Abstract
Human papillomavirus (HPV) is the most common sexually transmitted infectious agent and is the main cause of cervical cancer (CC). In Chile, CC is the second leading cause of death by cancer in women aged 20-44 years, four times higher than in developed countries. Currently, the detection of HPV infection using a cervical brush is recommended; however, this is an invasive procedure that many women try to avoid. The aim of this study was to evaluate the clinical performance of a self-collected, urine-based HPV detection method using conventional PCR followed by a reverse line blot. A PCR-based HPV genotyping was performed on 190 paired cervical and urine samples from gynecological exams at public health centers in the Araucania Region, Chile. HPV DNA detection and genotyping were performed by PCR and reverse line blot assay. Carcinogenic HPV types were present in 64.7% and 65.8% of the cervical and urine samples; the infection rates of HPV16 were 34.7% and 33.2%, respectively. The overall percent agreement between carcinogenic HPV detection in cervical and urine samples was 73.7%, with a moderate concordance rate of carcinogenic HPV detection (kappa = 0.42). Clinical sensitivities for cervical and urine-based sampling methods to diagnose cervical intraepithelial neoplasia 2/3 (CIN2/3) by histology were 93.4% and 90.2%, respectively. These results suggest that both cervical brush and urine-based sampling show a good clinical performance in the detection of HPV infection. The urine-based sampling method represents a valuable alternative with a great impact on public health, allowing increased cervical cancer screening coverage among women who do not undergo pelvic examinations.
Keywords: Human papillomavirus, cervical intraepithelial neoplasia, cervical cancer, urine-based sampling method
Introduction
Human papillomavirus (HPV) represents the most common sexually transmitted infectious agent throughout the world; the major risk factors are behaviors associated with sexual activity [1]. The etiological cause of nearly 100% of cervical cancers (CC) is being associated significantly with vulvar and vaginal cancers in women, penile cancer in men, and anal cancer [2-5]. In Chile, the incidence rate of CC was estimated at 12.8 per 100.000 women with a mortality rate of 6.64 per 100.000 women, making it the second leading cause of death by cancer in women aged 20-44 years, with a total of 584 deaths in 2012 [6,7]. The mortality rate for this neoplasm in our country is four times higher than in developed countries [8].
Screening efforts are focused on preventing CC, primarily by Pap test, which can detect abnormal cellular changes or pre-malignant lesions in cervical tissues [9]. Since the Pap test came into use, mortality rates from CC have decreased dramatically [10]. Nevertheless, the PAP test has several disadvantages; it requires a pelvic examination, a procedure that is invasive and uncomfortable for the patient, time consuming for healthcare providers, and cannot be carried out easily [11]. In addition, cervical cytology is susceptible to technical limitations such as the inadequate transfer of cells to the slide with homogenous distribution of abnormal cells, the presence of obscuring blood, and inflammation or thick areas of overlapping epithelial cells. Also, it is subject to interpretation errors, resulting in false negative results associated with relatively low sensitivity (less than 50%) [12,13]. In the last two decades, interest in molecular techniques for identifying HPV DNA present in infected tissues has increased, because it enables the identification of women at risk of developing CC due to its higher sensitivity of detection with only a small amount of viral DNA [14-16].
Actually, more than 200 HPV types have been reported, which can be grouped into high oncogenic risk (HR-HPV) and low oncogenic risk HPV (LR-HPV) [17,18]. HR-HPV types have been detected in more than 99% of invasive cancer cases and in the vast majority of pre-neoplastic lesions cases [19]. Therefore, detection of HR-HPV or individual HPV-16/HPV-18 is considered as the best method of CC screening, even better than liquid-based cytology [16,20,21].
In 2005, the International Agency for Research on Cancer (IARC) made recommendations that supported primary screening based on cytology or HPV-DNA testing [22]. In 2015, the Chilean Department of Health incorporated an algorithm in its clinical guide for the screening of CC in women aged 30-64 years using a method of detecting clinically validated HPV, followed by cytology [23]. However, detection of HPV DNA in cervical samples requires the same collection procedure used in Pap smears. Therefore, the development of a non-invasive self-sample collection method would have the potential to increase the screening of HPV infection in women [15]. Actually, several studies have demonstrated that urine-based HPV screening shows similar sensitivity and specificity to cervical-based sampling [15,24-26]. Our study was performed in the Araucania Region, a high-risk population due to its sociodemographic features; one of the highest native populations in the country, 30% of women living in non-urbanized areas, and high rates of incidence and mortality by CC [7,27].
The aim of this study was to evaluate the clinical performance of a self-collected, urine-based HPV detection method using conventional PCR followed by reverse line blot that could be used as a non-invasive method for the early detection of CC, thereby allowing an increase in screening among populations of women at a high risk of the disease.
