Abstract
Cervical cancer has been one of the leading causes of cancer-related deaths among women worldwide. However, few targeted drugs have been developed because of the poor understanding about the mechanisms of cervical cancer. A growing number of studies in recent years have shown that programmed cell death 4 (PDCD4), a new tumor suppressor, participates in the tumorigenesis and progression of various cancers. In this study, we investigated the influence of PDCD4 on cell migration, invasion, and apoptosis of cervical cancer cells. Siha cervical cancer cells were transfected with a recombinant lentivirus vector carrying the complete length of the PDCD4 gene and the normal controlled vector respectively and screened by puromycin. The expression of the mRNA and protein of PDCD4 were significantly elevated in Siha cells transfected with the recombinant vector carrying the PDCD4 gene. Then the overexpression of PDCD4 suppressed the cell migration and invasion in transwell migration and matrigel invasion assays respectively. Moreover, the overexpression of PDCD4 increased the proportion of cell apoptosis in flow cytometry analysis. In a multiple signal pathways assay, the upregulation of PDCD4 promoted the phosphorylation of some key proteins, such as p53 and STAT1. These results suggest that PDCD4 is a potential therapeutic target for cervical cancer.
Keywords: Programmed cell death 4, cervical cancer, cell migration, cell invasion, apoptosis
Introduction
Cervical cancer is one of the most common cancers among women worldwide, and the majority of the women with the disease are diagnosed in the later stages and miss the best opportunities for treatment [1]. The effective cure rate of carcinoma in situ is nearly 100%, but that in stage IV is only about 10%. It is understood that the conventional treatments for cervical cancer, including surgery, radiotherapy, and chemotherapy, generally end in failure due to metastasis and recurrence. Therefore, it is urgent to explore new, valuable therapeutic targets and to develop molecular targeted therapies for cervical cancer.
Programmed Cell Death Factor 4 (PDCD4) is a tumor suppressor implicated in the development of a broad spectrum of tumors and differentially expressed proteins during tumor cell apoptosis [2]. A number of recent studies have shown that PDCD4 is widely expressed in normal human tissues and frequently downregulated in various carcinomas, such as lung cancer [3], colorectal cancer [4], ovarian cancer [5], nasopharyngeal carcinoma [6], hepatocellular carcinoma [7], glioma [8], gastric cancer [9], and so on. A loss of PDCD4 has been correlated with a poor prognosis in patients who have cancer. PDCD4 has been reported to be involved in the modulation of the eukaryotic translation initiation factor eIF4A [10,11], resulting in the inhibition of procaspase-3 and p53 translation in tumor cell lines [12,13]. Besides, PDCD4 inhibits downstream gene expression by suppressing β-catenin/Tcf, mitogen-activated protein kinase kinase kinase kinase 1 (MAP4K1) and nuclear factor kappa B (NF-κB) [14-16].
Our previous study showed that the expression of PDCD4 in cervical cancer tissues is significantly decreased and is associated with the progression of cervical cancer [17]. However, the effects of PDCD4 in cervical cancer remain unknown. Hence, in the present study, we investigated the influence and the underlying mechanisms of PDCD4 in cervical cancer cells, to explore the application potential of the target in cancer treatments.
Materials and methods
Cell culture
Human cervical squamous carcinoma Siha cells were obtained from the Chinese Academic of Sciences (Shanghai, China), and cultured in a DMEM medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified incubator containing 5% CO2.
Lentiviral recombinant vector construction and cell transfection
The PDCD4-expressing lentivirus vector (Lv-PDCD4) and the negative universal control (Lv-NC), containing the green fluorescent protein EGFP and a puromycin resistant gene were constructed by Shanghai Genechem Co., Ltd. (Shanghai, China). Before infection with the virus, the Siha cells were seeded onto a 6-well plate (1.0×105 cells/well) and allowed to grow to a 30% confluency. The lentivirus Lv-PDCD4 and Lv-NC were then transfected into the cells with an enhanced infection solution (Shanghai Genechem Co., Ltd.) and polybrene (Sigma), The transfected Siha cells were selected with puromycin (3 μg/ml), and then the expression of the fluorescence protein EGFP was used to monitor the infection efficiency by fluorescence microscope after 72 h. The Lv-PDCD4 positive cells were designated as SihaPDCD4, and the Lv-NC cells were designated as SihaNC.
