Abstract
Zinc finger protein ZIC2 is a transcription factor encoded by the ZIC2 gene, which can interact with various DNAs and proteins. ZIC2 expression may promote cell proliferation and inhibit apoptosis. Recent studies have reported that ZIC2 acts as an oncogene in various cancers. The expression and distinct prognostic value of ZIC2 in NPC is not well established. The aim of this study was to investigate ZIC2 expression and its prognostic significance in nasopharyngeal carcinoma (NPC). The ZIC2 expressions at the mRNA levels in NPC tissues and normal tissues were investigated using Oncomine analysis. ZIC2 protein expression was analyzed in paraffin-embedded NPC tissues by immunohistochemistry. Statistical analyses were performed to evaluate the clinicopathological significance of ZIC2 expression. The result shows the expression of ZIC2 mRNA is significantly elevated in NPC tissue compared with normal nasopharynx tissues. In paraffin-embedded tissue samples, the immunoreactivity of ZIC2 was primarily seen in the nuclei and cytoplasms within tumor cells. High ZIC2 expression was obviously related to poor OS and DFS compared to low ZIC2 expression. In a multivariate analysis, the expression of ZIC2 was an independent prognostic factor for OS and DFS. ZIC2 is up-regulated in NPC and associated with histology and survival. ZIC2 may serve as a prognostic indicator for patients with NPC.
Keywords: NPC, ZIC2, overexpression, prognosis, nasopharyngeal carcinoma (NPC), Epstein-Barr virus
Introduction
Nasopharyngeal carcinoma (NPC), an Epstein-Barr virus-associated malignancy, arises from the nasopharynx and has an unbalanced distribution of incidence and morbidity in different regions of the world. It is well known that NPC is endemic in southern China, Hong Kong, North Africa [1], and Southeast Asia with an approximate incidence of 30 per 100,000, yet NPC is relatively rare among white populations [2-4]. What causes these variations in the incidence of NPC is unclear. Multiple factors, such as EBV infection, environmental factors, and genetic susceptibility are believed to account for NPC’s geographical distribution [5]. Since EBV infection is ubiquitous in the B lymphocytes but rare in epithelial cells [6], it is generally accepted that other environmental and genetic factors are also important determinants for NPC risk.
Currently, radiotherapy and concurrent chemoradiotherapy (CCRT) are the preferred treatment strategies for NPC. Despite a great improvement in diagnosis and treatment, the 5-year overall survival for locally-advanced NPC has remained poor [7]. Therefore, a novel target is in urgent need for early diagnosis and an efficient therapeutic option for NPC. Zinc finger protein ZIC2 (ZIC2) is a transcription factor encoded by the ZIC2 gene, which contains C2H2 zinc fingers and can interact with various DNA and proteins [8].
ZIC2 expression can also promote cell proliferation and inhibit apoptosis during the development of pancreatic ductal adenocarcinoma [9]. Recent studies have reported that ZIC2 acts as an oncogene in various cancers, such as ovarian cancer [10], liver cancer [11], and pancreatic cancer [9]. However, the clinical significance of ZIC2 in NPC remains unclear. In the present study, we examined the ZIC2 expression in NPC tissue samples and revealed its significance in NPC prognosis and clinical pathology.
Material and methods
Oncomine analysis
Oncomine (www.oncomine.org) is an online database of cancer microarrays that aims at collecting, standardizing, and analyzing cancer transcriptome data to the biomedical research community and providing gene expression as well as clinical and pathological profiles for the major types of cancer contrasted with respective normal tissues [12]. It contains 65 gene expression datasets covering a wide variety of human malignant cancers [12]. In this study, we took advantage of this high-throughput database to mine ZIC2 expression in nasopharyngeal carcinoma tissues and normal tissues. The threshold value of this study was a 2.0 fold change, a cut-off p-value <0.05, and top 10% gene rank.
Patients and tissue specimens
All clinical samples used for the expression of ZIC2 studies by immunohistochemistry (IHC) assay were collected from the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China, between April 2010 and December 2017. The original site of NPC was involved in selecting retrievable biopsy samples. These cases included 78 (72.2%) men and 30 (27.8%) women, with a median age of 48 years (range, 16 to 80 years old). The median follow-up time was 46 months (range, 3.0 to 89 months). All the patients were followed from diagnosis until death, or relapse, or the last census date. The clinicopathologic characteristics for these patients including age, gender, histology, clinical stage T classification, N classification, carotid sheath involvement, nasal cavity involvement, maxillary sinus involvement, neck lymph node level involvement, and maximum neck lymph node diameter are summarized in Table 1. All the NPC patients were treated with standard radical radiotherapy, except 4 patients with distant metastasis at the time of diagnosis (with or without chemotherapy). The TNM stage was defined on the basis of the 8th AJCC (American Joint Committee on Cancer) Staging System. Based on the primary tumor sites and lymph nodes, there were 3, 23, 47, 25 and 4 patients belonging to the categories of stage I, II, III, IVa, and IVb respectively. For the use of these clinical materials on research purposes, prior consent was obtained from each of the patients, and the protocol of this study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University.
Table 1.