Material and methods
Study design
We analyzed a cohort of 190 women with cytology and histological diagnosis of cervical lesions conducted by specialized clinicians performed at the Unit of Pathological Anatomy and Cytology of the Hernán Henríquez Aravena Hospital (Temuco, Chile). To perform this study, three paired samples were collected from women referred for a gynecological exam at public health centers in the Araucania Region; one for diagnostic purposes and the other two for research purposes (cervical and urine samples). Subsequently, these were classified as no cervical Intraepithelial neoplasia (No CIN), cervical intraepithelial neoplasia grade 1 (CIN1), grade 2 (CIN2), grade 3 (CIN3) and squamous cervical cancer (SCC), according to the results of the histological diagnosis. At the same time - without knowing the diagnosis -DNA was extracted from each sample for HPV genotyping (Figure 1).
Figure 1.
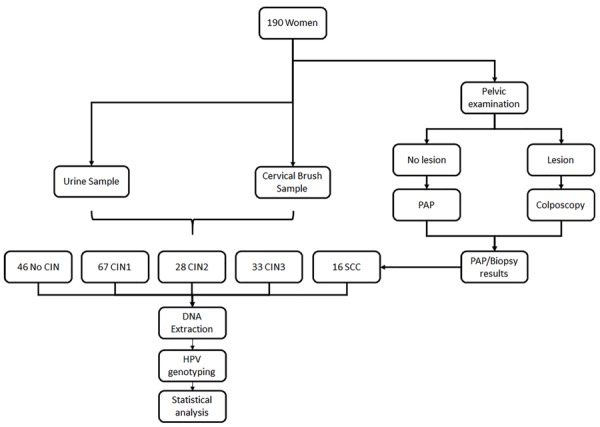
Flowchart of cervical and urine samples included in this study. Note: CIN: cervical intraepithelial neoplasia; SCC: squamous cervical cancer.
Clinical sample collections
Cervical samples were collected by a physician using a cervical brush and placed in a Tris-EDTA buffer solution and transported immediately to the lab for further processing. On the same day, prior to undergoing a pelvic examination, 60 mL of first-stream urine samples were collected at home in a sterile container with crystal violet (10%) and then transported immediately to the lab.
DNA extraction from cervical and urine samples
DNA extraction from the cervical and urine samples was performed using the Tissue DNA Kit E.Z.N.A (Zymo Research), according to the manufacturer’s instructions. DNA was evaluated by amplification of a 268 bp fragment of the β-globin gene using the GH20 and PCO4 primers and PCR conditions previously described [28,29].
HPV genotyping
HPV genotyping was performed by conventional PCR amplification followed by non-radioactive hybridization, which enables the detection of HPV carcinogenic genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66) and HPV non-carcinogenic genotypes (6, 11, 42, 53, 70). The PCR reaction for the L1 gene was performed using the specific primers GP5+ and biotinylated GP6+ as previously described [30]. Subsequently, a reverse line blot was performed to identify the HPV genotype, as previously reported [29]. The PCR product of GP5+/biotinylated GP6+ was denaturalized at 96ºC and cooled in ice before starting the hybridization step. Then, the PCR products were placed in a pre-treated Biodyne C membrane (Pall Bio-Support West Chester, USA) containing non-radioactive labeled oligoprobes specific to each viral genotype. A colorimetric reaction using anti-digoxigenin conjugate with alkaline phosphatase was used to detect the presence/absence of each HPV genotype. Finally, the membranes were developed using a NBT/BCIP substrate solution (Thermo Fisher), according to the manufacturer’s protocol.
Ethics statement
This study was approved by Ethics Committee of the Faculty of Medicine of the University of La Frontera, Temuco, Chile (Resolution Number 246/006, august 25th 2010). All the women were informed of the purposes of this study and written informed consent was obtained.
Statistical analysis
The overall percent agreement (OPA), positive and negative predictive agreement (PPA and NPA), and agreement beyond chance (Cohen’s kappa) were calculated as percentages with 95% confidence intervals (95% CI) for carcinogenic HPV detection in the cervical and urine samples. The kappa concordance test was interpreted according to a previous study [25]. McNemar’s test was used to calculate the differences between paired proportions, with a p value less than 0.05 being considered statistically significant.
In addition, we calculated the sensitivity, specificity, positive and negative predictive values, and Youden’s index with a 95% CI in the cervical and urine samples for the detection of carcinogenic HPV DNA against two histologically confirmed cervical disease endpoints: CIN2/3 and CIN3.