RNA extraction and RT-PCR
Total RNA was extracted from the Siha cells using TRIzol reagent (Takara) according to the manufacturer’s protocol.The RNA concentration and purity (OD260/OD280) were assessed by a Nanodrop 2000TM spectrophotometer (Thermo Scientific, USA). First strand cDNA synthesis was generated from 1 μg of total RNA according to the PrimeScript RT reagent kit’s instructions (Takara, Japan). The target and reference genes (GAPDH) were quantified in triplicate using the Light-Cycler® 480 II Real-Time PCR System (Roche, Applied Science). The following primers were employed in each reaction: GAPDH, forward 5’-GCA CCG TCA AGG CTG AGA AC-3’ and reverse 5’-TGG TGA AGA CGC CAG TGG A-3’; PDCD4, forward 5’-TGG ATG TCC CAC ATT CAT ACT CTG-3’ and reverse 5’-TCT GGT TTA AGA CGA CCT CCA TCT-3’. Following normalization to the GAPDH gene, the expression levels of each target gene were calculated using the comparative threshold cycle (CT) method. The Δct values were calculated according to the formula Δct = ct (gene of interest) - ct (GAPDH) in the correlation analysis, and 2ΔΔct was calculated according to the formula ΔΔct = Δct (control group) - Δct (experimental group) for the determination of relative expression.
Western blotting analysis
Total proteins were extracted from Siha cells according to the manufacturer’s protocol of KeyGEN Whole Cell Lysis Assay kit (KeyGEN BioTECH, China) and used for the Western blot analysis. Equal amounts of protein samples were loaded per well and separated by SDS-PAGE and then transferred onto PVDF membranes (EMD Millipore, USA) as described previously [18]. After blocking, the membranes were probed using an anti-PDCD4 mAb (1:1000 dilution, Abcam) overnight at 4°C, and the GAPDH mAb (1:1000; Santa Cruz) was used as an internal loading control. Thereafter, membranes were incubated with HRP-conjugated secondary antibodies (1:5000 dilution, Santa Cruz) for 1 h at room temperature. Samples running in the same gel were compared. The specific immune complexes were detected by ECL enhanced chemiluminescence substrate (Fdbio Science, China) and the images were captured with the Kodak In-Vivo Imaging System F (Kodak, USA).
Transwell migration and matrigel invasion assays
Transwell migration and matrigel invasion assays were performed using a transwell membrane (Corning, USA) in a 24-well plate according to the manufacturer’s instructions. The polycarbonate filters (8 μm pore size, Corning) were pre-coated with Matrigel matrix (BD, USA) for the transwell invasion assay, and uncoated filters were used for the migration assay. SihaPDCD4 or SihaNC cells were adjusted at a density of 5×105 cells/ml with serum-free DMEM and then a 100 μl cell suspension was added into the upper chamber, while a 600 μl complete medium containing 30% FBS was added to the lower chamber. After incubation of 24 h for the migration assay and 48 h for the invasion assay, the cells migrated through the Matrigel and adhered onto the lower chamber were then fixed in 4% paraformaldehyde for 20 min, stained with crystal violet and counted under an upright microscope (Olympus, Japan) for five fields per chamber.
Apoptosis assay
The Siha cells growing in the exponential phase were trypsinized, and then they underwent centrifugation at 1300 rpm for 5 min and then washed with PBS twice. The cells were collected and then suspended in a binding buffer, followed by Annexin V-APC staining for 15 min. The samples were analyzed by a LSRFortessaTM flow cytometer (BD, USA).
Signaling array
The Siha cell lysate was probed with 17 validated antibody cocktails of the MAPK signaling pathway specific antibodies, including antibodies against ERK1/2, Stat1, Stat3, Akt, AMPKα, S6 Ribosomal Protein, mTOR, HSP27, Bad, p70 S6 Kinase, PRAS40, p53, p38, SAPK/JNK, PARP, Caspase-3 and GSK-3β, and then they were analyzed using with the PathScan Intracellular Signaling Array Kit. The antibodies were specific to the intracellular signaling molecules. In brief, the array blocking buffer was added into each well followed by incubation for 15 min at room temperature. Subsequently, cell lysates were added into each well and incubated for 2 h. After washing, each antibody was loaded in a separate well for 1 h, followed by HRP-linked streptavidin for 30 min. Specific immune complexes were detected using CLINX Chemiscope 5300 (Shanghai, China).