Correlation of ZIC2 expression with clinicopathological features
| Characteristics | Total (n=108) | ZIC2 expression | P value | |
|---|---|---|---|---|
|
| ||||
| Low (n=99) | High (n=9) | |||
| Age (years) | 0. 305 | |||
| ≤ 46 | 44 (40.7%) | 42 (95.5%) | 2 (4.5%) | |
| > 46 | 64 (59.3%) | 57 (89.1%) | 7 (10.9%) | |
| Gender | 0.112 | |||
| Male | 78 (72.2%) | 74 (94.9%) | 4 (5.1%) | |
| Female | 30 (27.8%) | 25 (83.3%) | 5 (16.7%) | |
| Histology | 0.478 | |||
| WHO I | 0 (0%) | 0 (0%) | 0 (0%) | |
| WHO II | 41 (38.0%) | 39 (95.1%) | 2 (6.9%) | |
| WHO III | 67 (62.0%) | 60 (89.6%) | 7 (10.4%) | |
| Clinical stage (UICC) | 0.683 | |||
| I | 3 (2.9%) | 3 (100.0%) | 0 (0.0%) | |
| II | 23 (22.5%) | 22 (95.7%) | 1 (4.3%) | |
| III | 47 (46.1%) | 43 (91.5%) | 4 (8.5%) | |
| IV | 25 (24.5%) | 21 (84.0%) | 4 (16.0%) | |
| V | 4 (3.9%) | 4 (100.0%) | 0 (0.0%) | |
| T classification | 0.463 | |||
| T1 | 11 (10.8%) | 10 (90.9%) | 1 (9.1%) | |
| T2 | 40 (39.2%) | 36 (90.0%) | 4 (10.0%) | |
| T3 | 31 (30.4%) | 30 (96.8%) | 1 (3.2%) | |
| T4 | 20 (19.6%) | 17 (85.0%) | 3 (15.0%) | |
| N classification | 0.233 | |||
| N0 | 29 (28.4%) | 25 (86.2%) | 4 (13.8%) | |
| N1 | 25 (24.5%) | 25 (100.0%) | 0 (0.0%) | |
| N2 | 38 (37.3%) | 34 (89.5%) | 4 (10.5%) | |
| N3 | 10 (9.8%) | 9 (90.0%) | 1 (10.0%) | |
| Carotid sheath involvement | 0.617 | |||
| No | 15 (14.7%) | 13 (86.7%) | 2 (13.3%) | |
| Yes | 87 (85.3%) | 80 (92.0%) | 7 (8.0%) | |
| Nasal cavity involvement | 0.055 | |||
| No | 72 (70.6%) | 63 (87.5%) | 9 (12.5%) | |
| Yes | 30 (29.4%) | 30 (100.0%) | 0 (0.0%) | |
| Maxillary sinus involvement | 0.680 | |||
| No | 80 (78.4%) | 72 (90.0%) | 8 (10.0%) | |
| Yes | 22 (21.6%) | 21 (95.5%) | 1 (4.5%) | |
| Neck lymph node level involvement | 0.827 | |||
| No | 29 (28.4%) | 25 (86.2%) | 4 (13.8%) | |
| Level I-III | 63 (61.8%) | 59 (93.7%) | 4 (6.3%) | |
| Level IV | 10 (9.8%) | 9 (90.0%) | 1 (10.0%) | |
| Maximum neck lymph node diameter | 0.488 | |||
| <20 mm | 52 (51.0%) | 46 (88.5%) | 6 (11.5%) | |
| ≥20 mm | 50 (49.0%) | 47 (94.0%) | 3 (6.0%) | |
WHO, World Health Organization.
Immunohistochemistry (IHC) staining
Formalin-fixed and paraffin-embedded NPC samples were cut into sequential sections, each 4 μm thick. Then the slides were baked for 3 hours at 60°C. After being deparaffinized in xylenes and rehydrated through graded alcohol to distilled water, these slides were immersed into 3% hydrogen peroxide to block endogenous peroxidase activity at room temperature for 20 min, and then we boiled the slides in an ethylenediamine tetraacetic acid (EDTA) buffer (pH=8.0) for 3 min in a high-pressure cooker for antigen retrieval. After the retrieval solution returned to room temperature, the slides were incubated with diluted rabbit polyclonal anti-ZIC2 antibody (1:200 dilutions; Affinity, USA) in a moist chamber at 4°C overnight. Subsequently, after we washed the samples 3 times in phosphate buffered saline, we added Tween-20 (TBST), and then the slides were incubated with a secondary antibody at room temperature for 1 hour and then washed in PBST 3 times, and sequentially stained for 2 min in DAB (3,3-diaminobenzidine) at room temperature for protein detection. Finally, the slides were counterstained with Mayer’s hematoxylin and dehydrated and mounted. A negative control was obtained by replacing the primary antibody with a normal rabbit IgG.
IHC evaluation
To ensure the accuracy and objectivity of the experiment, the evaluation of the IHC staining for each sample was conducted separately by three pathologists blinded to the clinicopathological data. The expression of ZIC2 was located in the nuclei and cytoplasms. Criteria were established such that tissue with BSG expression in more than 25% of tumor cells would be considered as BSG-positive. The immunostaining of EGFR and P53 expression was assessed according to the proportion of cells with a positive expression to the total number of cells. At least 10 typical fields in high magnification (40×40) from necrotic areas were randomly selected for counting. Results of the immunostaining were divided into four groups: (-) for negative staining, (+) for weak staining, (++) for moderate staining and (+++) for strong staining.
Evaluation of IHC
The tumor cell percentage was scored as follows: 0 for less than 10% positive cells; 1 for 10-25% positive cells; 2 for 26-50% positive cells; 3 for 51-75% positive cells; 4 for more than 75% positive cells. The staining intensity was categorized: 0 for no staining; 1 for weak staining; 2 for moderate staining and 3 for strong staining. The two individual parameters were multiplied to get a final immunoreactivity score (IRS) ranging from 0 to 12. An optimal cut-off value for the high and low expression of ZIC2 was decided on the basis of a measure of heterogeneity with a log-rank test statistical analysis with respect to the overall survival (OS). For ZIC2, the optimal cutoff value was determined: an IRS ≤ 4 was defined as low expression and an IRS > 4 was defined as high expression.
Statistical analysis
The statistical analysis was performed by the Statistical Program for Social Sciences (SPSS, Chicago, IL, USA; 19.0). The duration from the start of randomization to death caused by any event was defined as overall survival (OS). The duration from the start of randomization to tumor recurrence or relapse or death was defined as disease-free survival (DFS). The correlation between the ZIC2 expression and the clinicopathological characteristics of these NPC patients was assessed by a chi-square test. Survival curves for the ZIC2 high-expression and the ZIC2 low expression patients were plotted by the Kaplan-Meier analysis and a log-rank test. The univariate and multivariate survival analyses were performed using the Cox proportional hazard model to determine effect of the particular prognostic factors on survival. All the P-values in the statistical analysis were two-tailed, and differences were considered significant if the P-value was <0.05.
