation (Figure 5), as indicated by HE staining of the scala tympani, which showed undifferentiated cells, stromal cells, and duct-like structures. Nishimura et al. confirmed teratoma formation in one of five cochleae transplanted with TTF-derived iPSCs [19], which is in line with our results. Miura et al. had previously indicated that association with undifferentiated cells is a key feature in tumorigenesis of iPSCs [20]. Because the sample size in this study was small, and undifferentiated iPSCs with specific tumorigenicity were observed after the cochlear transplant, further investigation is needed.
This study confirmed that undifferentiated iPSCs can be processed in the cochlea and live for at least 4 weeks, and mouse iPSCs can differentiate into cells expressing neural cell markers after transplantation. This study also examined the potential of iPSCs as a source of transplants for SNHL. However, the risk for tumorigenesis resulting from transplanting undifferentiated iPSCs into the cochlea is a crucial problem that remains to be examined. The eradication of iPSC tumorigenesis remains to be further studied.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC) (Grant No 81160126).
Disclosure of conflict of interest
None.
References
- 1.Booth KT, Azaiez H, Kahrizi K, Simpson AC, Tollefson WT, Sloan CM, Meyer NC, Babanejad M, Ardalani F, Arzhangi S, Schnieders MJ, Najmabadi H, Smith RJ. PDZD7 and hearing loss: more than just a modifier. Am J Med Genet A. 2015;167A:2957–65. doi: 10.1002/ajmg.a.37274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCall AA, Swan EE, Borenstein JT, Sewell WF, Kujawa SG, McKenna MJ. Drug delivery for treatment of inner ear disease: current state of knowledge. Ear Hear. 2010;31:156–65. doi: 10.1097/AUD.0b013e3181c351f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsui JI, Parker MA, Ryals BM, Cotanche DA. Regeneration and replacement in the vertebrate inner ear. Drug Discov Today. 2005;10:1307–12. doi: 10.1016/S1359-6446(05)03577-4. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Jiang H, Yan Y, Wang Y, Shen Y, Li W, Li H. Characterization of proliferating cells from newborn mouse cochleae. Neuroreport. 2006;17:767–71. doi: 10.1097/01.wnr.0000215781.22345.8b. [DOI] [PubMed] [Google Scholar]
- 5.Lindvall O. Treatment of parkinson’s disease using cell transplantation. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140370. doi: 10.1098/rstb.2014.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilgimol JC, Ragupathi S, Vengadassalapathy L, Senthil NS, Selvakumar K, Ganesan M, Manjunath SR. Stem cells: an eventual treatment option for heart diseases. World J Stem Cells. 2015;7:1118–26. doi: 10.4252/wjsc.v7.i8.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, Milo M, Thurlow JK, Andrews PW, Marcotti W, Moore HD, Rivolta MN. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490:278–282. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa M, Ohnishi H, Skerleva D, Sakamoto T, Yamamoto N, Hotta A, Ito J, Nakagawa T. Transplantation of neurons derived from human iPS cells cultured on collagen matrix into guinea-pig cochleae. J Tissue Eng Regen Med. 2017;11:1766–1778. doi: 10.1002/term.2072. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Kimbrel EA, Lanza R. Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat Rev Drug Discov. 2015;14:681–92. doi: 10.1038/nrd4738. [DOI] [PubMed] [Google Scholar]
- 11.Guan L, Chen Y, Zhu H, Chen J, Jiang H. Study on the induced differentiation of induced pluripotent stem cells into cochlear hair cell-like cells and spiral ganglion neuron-like cells in vitro. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;49:680–686. [PubMed] [Google Scholar]
- 12.Chen J, Liu J, Yang J, Chen Y, Chen J, Ni S, Song H, Zeng L, Ding K, Pei D. BMPs functionally replace Klf4 and support efficient reprogramming of mouse fibroblasts by Oct4 alone. Cell Res. 2011;21:205–212. doi: 10.1038/cr.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang H, Schulte BA, Goddard JC, Hedrick M, Schulte JB, Wei L, Schmiedt RA. Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury. J Assoc Res Otolaryngol. 2008;9:225–40. doi: 10.1007/s10162-008-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iguchi F, Nakagawa T, Tateya I, Endo T, Kim TS, Dong Y, Kita T, Kojima K, Naito Y, Omori K, Ito J. Surgical techniques for cell transplantation into the mouse cochlea. Acta Otolaryngol Suppl. 2004:43–7. doi: 10.1080/03655230310016816. [DOI] [PubMed] [Google Scholar]
- 15.Backhouse S, Coleman B, Shepherd R. Surgical access to the mammalian cochlea for cellbased therapies. Exp Neurol. 2008;214:193–200. doi: 10.1016/j.expneurol.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekiya T, Holley MC, Kojima K, Matsumoto M, Helyer R, Ito J. Transplantation of conditionally immortal auditory neuroblasts to the auditory nerve. Eur J Neurosci. 2007;25:2307–18. doi: 10.1111/j.1460-9568.2007.05478.x. [DOI] [PubMed] [Google Scholar]
- 17.Bogaerts S, Douglas S, Corlette T, Pau H, Saunders D, McKay S, Oleskevich S. Microsurgical access for cell injection into the mammalian cochlea. J Neurosci Methods. 2008;168:156–63. doi: 10.1016/j.jneumeth.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura K, Nakagawa T, Ono K, Ogita H, Sakamoto T, Yamamoto N, Okita K, Yamanaka S, Ito J. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport. 2009;20:1250–4. doi: 10.1097/WNR.0b013e32832ff287. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura K, Nakagawa T, Sakamoto T, Ito J. Fates of murine pluripotent stem cell-derived neural progenitors following transplantation into mouse cochleae. Cell Transplant. 2012;21:763–71. doi: 10.3727/096368911X623907. [DOI] [PubMed] [Google Scholar]
- 20.Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, Higashi H, Nagai T, Katoh H, Kohda K, Matsuzaki Y, Yuzaki M, Ikeda E, Toyama Y, Nakamura M, Yamanaka S, Okano H. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:12704–9. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]