Statistical analysis
The results were expressed as the mean ± standard deviation (SD) of the three independent experiments unless otherwise specified. Data were analyzed by a two-tailed unpaired Student’s t-test between any two groups and were analyzed using SPSS Statistics 13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered as statistically significant.
Results
Transfection of the recombinant lentiviral vector carrying the PDCD4 gene upregulated PDCD4 expression in Siha cells
Considering the low PDCD4 level in cervical cancer, the influence of PDCD4 overexpression was determined in Siha cervical cancer cells. Hence, we constructed the recombinant lentiviral vector carrying the PDCD4 gene (Lv-PDCD4) and the normal controlled lentiviral vector (Lv-NC). After viral infection and screening by puromycin, the expression of the fluorescence protein EGFP under fluorescence microscopy was observed (Figure 1A), and we found that the infection efficiencies of Lv-PDCD4 and Lv-NC in Siha cells were more than 90%, indicating the infection effectiveness of the two lentiviral vectors in the Siha cells. Moreover, RT-PCR (Figure 1B) and Western blot assays (Figure 1C) confirmed that the level of PDCD4 in the SihaPDCD4 cells was significantly upregulated compared to the level in the SihaNC cells. These results demonstrated that the two lentiviral vectors and the two corresponding cells could be used in the following functional experiments.
Figure 1.
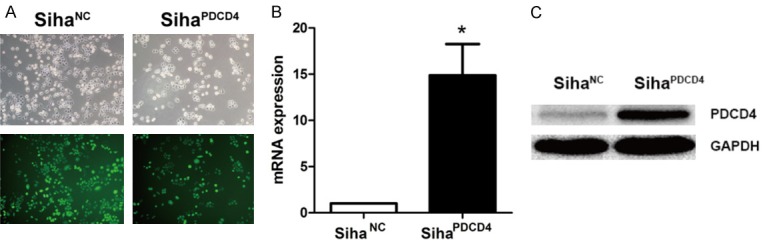
Determination of PDCD4 expression in Siha cells transfected with a recombinant lentiviral vector carrying the PDCD4 gene. Representative images of Siha cells transfected with the Lv-NC vector or the Lv-PDCD4 vector are shown at a ×100 magnification respectively (A). The mRNA and protein levels of PDCD4 in Siha cells were detected by RT-PCR (B) and Western blotting assays (C), P<0.05.
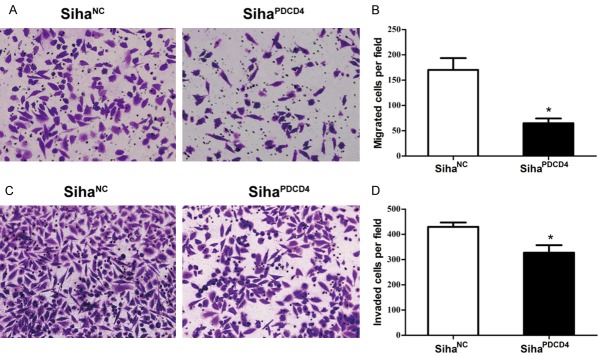
Overexpression of PDCD4 inhibited cell migration and invasion in Siha cells
PDCD4 is associated with the suppression of the migration and invasion of various tumor cells [15,19,20]. Our previous work also revealed that PDCD4 has a negative correlation with lymph node metastasis [17]. Thus, we further investigated the impact of PDCD4 on the tumor migration and invasion ability of Siha cells. A transwell migration assays showed that the number of migrated SihaPDCD4 cells was dramatically decreased compared to the number of SihaNC cells in the same cultural conditions (P = 0.01, Figure 2A and 2B). Similarly, transwell and matrigel invasion assays showed that the transferred SihaPDCD4 cells showed a more remarkable decrease than the SihaNC cells (P = 0.041, Figure 2C and 2D). These data indicated that the overexpression of PDCD4 markedly inhibited the migration and invasive ability of Siha cells.
Figure 2.