Results
The expression level of ZIC2 was elevated in NPC patients
In the Oncomine database, the distribution of ZIC2 expression in NPC tissues and normal nasopharynx tissues were available from a microarray chips including 41 samples. We compared the expression levels of ZIC2 between NPC tissues and the normal nasopharynx tissue in the Oncomine database. The results showed that the expression of ZIC2 is significantly elevated in NPC tissue compared with normal nasopharynx tissues (Figure 1).
Figure 1.
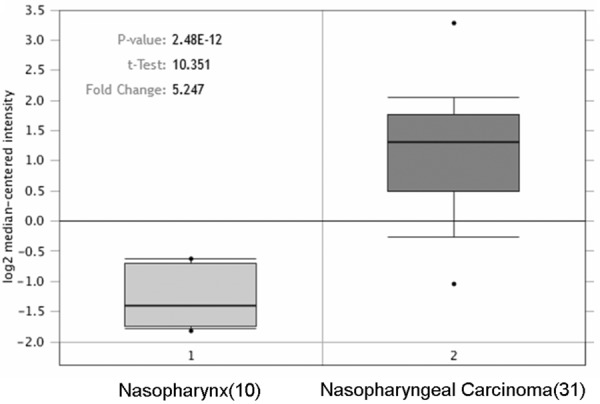
ZIC2 expression in NPC and normal nasopharynx tissues. The figure was derived from gene expression data in the Oncomine database comparing expression levels of ZIC2 in normal (left plot) and cancer tissue (right plot).
The expression patterns of ZIC2 in NPCs by IHC
To examine the expression pattern of ZIC2 in archived NPC tissues, we performed an IHC analysis with the specific antibody in 108 NPC tissues. Four representative images of immunostaining illustrated the ZIC2 expression pattern in the NPC tissues are shown in Figure 2. The Immunoreactivity of the ZIC2 expression was primarily seen in the nuclei and cytoplasms within the tumor cells. Among them, 29 (26.9%) cases showed weak intensity (Figure 2A and 2B), 70 (64.8%) cases showed moderate staining (Figure 2C), and 9 (8.3%) cases showed strong intensity (Figure 2D).
Figure 2.
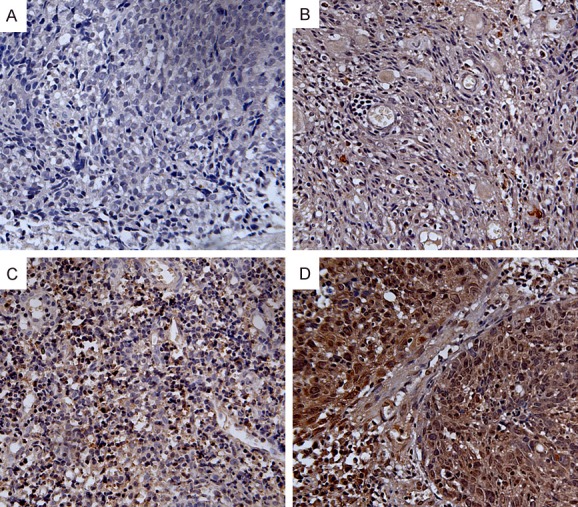
Expression analysis of ZIC2 protein as determined by immunohistochemistry (200×). ZIC2 expression was localized in the nuclei and cytoplasms of NPC cells. A. Negative staining of ZIC2 in NPC tissues. B. “+” (score 1-4, weakly positive) expression of ZIC2 in NPC tissues. C. “++” (score 5-8, positive) expression of ZIC2 in NPC tissues. D. “+++” (score 9-12, strongly positive) expression of ZIC2 in NPC tissues.
Furthermore, we investigated the relationship between ZIC2 expression and the clinicopathological parameters of NPC. Unfortunately, neither age, gender, clinical stage (UICC), Histology, T classification, N classification, carotid sheath involvement, nasal cavity involvement, maxillary sinus involvement, neck lymph node level involvement, or maximum neck lymph node diameter had any statistical relationship with the expression level of ZIC2 (Table 1).
The association between ZIC2 expression and patient prognosis
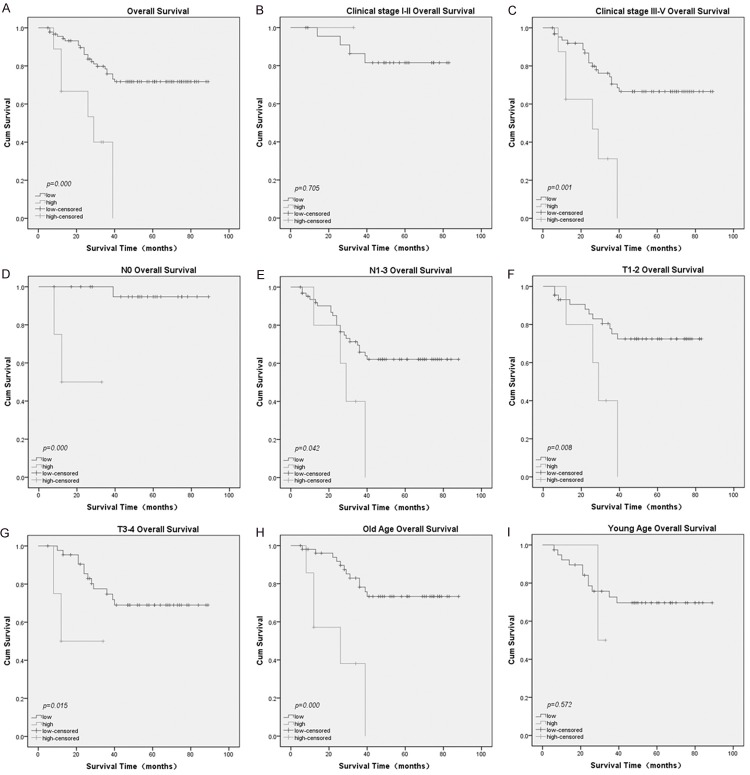
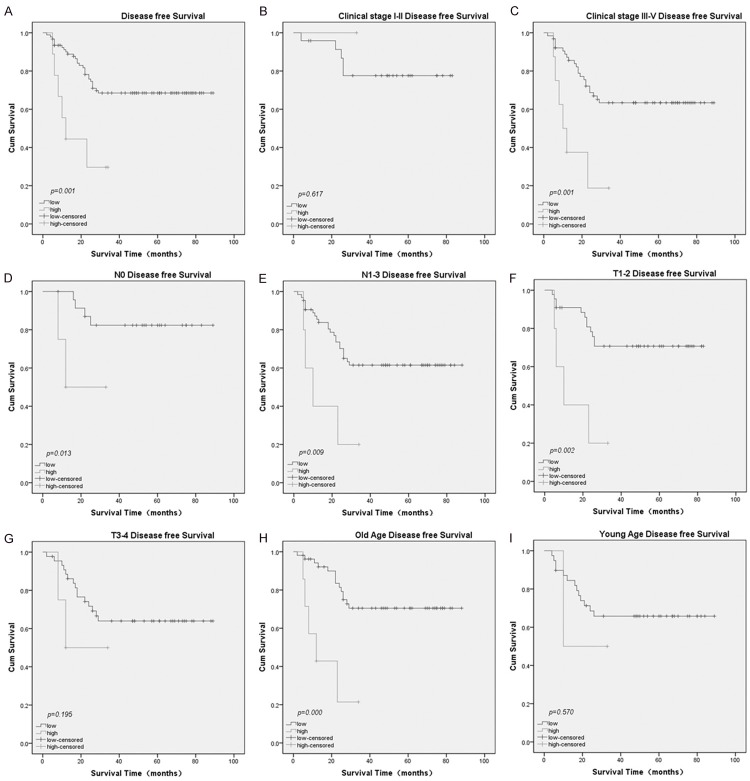
The prognostic value of ZIC2 was evaluated through estimation of OS and DFS using a Kaplan- Meier survival analysis (log-rank test). It is obviously shown that a high ZIC2 expression was significantly related to poor OS (P<0.001, Figure 3A) and DFS (P=0.001, Figure 4A) compared to low ZIC2 expression. In addition, a Univariate Cox proportional hazard regression analysis showed that clinical stage (UICC) (P<0.001), N classification (P=0.001), neck lymph node level involvement (P=0.003), maximum neck lymph node diameter (P=0.020), and ZIC2 expression (P=0.001) were significantly associated with survival (Table 2). AS for DFS, clinical stage (8th AJCC) (P=0.026), N stage (P=0.010), neck lymph node level involvement (P=0.016), maximum neck lymph node diameter (P=0.045), and ZIC2 expression (P=0.003) were significantly associated with survival (Table 3).
Figure 3.
The prognostic value (OS) of ZIC2 expression analyzed using a Kaplan-Meier Plotter. A. OS rates for patients with high ZIC2 expression versus those with low ZIC2 expression levels. B. OS rate for patients with early clinical stage tumors (stage I/ II) with high ZIC2 expression versus those with low ZIC2 expression. C. OS rate for patients with late clinical stage tumors (stage III/ IV) with high ZIC2 expression versus those with low ZIC2 expression. D. OS rate for patients with N0 Stage tumors with high ZIC2 expression versus those patients with low ZIC2 expression. E. OS rate for patients with N1-3 Stage tumors with high ZIC2 expression versus those patients with low ZIC2 expression. F. OS rate for patients with T1-2 Stage tumors with high ZIC2 expression versus those patients with low ZIC2 expression. G. OS rate for patients with T3-4 Stage tumors with high ZIC2 expression versus those patients with low ZIC2 expression. H. OS rate for older patients with high ZIC2 expression versus those patients with low ZIC2 expression. I. OS rate for younger patients with high ZIC2 expression versus those patients with low ZIC2 expression.
Figure 4.
The prognostic value (DFS) of ZIC2 expression analyzed using a Kaplan-Meier plotter. A. DFS rates for patients with high ZIC2 expression versus those with low ZIC2 expression levels. B. DFS rates for patients with early clinical stage tumor (stage I/ II) with high ZIC2 expression versus those with low ZIC2 expression. C. DFS rate for patients with late clinical stage tumor (stage III/ IV) with high ZIC2 expression versus those with low ZIC2 expression. D. DFS rate for patients with N0 Stage tumor with high ZIC2 expression versus those patients with low ZIC2 expression. E. DFS rate for patients with N1-3 Stage tumor with high ZIC2 expression versus those patients with low ZIC2 expression. F. DFS rate for patients with T1-2 Stage tumor with high ZIC2 expression versus those patients with low ZIC2 expression. G. DFS rate for patients with T3-4 Stage tumor with high ZIC2 expression versus those patients with low ZIC2 expression. H. DFS rate for patients of old age with high ZIC2 expression versus those patients with low ZIC2 expression. I. DFS rate for patients of young age with high ZIC2 expression versus those patients with low ZIC2 expression.
Table 2.