The influence of PDCD4 overexpression on cell migration and invasion. Siha cells infected with Lv-NC or Lv-PDCD4 were harvested to perform the migration assays (A, B) and the invasion assay (C, D). Cells migrated into the lower chamber (A) or invaded through the matrigel into the underside of the filter (C) were fixed, stained, and photographed at 200× magnification. The numbers of migrated (B) and invaded cells (D) were recorded. Data represent the average of three independent experiments. *P<0.05 compared with negative control.
Overexpression of PDCD4 promoted cell apoptosis in Siha cells
AnnexinV-APC staining showed that PDCD4 triggered a significantly higher proportion of Siha cell apoptosis than the negative control (5.77% vs 4.23%, P = 0.02, Figure 3A and 3B), indicating the promotion of cell apoptosis by PDCD4 in Siha cells.
Figure 3.
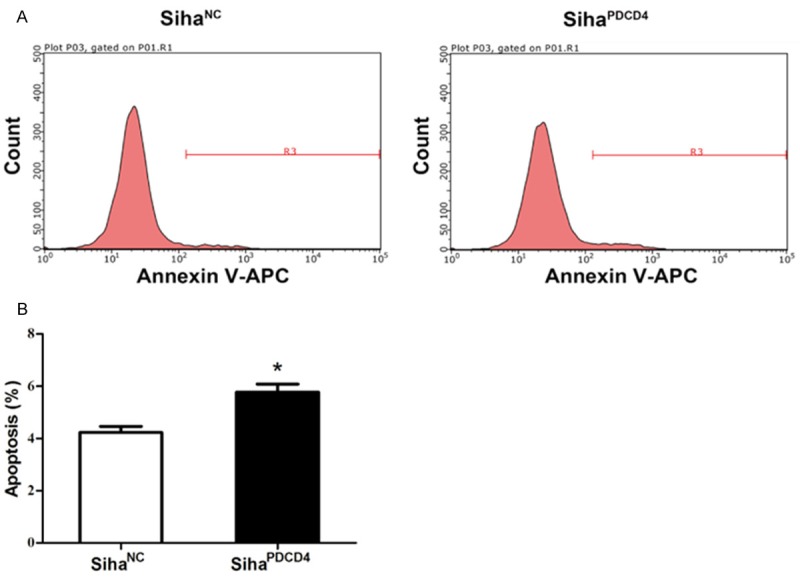
The influence of PDCD4 overexpression on cell apoptosis. SihaPDCD4 cells and SihaNC cells were stained with Annexin V-APC and analyzed by FCM (A). The average percentage of apoptotic cells was further calculated (B). *P<0.05 compared with negative control.
Overexpression of PDCD4 activated MAPK signaling pathways in Siha cells
The phosphorylation of key proteins in the signaling pathways was involved in the basic cellular functions, including cell apoptosis, migration and invasion. To further explore the mechanism of PDCD4 in Siha cell apoptosis and the suppression of Siha cell motility, we detected the phosphorylation levels of key proteins that are correlated with multiple diseases between SihaPDCD4 and SihaNC cells by signaling array assays. The results revealed that almost all the key proteins’ phosphorylation levels in Siha cells were upregulated after infection with Lv-PDCD4 (Figure 4A). The phosphorylation levels of Akt, mTOR, S6 Ribosomal Protein, PRAS40 and GSK-3β were increased by 1.52, 1.35, 1.41, 1.85, and 1.32 folds respectively, demonstrating that Akt/mTOR pathway was activated in SihaPDCD4 cells. Moreover, the phosphorylation levels of AMPKα, JNK, p38, STAT1 and p53 were also slightly increased, indicating that PDCD4 could promote the activation of the AMPKα, JNK/MAPK, p38/MAPK, STAT1, and p53 pathways (Figure 4A). Although the total protein levels of p53 and STAT1 showed no significant differences, the phosphorylation levels were markedly upregulated after infection with Lv-PDCD4 (Figure 4B).
Figure 4.