Cox-regression analysis of parameters associated with overall survival of all patients
| Factor | Univariate | Multivariate | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | ||||
| ≤ 46 | Reference | |||
| > 46 | 1.019 (0.487-2.134) | 0.960 | - | - |
| Gender | ||||
| Male | Reference | |||
| Female | 1.160 (0.495-2.718) | 0.732 | - | - |
| Histology | ||||
| WHO I | ||||
| WHO II | Reference | - | - | |
| WHO III | 1.456 (0.700-3.027) | 0.315 | - | - |
| Clinical stage (UICC) | ||||
| I | Reference | 0.000 | Reference | 0.000 |
| II | 0.036 (0.006-0.221) | 0.000 | 0.020 (0.003-0.134) | 0.000 |
| III | 0.046 (0.009-0.239) | 0.000 | 0.018 (0.003-0.111) | 0.000 |
| IV | 0.147 (0.030-0.726) | 0.019 | 0.073 (0.013-0.397) | 0.002 |
| T classification | ||||
| T1 | Reference | 0.263 | ||
| T2 | 1.103 (0.360-3.377) | 0.863 | - | - |
| T3 | 0.546 (0.215-1.385) | 0.202 | - | - |
| T4 | 0.438 (0.152-1.263) | 0.126 | - | - |
| N classification | ||||
| N0 | Reference | 0.001 | ||
| N1 | 0.081 (0.021-0.316) | 0.000 | - | - |
| N2 | 0.237 (0.083-0.682) | 0.008 | - | - |
| N3 | 0.257 (0.100-0.660) | 0.005 | - | - |
| Carotid sheath involvement | ||||
| No | Reference | |||
| Yes | 0.903 (0.314-2.594) | 0.849 | - | - |
| Nasal cavity involvement | ||||
| No | Reference | |||
| Yes | 0.740 (0.344-1.593) | 0.441 | - | - |
| Maxillary sinus involvement | ||||
| No | Reference | |||
| Yes | 0.754 (0.334-1.702) | 0.496 | - | - |
| Neck lymph node level involvement | ||||
| No | Reference | 0.003 | ||
| Level I-III | 0.211 (0.026-1.723) | 0.147 | - | - |
| Level IV-V | 0.241 (0.094-0.617) | 0.003 | - | - |
| Maximum neck lymph node diameter | ||||
| <20 mm | Reference | Reference | ||
| ≥20 mm | 0.394 (0.179-0.865) | 0.020 | 0.378 (0.166-0.859) | 0.020 |
| ZIC2 expression | ||||
| Low | Reference | Reference | ||
| High | 0.219 (0.087-0.551) | 0.001 | 0.146 (0.054-0.399) | 0.000 |
Table 3.
Cox-regression analysis of parameters associated with the disease free survival of all patients
| Factor | Univariate | Multivariate | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | ||||
| ≤ 46 | Reference | |||
| > 46 | 1.080 (0.542-2.155) | 0.826 | - | - |
| Gender | ||||
| Male | Reference | |||
| Female | 1.194 (0.539-2.648) | 0.662 | - | - |
| Histology | ||||
| WHO I | ||||
| WHO II | Reference | - | - | |
| WHO III | 1.362 (0.683-2.719) | 0.380 | - | - |
| Clinical stage (UICC) | ||||
| I | Reference | 0.026 | ||
| II | 1.038 (0.093-6.314) | 0.931 | - | - |
| III | 6.490 (0.094-8.713) | 0.929 | - | - |
| IV | 1.340 (0.334-1.979) | 0.914 | - | - |
| T classification | ||||
| T1 | Reference | 0.562 | ||
| T2 | 0.534 (0.185-1.539) | 0.245 | - | - |
| T3 | 0.574 (0.192-1.714) | 0.320 | - | - |
| T4 | 0.855 (0.279-2.613) | 0.783 | - | - |
| N classification | ||||
| N0 | Reference | 0.010 | ||
| N1 | 0.172 (0.057-0.514) | 0.002 | - | - |
| N2 | 0.290 (0.104-0.805) | 0.017 | - | - |
| N3 | 0.283 (0.110-0.724) | 0.008 | - | - |
| Carotid sheath involvement | ||||
| No | Reference | |||
| Yes | 0.780 (0.274-2.219) | 0.642 | - | - |
| Nasal cavity involvement | ||||
| No | Reference | |||
| Yes | 0.594 (0.295-1.195) | 0.144 | - | - |
| Maxillary sinus involvement | ||||
| No | Reference | |||
| Yes | 0.739 (0.343-1.591) | 0.439 | - | - |
| Neck lymph node level involvement | ||||
| No | Reference | 0.016 | ||
| Level I-III | 0.172 (0.057-0.514) | 0.002 | - | - |
| Maximum neck lymph node diameter | ||||
| <20 mm | Reference | |||
| ≥20 mm | 0.483 (0.237-0.982) | 0.045 | - | - |
| ZIC2 expression | ||||
| Low | Reference | Reference | ||
| High | 0.251 (0.102-0.615) | 0.003 | 0.187 (0.070-0.498) | 0.001 |
After multivariate adjustment for the above significant clinicopathological features, the clinical stage (8th AJCC) (HR: 0.020; 95% confidence interval: 0.003-0.134; P<0.001), maximum neck lymph node diameter (HR: 0.378; 95% confidence interval: 0.166-0.859; P=0.020). and ZIC2 expression (HR: 0.146; 95% confidence interval: 0.054-0.399; P<0.001) were independent prognostic factors for OS (Table 2), while only ZIC2 expression was an independent prognostic factor for DFS (HR: 0.187; 95% confidence interval: 0.070-0.498; P=0.001, Table 3).
Furthermore, we analyzed the prognostic value of ZIC2 in selective patient subgroups stratified by clinical stage (8th AJCC), N stage, T stage, and age. The expression of ZIC2 was strongly associated with OS in patients of advanced stage tumor (Figure 3C, log-rank test, P=0.001), but not in patients of early stage tumor (Figure 3B, log-rank test, P=0.706). The expression of ZIC2 was also strongly associated with OS duration in the patients of both N0 tumors (Figure 3D, log-rank test, P<0.001) and N1-3 tumors (Figure 3E, log-rank test, P=0.042), and both T1-2 tumors (Figure 3F, log-rank test, P=0.008) and T3-4 tumors (Figure 3G, log-rank test, P=0.015). As for the age subgroup, the expression of ZIC2 was strongly associated with OS duration in older patients (Figure 3H, log-rank test, P<0.001), but not in younger patients (Figure 3I, log-rank test, P=0.572). When evaluated according to DFS, the expression of ZIC2 was strongly associated with DFS duration in advanced-stage tumor patients (Figure 4C, log-rank test, P=0.001), but not in early-stage tumor patients (Figure 4B, log-rank test, P=0.617). The expression of ZIC2 was also strongly associated with DFS duration in the patients of both N0 tumors (Figure 4D, log-rank test, P=0.013) and N1-3 tumors (Figure 4E, log-rank test, P=0.009). As for the T Stage subgroup, the expression of ZIC2 was strongly associated with DFS duration in patients with T1-2 tumors (Figure 4F, log-rank test, P=0.002), but not in patients with T3-4 tumors (Figure 4G, log-rank test, P=0.195). In older patients, the expression of ZIC2 was strongly associated with DFS duration (Figure 4H, log-rank test, P<0.001), but not in younger patients (Figure 4I, log-rank test, P=0.570).
Discussion
The human ZIC (zinc finger of the cerebellum) proteins-ZIC1, ZIC2, ZIC3, ZIC4, and ZIC5, which share five highly-conserved Cys2His2 zinc-finger motifs, function in biological processes [13] as transcription factors (TFs) which contribute to the pathogenesis of multiple human cancers [14]. As zinc finger transcription factors, these proteins can bind with the GC-rich sequence in target genes [15]. ZIC proteins are critical to the developing vertebrate embryo and to human cancers [16]. The Cys2His2 zinc-finger motifs interact with the Gli family proteins via homologous structures in both an antagonistic and synergistic manner, which are quite essential for the development of human nervous system [17]. These five proteins, which are homologues of the Drosophila odd-paired gene (OPA), are structurally similar to each other, which imply that the ZIC proteins share some, but not all, functions [8]. ZIC genes play important roles in a wide array of developmental systems, such as the central nervous system, muscle and skeletal development [18]. In recent years, growing evidence has indicated that ZICs may play different roles in the pathological events of various diseases, including cancer.
ZIC1 has been found to inhibit the growth and development of various carcinomas, including GC [19], colorectal cancer [20], medulloblastoma [21], thyroid cancer, mesenchymal neoplasms [22], breast cancer [23] and endometrial cancer [24], and has also become a putative indicator of good prognosis [20,25-28]. Genome-wide analysis of CpG island methylation in pTa-bladder cancer suggested that ZIC4 [29] was correlated with poor progression [29,30]. Pavlova et al. [31] also identified that methylation of ZIC4 might participate in the development of breast cancer. However, ZIC2, ZIC3, and ZIC5 are overexpressed in cancer cells and may function as oncogenes by promoting the proliferation and inhibiting the apoptosis of tumor cells [32-34]. But the clinicopathologic and prognostic significance of the ZIC proteins still requires further illumination.
ZIC2 is only found in the testis and brain in normal tissues, but it is widely expressed in tumors. It has C2H2 zinc fingers and can interact with various DNAs and proteins [8]. ZIC2 expression could also promote cell proliferation and inhibit apoptosis during the development of pancreatic ductal adenocarcinoma [9] Recent studies have reported that ZIC2 acts as an oncogene in various cancers, such as ovarian and cervical cancer [10,35], liver cancer [11], pancreatic cancer [9], and small cell lung carcinoma [32]. Marchini et al. have reported that the overexpression of ZIC2 increased the growth rate and foci formation of ovarian cancer and stimulated anchorage- independent colony formation [10]. Chan and colleagues have revealed that in cervical cancer, ZIC2 could promote cell growth by enhancing Hedgehog signaling [35]. It can also up-regulate the expression of OCT4 by recruiting the nuclear remodeling factor complex (NURF) to the OCT4 promoter in liver cancer cells [11]. ZIC2 can enhance cellular proliferation and reduce apoptosis in pancreatic cancer via activating the transcription of fibroblast growth factor receptor 3 (FGFR3) and Annexin A8 (ANXA8) [9]. ZIC2 expression seems to increase stepwise during tumor progression and targeting ZIC2 should be a promising strategy for the clinical management of multiple cancers. However, the roles of ZIC2 in the development of cancer have not yet been fully elucidated, and the clinical significance of ZIC2 in NPC remains unclear. A comparison of ZIC2 expression and the clinical significance in NPC is required.
In this study, we compared the expression level of ZIC2 between NPC and normal nasopharynx tissues in the Oncomine database and found that the expression of ZIC2 is significantly elevated in NPC tissue compared with normal nasopharynx tissues. So, we consider that ZIC2 is an important molecular marker of NPC that can help provide precise diagnoses. ZIC2 overexpression in NPC may reflect the aberrant regulation of transcription factors (TFs). However, to understand the precise signaling pathways of ZIC2 in NPC requires further studies.
Reports have proved the prognostic value of ZIC2 in human cancers. Marchini et al. have reported that the upregulation of ZIC2 in stage I ovarian cancer was related to a poorer outcome [10]. Chan et al. also demonstrated its over-expression in cervical cancer was associated with a worse overall survival of patients [35]. Bidus et al. have illustrated that ZIC2 overexpression was found in endometrial cancer patients with metastasis in lymph nodes [36]. However, the prognostic implication of ZIC2 in NPC has not been investigated. In our study, a high ZIC2 expression was significantly related to poor OS and DFS, compared to low ZIC2 expression group. In addition, a Cox regression revealed that clinical stage (UICC), maximum neck lymph node diameter and ZIC2 expression were independent prognostic factors for OS, while only ZIC2 expression was an independent prognostic factor for DFS. This finding indicates the possibility of using ZIC2 as a predictor for prognosis and survival. A sub-group analysis revealed that ZIC2 overexpression patients with an obviously poor OS among patients whose tumors demonstrated the features of late stage disease, and old age. When evaluated according to DFS, the expression of ZIC2 was strongly associated with DFS duration in late stage tumor patients, T1-2 tumors, and older patients.