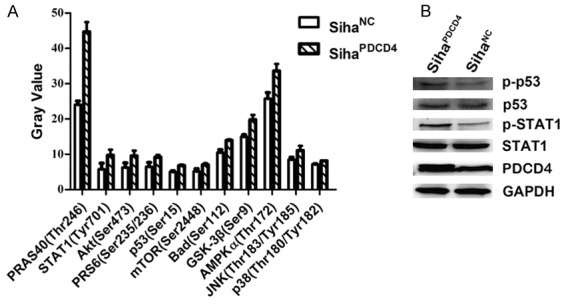
Signaling array of phosphorylation of key proteins in signaling pathways. Intracellular signaling array of Siha cells were conducted after Lv-NC or Lv-PDCD4 infection (A). PDCD4 induces rapid p53 and STAT1 phosphorylation and then the expression of p-p53, p53, p-STAT1 and STAT1 was analyzed by Western blotting (B).
Discussion
To explore the effects of PDCD4 in cervical cancer cells, we first constructed PDCD4 overexpression (Lv-PDCD4) and PDCD4 negative control (Lv-NC) lentiviral vectors, which were then proved to have high infection efficiency (Figure 1A-C). The recombination vector Lv-PDCD4 significantly elevated PDCD4 expression in Siha cells. Our previous work revealed that low PDCD4 expression was closely correlated with the lymph node metastasis of cervical cancer [17]. It was reported that PDCD4 could directly interact with Twist1, which is a key transcription factor during the EMT transformation process of various tumor cells [21]. In accordance with this, we found that the overexpression of PDCD4 significantly suppressed the migration and invasion of Siha cells. Furthermore, PDCD4 overexpression has been proven to induce the apoptosis of tumor cells [22]. Our study data showed that the overexpression of PDCD4 could promote Siha cell apoptosis. Thus, our results revealed that low PDCD4 expression in cervical cancer might in turn be attributed to cell migration, cell invasion, and anti-apoptosis, indicating PDCD4 as a potential new diagnostic and therapeutic target for cervical cancer.
The molecular mechanisms of PDCD4 underlying the regulatory functions of cervical cancer cells remain unclear, and hence should be further investigated. A signaling array assay showed that the phosphorylation levels of Akt, mTOR, S6 Ribosomal Protein, PRAS40, and GSK-3β were increased, demonstrating that the Akt/mTOR pathway was activated in SihaPDCD4 cells. In addition, the phosphorylation levels of AMPKα, JNK, p38, STAT1, and p53 were also elevated, indicating that PDCD4 could promote the activation of the AMPKα, JNK/MAPK, p38/MAPK, STAT1, and p53 pathways (Figure 4A). In other words, the PI3K/Akt/mTOR signaling pathway, p38, and JNK were related to cell migration and invasion in some cancers, making us confused [23-25]. The effects of PI3K/Akt/mTOR signaling, p38 and JNK in the suppression of cervical cancer cell migration and invasion by PDCD4 should be investigated in future studies. It was reported that the activated STAT1 pathway downregulated the expression levels of bFGF, MMP-2, MMP-9 and VEGF to inhibit tumor metastasis [26]. Previous studies reported that PDCD4 deficiency facilitated the IL-6/STAT3 pathway in mice and promoted colorectal cancer development [27,28]. Phosphorylation levels of STAT1 in Western blotting and the activation of the STAT1 pathway in the signaling array assay indicated that PDCD4 might have inhibited cell migration and invasion through the activation of STAT1 signaling.
As a tumor suppressor protein, p53 upregulation could trigger the apoptosis of various cancer cells. Our previous study also revealed that the expression level of PDCD4 was significantly associated with p53 status in cervical cancer [17]. The activated STAT1 pathway could upregulate the expression of Caspase 1 and Caspase 11 to induce cell apoptosis [29]. Considering the enhanced phosphorylation of p53 and STAT1 in Western blotting and the activation of the STAT1 pathway in the signaling array assay, we deduced that PDCD4 might induce the apoptosis of Siha cells through p53 and STAT1 signaling.
In summary, our results demonstrated that PDCD4 is closely associated with cell migration, cell invasion and cell apoptosis in cervical cancer cells through the STAT1 and p53 pathways. In vivo and in vitro studies exploring the roles and mechanisms of PDCD4 should be conducted to assess these potential molecular targets for cervical cancer treatment.
Acknowledgements
This work was supported by the Guangdong Planning Project of Science and Technology (No. 2014A020212190 and No. 2016A020215118), the Social Science and Technology Development Program of Dongguan (No. 2013108101061), the Guangdong Natural Science Foundation (No. 2016A030313525), and the Science and Technology Program of Guangzhou (No. 201607010015).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Shibahara K, Asano M, Ishida Y, Aoki T, Koike T, Honjo T. Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene. 1995;166:297–301. doi: 10.1016/0378-1119(95)00607-9. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Knosel T, Kristiansen G, Pietas A, Garber ME, Matsuhashi S, Ozaki I, Petersen I. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J Pathol. 2003;200:640–646. doi: 10.1002/path.1378. [DOI] [PubMed] [Google Scholar]
- 4.Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH, Post S, Jansen A, Colburn NH, Allgayer H. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer. 2007;110:1697–1707. doi: 10.1002/cncr.22983. [DOI] [PubMed] [Google Scholar]
- 5.Wei NA, Liu SS, Leung TH, Tam KF, Liao XY, Cheung AN, Chan KK, Ngan HY. Loss of Programmed cell death 4 (Pdcd4) associates with the progression of ovarian cancer. Mol Cancer. 2009;8:70. doi: 10.1186/1476-4598-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhen Y, Liu Z, Yang H, Yu X, Wu Q, Hua S, Long X, Jiang Q, Song Y, Cheng C, Wang H, Zhao M, Fu Q, Lyu X, Chen Y, Fan Y, Liu Y, Li X, Fang W. Tumor suppressor PDCD4 modulates miR-184-mediated direct suppression of C-MYC and BCL2 blocking cell growth and survival in nasopharyngeal carcinoma. Cell Death Dis. 2013;4:e872. doi: 10.1038/cddis.2013.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Ozaki I, Mizuta T, Hamajima H, Yasutake T, Eguchi Y, Ideguchi H, Yamamoto K, Matsuhashi S. Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene. 2006;25:6101–6112. doi: 10.1038/sj.onc.1209634. [DOI] [PubMed] [Google Scholar]
- 8.Gao F, Zhang P, Zhou C, Li J, Wang Q, Zhu F, Ma C, Sun W, Zhang L. Frequent loss of PDCD4 expression in human glioma: possible role in the tumorigenesis of glioma. Oncol Rep. 2007;17:123–128. [PubMed] [Google Scholar]
- 9.Guo PT, Yang D, Sun Z, Xu HM. PDCD4 functions as a suppressor for pT2a and pT2b stage gastric cancer. Oncol Rep. 2013;29:1007–1012. doi: 10.3892/or.2013.2232. [DOI] [PubMed] [Google Scholar]
- 10.Yang HS, Cho MH, Zakowicz H, Hegamyer G, Sonenberg N, Colburn NH. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol. 2004;24:3894–3906. doi: 10.1128/MCB.24.9.3894-3906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, Lockett SJ, Sonenberg N, Colburn NH. The transformation suppressor pdcd4 is a novel eukaryotic translation initiation factor 4a binding protein that inhibits translation. Mol Cell Biol. 2003;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eto K, Goto S, Nakashima W, Ura Y, Abe SI. Loss of programmed cell death 4 induces apoptosis by promoting the translation of procaspase-3 mRNA. Cell Death Differ. 2012;19:573–581. doi: 10.1038/cdd.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakimoto T, Shiraishi R, Iwakiri R, Fujimoto K, Takahashi H, Hamajima H, Mizuta T, Ideguchi H, Toda S, Kitajima Y. Expression patterns of the tumor suppressor PDCD4 and correlation with β-catenin expression in gastric cancers. Oncol Rep. 2011;26:1385–1392. doi: 10.3892/or.2011.1450. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Sun ZX, Allgayer H, Yang HS. Down-regulation of E-cadherin is an essential event in activating β-catenin/Tcf dependent transcription and expression of its target genes in Pdcd4 knock-down cells. Oncogene. 2010;29:128–138. doi: 10.1038/onc.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, Li CC, Tan TH, Colburn NH. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26:1297–1306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang SK, Baker AR, Young MR, Colburn NH. Tumor suppressor PDCD4 inhibits NF-kappaB-dependent transcription in human glioblastoma cells by direct interaction with p65. Carcinogenesis. 2014;35:1469–1480. doi: 10.1093/carcin/bgu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu XB, Wu KH, Huang SL, Zhang YZ, Zeng T, Qiu YR. Down-regulation of programmed cell death 4 (PDCD4) associates with the progression of cervical cancer. International Journal of Clinical and Experimental Pathology. 2016;9:4424–4431. [Google Scholar]