In conclusion, to our knowledge, this is the first report addressing ZIC2 expression and its clinicopathological and prognostic significance in NPC. Our findings suggest that ZIC2 is up-regulated in NPC and closely correlated with histology, and a Cox regression analysis revealed that ZIC2 might be an independent molecular biomarker for the prediction of NPC prognosis and survival. ZIC2 may serve as a prognostic indicator for patients with NPC.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81672661, 81502268), the Guangdong Province Natural Science Foundation (2015A030310126, 2016A030313598) and the Guangzhou Science and Technology Project (201804010199).
Disclosure of conflict of interest
None.
References
- 1.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 2.Wang JG, Tang WP, Liao MC, Liu YP, Ai XH. MiR-99a suppresses cell invasion and metastasis in nasopharyngeal carcinoma through targeting HOXA1. Onco Targets Ther. 2017;10:753–761. doi: 10.2147/OTT.S126781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiang G, Li X, Cao L, Zhu C, Dai Z, Pan S, Lin S. Frequent overexpression of PDK1 in primary nasopharyngeal carcinoma is associated with poor prognosis. Pathol Res Pract. 2016;212:1102–1107. doi: 10.1016/j.prp.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Z, Meng M, Ni H. Chemosensitizing effect of astragalus polysaccharides on nasopharyngeal carcinoma cells by inducing apoptosis and modulating expression of Bax/Bcl-2 ratio and caspases. Med Sci Monit. 2017;23:462–469. doi: 10.12659/MSM.903170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao SW, Yip YL, Tsang CM, Pang PS, Lau VM, Zhang G, Lo KW. Etiological factors of nasopharyngeal carcinoma. Oral Oncol. 2014;50:330–338. doi: 10.1016/j.oraloncology.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Li G, Yang N, Su Z, Zhang S, Deng T, Ren S, Lu S, Tian Y, Liu Y, Qiu Y. miR-324-3p suppresses migration and invasion by targeting WNT2B in nasopharyngeal carcinoma. Cancer Cell Int. 2017;17:2. doi: 10.1186/s12935-016-0372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali RG, Bellchambers HM, Arkell RM. Zinc fingers of the cerebellum (Zic): transcription factors and co-factors. Int J Biochem Cell Biol. 2012;44:2065–2068. doi: 10.1016/j.biocel.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Inaguma S, Ito H, Riku M, Ikeda H, Kasai K. Addiction of pancreatic cancer cells to zinc-finger transcription factor ZIC2. Oncotarget. 2015;6:28257–28268. doi: 10.18632/oncotarget.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchini S, Poynor E, Barakat RR, Clivio L, Cinquini M, Fruscio R, Porcu L, Bussani C, D’Incalci M, Erba E, Romano M, Cattoretti G, Katsaros D, Koff A, Luzzatto L. The zinc finger gene ZIC2 has features of an oncogene and its overexpression correlates strongly with the clinical course of epithelial ovarian cancer. Clin Cancer Res. 2012;18:4313–4324. doi: 10.1158/1078-0432.CCR-12-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu P, Wang Y, He L, Huang G, Du Y, Zhang G, Yan X, Xia P, Ye B, Wang S, Hao L, Wu J, Fan Z. ZIC2-dependent OCT4 activation drives selfrenewal of human liver cancer stem cells. J Clin Invest. 2015;125:3795–3808. doi: 10.1172/JCI81979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houtmeyers R, Souopgui J, Tejpar S, Arkell R. The ZIC gene family encodes multi-functional proteins essential for patterning and morphogenesis. Cell Mol Life Sci. 2013;70:3791–3811. doi: 10.1007/s00018-013-1285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhagwat AS, Vakoc CR. Targeting transcription factors in cancer. Trends Cancer. 2015;1:53–65. doi: 10.1016/j.trecan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai H, Kikuchi K, Tsuchiya T, Kanazawa H, Tsuda M. Developmentally and regionally regulated alterations of octamer- and GC-boxbinding activities during the postnatal development of mouse cerebellum. Brain Res Dev Brain Res. 1991;61:161–168. doi: 10.1016/0165-3806(91)90127-5. [DOI] [PubMed] [Google Scholar]
- 16.Chervenak AP, Hakim IS, Barald KF. Spatiotemporal expression of Zic genes during vertebrate inner ear development. Dev Dyn. 2013;242:897–908. doi: 10.1002/dvdy.23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aruga J, Nagai T, Tokuyama T, Hayashizaki Y, Okazaki Y, Chapman VM, Mikoshiba K. The mouse zic gene family. homologues of the drosophila pair-rule gene odd-paired. J Biol Chem. 1996;271:1043–1047. doi: 10.1074/jbc.271.2.1043. [DOI] [PubMed] [Google Scholar]