- 18.Zhang YZ, Huang SL, Gao HY, Wu KH, Ouyang XM, Zhu Z, Yu XB, Zeng T. Upregulation of KIN17 is associated with non-small cell lung cancer invasiveness. Oncol Lett. 2017;13:2274–2280. doi: 10.3892/ol.2017.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei N, Liu SS, Chan KK, Ngan HY. Tumour suppressive function and modulation of programmed cell death 4 (PDCD4) in ovarian cancer. PLoS One. 2012;7:e30311. doi: 10.1371/journal.pone.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santhanam AN, Baker AR, Hegamyer G, Kirschmann DA, Colburn NH. Pdcd4 repression of lysyl oxidase inhibits hypoxia-induced breast cancer cell invasion. Oncogene. 2010;29:3921–3932. doi: 10.1038/onc.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiota M, Izumi H, Tanimoto A, Takahashi M, Miyamoto N, Kashiwagi E, Kidani A, Hirano G, Masubuchi D, Fukunaka Y, Yasuniwa Y, Naito S, Nishizawa S, Sasaguri Y, Kohno K. Programmed cell death protein 4 down-regulates Y-box binding protein-1 expression via a direct interaction with Twist1 to suppress cancer cell growth. Cancer Res. 2009;69:3148–3156. doi: 10.1158/0008-5472.CAN-08-2334. [DOI] [PubMed] [Google Scholar]
- 22.Wei ZT, Zhang X, Wang XY, Gao F, Zhou CJ, Zhu FL, Wang Q, Gao Q, Ma CH, Sun WS, Fu QZ, Chen YH, Zhang LN. PDCD4 inhibits the malignant phenotype of ovarian cancer cells. Cancer Sci. 2009;100:1408–1413. doi: 10.1111/j.1349-7006.2009.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loesch M, Zhi HY, Hou SW, Qi XM, Li RS, Basir Z, Iftner T, Cuenda A, Chen G. p38γ MAPK Cooperates with c-Jun in trans-Activating Matrix Metalloproteinase 9. J Biol Chem. 2010;285:15149–15158. doi: 10.1074/jbc.M110.105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi Y, Ko YS, Park J, Choi Y, Kim Y, Pyo JS, Jang BG, Hwang DH, Kim WH, Lee BL. HER2-induced metastasis is mediated by AKT/JNK/EMT signaling pathway in gastric cancer. World J Gastroenterol. 2016;22:9141–9153. doi: 10.3748/wjg.v22.i41.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Shi H, Tang H, Fang Z, Wang J, Cui S. miR-218 inhibits the invasion and migration of colon cancer cells by targeting the PI3K/Akt/mTOR signaling pathway. Int J Mol Med. 2015;35:1301–1308. doi: 10.3892/ijmm.2015.2126. [DOI] [PubMed] [Google Scholar]
- 26.Huang S, Bucana CD, Van Arsdall M, Fidler IJ. Stat1 negatively regulates angiogenesis, tumorigenicity and metastasis of tumor cells. Oncogene. 2002;21:2504–2512. doi: 10.1038/sj.onc.1205341. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Uzair ur R, Guo Y, Liang H, Cheng R, Yang F, Hong Y, Zhao C, Liu M, Yu M, Zhou X, Yin K, Chen J, Zhang J, Zhang CY, Zhi F, Chen X. miR-181b functions as an oncomiR in colorectal cancer by targeting PDCD4. Protein Cell. 2016;7:722–734. doi: 10.1007/s13238-016-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Zhao M, Guo C, Wang G, Zhu F, Wang J, Wang X, Wang Q, Zhao W, Shi Y. PDCD4 deficiency aggravated colitis and colitis-associated colorectal cancer Via promoting IL-6/STAT3 pathway in mice. Inflamm Bowel Dis. 2016;22:1107–1118. doi: 10.1097/MIB.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 29.Lee CK, Smith E, Gimeno R, Gertner R, Levy DE. STAT1 affects lymphocyte survival and proliferation partially independent of its role downstream of IFN-gamma. J Immunol. 2000;164:1286–1292. doi: 10.4049/jimmunol.164.3.1286. [DOI] [PubMed] [Google Scholar]