- 18.Bataller L, Wade DF, Fuller GN, Rosenfeld MR, Dalmau J. Cerebellar degeneration and autoimmunity to zinc-finger proteins of the cerebellum. Neurology. 2002;59:1985–1987. doi: 10.1212/01.wnl.0000038352.01415.ce. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Lin Z, Xue M, Si J, Chen S. Zic1 Promoter hypermethylation in plasma DNA is a potential biomarker for gastric cancer and intraepithelial neoplasia. PLoS One. 2015;10:e0133906. doi: 10.1371/journal.pone.0133906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gan L, Chen S, Zhong J, Wang X, Lam EK, Liu X, Zhang J, Zhou T, Yu J, Si J, Wang L, Jin H. ZIC1 is downregulated through promoter hypermethylation, and functions as a tumor suppressor gene in colorectal cancer. PLoS One. 2011;6:e16916. doi: 10.1371/journal.pone.0016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacroix J, Schlund F, Leuchs B, Adolph K, Sturm D, Bender S, Hielscher T, Pfister SM, Witt O, Rommelaere J, Schlehofer JR, Witt H. Oncolytic effects of parvovirus H-1 in medulloblastoma are associated with repression of master regulators of early neurogenesis. Int J Cancer. 2014;134:703–716. doi: 10.1002/ijc.28386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pourebrahim R, Van Dam K, Bauters M, De Wever I, Sciot R, Cassiman JJ, Tejpar S. ZIC1 gene expression is controlled by DNA and histone methylation in mesenchymal proliferations. FEBS Lett. 2007;581:5122–5126. doi: 10.1016/j.febslet.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 23.Nakakido M, Tamura K, Chung S, Ueda K, Fujii R, Kiyotani K, Nakamura Y. Phosphatidylinositol glycan anchor biosynthesis, class X containing complex promotes cancer cell proliferation through suppression of EHD2 and ZIC1, putative tumor suppressors. Int J Oncol. 2016;49:868–876. doi: 10.3892/ijo.2016.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong YF, Cheung TH, Lo KW, Yim SF, Siu NS, Chan SC, Ho TW, Wong KW, Yu MY, Wang VW, Li C, Gardner GJ, Bonome T, Johnson WB, Smith DI, Chung TK, Birrer MJ. Identification of molecular markers and signaling pathway in endometrial cancer in Hong Kong chinese women by genome-wide gene expression profiling. Oncogene. 2007;26:1971–1982. doi: 10.1038/sj.onc.1209986. [DOI] [PubMed] [Google Scholar]
- 25.Zhong J, Chen S, Xue M, Du Q, Cai J, Jin H, Si J, Wang L. ZIC1 modulates cell-cycle distributions and cell migration through regulation of sonic hedgehog, PI(3)K and MAPK signaling pathways in gastric cancer. BMC Cancer. 2012;12:290. doi: 10.1186/1471-2407-12-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiang W, Zhao Y, Yang Q, Liu W, Guan H, Lv S, Ji M, Shi B, Hou P. ZIC1 is a putative tumor suppressor in thyroid cancer by modulating major signaling pathways and transcription factor FOXO3a. J Clin Endocrinol Metab. 2014;99:E1163–1172. doi: 10.1210/jc.2013-3729. [DOI] [PubMed] [Google Scholar]
- 27.Wang YY, Jiang JX, Ma H, Han J, Sun ZY, Liu ZM, Xu ZG. Role of ZIC1 methylation in hepatocellular carcinoma and its clinical significance. Tumour Biol. 2014;35:7429–7433. doi: 10.1007/s13277-014-1971-4. [DOI] [PubMed] [Google Scholar]
- 28.Ma G, Dai W, Sang A, Yang X, Li Q. Roles of ZIC family genes in human gastric cancer. Int J Mol Med. 2016;38:259–266. doi: 10.3892/ijmm.2016.2587. [DOI] [PubMed] [Google Scholar]
- 29.Kandimalla R, van Tilborg AA, Kompier LC, Stumpel DJ, Stam RW, Bangma CH, Zwarthoff EC. Genome-wide analysis of CpG island methylation in bladder cancer identified TBX2, TBX3, GATA2, and ZIC4 as pTa-specific prognostic markers. Eur Urol. 2012;61:1245–1256. doi: 10.1016/j.eururo.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Beukers W, Kandimalla R, Masius RG, Vermeij M, Kranse R, van Leenders GJ, Zwarthoff EC. Stratification based on methylation of TBX2 and TBX3 into three molecular grades predicts progression in patients with pTa-bladder cancer. Mod Pathol. 2015;28:515–522. doi: 10.1038/modpathol.2014.145. [DOI] [PubMed] [Google Scholar]
- 31.Pavlova TV, Kashuba VI, Muravenko OV, Yenamandra SP, Ivanova TA, Zabarovskaia VI, Rakhmanaliev ER, Petrenko LA, Pronina IV, Loginov VI, Iurkevich O, Kiselev LL, Zelenin AV, Zabarovskii ER. [Technology of analysis of epigenetic and structural changes of epithelial tumors genome with NotI-microarrays by the example of human chromosome] . Mol Biol (Mosk) 2009;43:339–347. [PubMed] [Google Scholar]
- 32.Vural B, Chen LC, Saip P, Chen YT, Ustuner Z, Gonen M, Simpson AJ, Old LJ, Ozbek U, Gure AO. Frequency of SOX group B (SOX1, 2, 3) and ZIC2 antibodies in turkish patients with small cell lung carcinoma and their correlation with clinical parameters. Cancer. 2005;103:2575–2583. doi: 10.1002/cncr.21088. [DOI] [PubMed] [Google Scholar]
- 33.Yang B, Jia L, Guo Q, Ren H, Hu D, Zhou X, Ren Q, Hu Y, Xie T. MiR-564 functions as a tumor suppressor in human lung cancer by targeting ZIC3. Biochem Biophys Res Commun. 2015;467:690–696. doi: 10.1016/j.bbrc.2015.10.082. [DOI] [PubMed] [Google Scholar]
- 34.Sun Q, Shi R, Wang X, Li D, Wu H, Ren B. Overexpression of ZIC5 promotes proliferation in non-small cell lung cancer. Biochem Biophys Res Commun. 2016;479:502–509. doi: 10.1016/j.bbrc.2016.09.098. [DOI] [PubMed] [Google Scholar]
- 35.Chan DW, Liu VW, Leung LY, Yao KM, Chan KK, Cheung AN, Ngan HY. Zic2 synergistically enhances hedgehog signalling through nuclear retention of Gli1 in cervical cancer cells. J Pathol. 2011;225:525–534. doi: 10.1002/path.2901. [DOI] [PubMed] [Google Scholar]
- 36.Bidus MA, Risinger JI, Chandramouli GV, Dainty LA, Litzi TJ, Berchuck A, Barrett JC, Maxwell GL. Prediction of lymph node metastasis in patients with endometrioid endometrial cancer using expression microarray. Clin Cancer Res. 2006;12:83–88. doi: 10.1158/1078-0432.CCR-05-0835. [DOI] [PubMed] [Google Scholar]