Results
Comparison of carcinogenic HPV detection in cervical and urine samples
All 190 paired cervical and urine samples collected were suitable for analysis in this study, and the β-globin gene was amplified in all of those. The median age of the study participants was 34 years (interquartile range: 16 years; range: 15-75 years). At least one of the detectable HPV genotypes was present in 70.5% (134/190) of the cervical samples and 71.1% (135/190) of the urine samples. At least one of the detectable HR-HPV types was present in 65.8% (125/190) of the cervical samples and 64.7% (123/190) of the urine samples (P-value = 0.89). The detection of LR-HPV type was practically equal in the cervical and urine samples (4.7% and 6.3%; P-value = 0.63, respectively). The HPV16 frequency was 34.7% and 33.2% (P-value = 0.79) in cervical and urine samples, respectively (Figure 2). In summary, there were no significant differences in HPV detection and genotyping between the cervical and urine samples.
Figure 2.
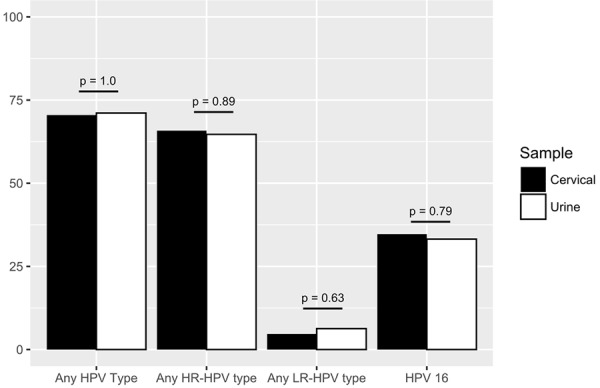
Prevalence of HPV genotypes detected in cervical and urine samples. Note: HR-HPV: high risk HPV; LR-HPV: low risk HPV; HPV 16: HPV genotype 16.
The OPA between HR-HPV detection in the cervical and urine samples was 73.7% (95% CI: 67.0%-79.4%). The PPA between the cervical and urine samples was 80.5% (95% CI: 72.6%-86.5%). A moderate concordance rate of HR-HPV detection in the two samples was observ-ed (kappa = 0.42; 95% CI: 0.35-0.49) (Table 1).
Table 1.
Comparative table of agreement between carcinogenic HPV detection in cervical and urine samples
| Cervical samples | Value (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Negative | Positive | Total | OPA (%) | PPA (%) | NPA (%) | Cohen’s kappa | ||
| Urine samples | Negative | 41 | 24 | 65 | 73.7 (67.0-79.4) | 80.5 (72.6-86.5) | 61.2 (49.2-72.0) | 0.42 (0.35-0.49) |
| Positive | 26 | 99 | 125 | |||||
| Total | 67 | 123 | 190 | |||||
Note: OPA: Overall Percent Agreement; PPA: Positive Percent Agreement; NPA: Negative Percent Agreement.
There were 50/190 (26.3%) discrepancies in the results obtained for the two types of samples. In 24 of them, the detection of carcinogenic HPV was positive in the cervical but negative in the urine samples. Conversely, 26 samples were positive for HR-HPV detection in urine but negative in cervical samples (see Supplementary Table 1).
HR-HPV detection in cervical and urine samples according to grade lesion endpoint
Histology results were available for all 190 women, which 46/190 (24.2%) were No CIN, 67/190 (35.3%) CIN1, 28/190 (14.7%) CIN2, 33/190 (17.4%) CIN3, and 16/190 (8.4%) SCC.
HR-HPV prevalence in women with CIN 2 showed similar proportions in both cervical and urine samples (89.3% and 96.4%, respectively). Nevertheless, women with CIN3 showed a higher prevalence of HR-HPV in the cervical samples than in the urine samples (90.9% versus 72.7%, respectively). Meanwhile, women with SCC have a prevalence of 96.4% in the urine samples compared to 75.0% in the cervical samples (Table 2).
Table 2.
HR-HPV prevalence in cervical and urine samples according different stages of the disease by histology
| No CIN (%) | CIN 1 (%) | CIN 2 (%) | CIN 3 (%) | SCC (%) | Overall (%) | |
|---|---|---|---|---|---|---|
| Cervical | 8/46 (17.4) | 50/67 (74.6) | 25/28 (89.3) | 30/33 (90.9) | 12/16 (75.0) | 125/190 (65.8) |
| Urine | 10/46 (21.7) | 47/67 (70.1) | 27/28 (96.4) | 24/33 (72.7) | 15/16 (93.8) | 123/190 (64.7) |
Note: CIN: Cervical intraepithelial neoplasia; SCC: Squamous cervical cancer.
The three cases negative for CIN2 in the cervical samples were positive for HPV 18 or 16/18 in urine samples. Similarly, the only case negative for CIN2 in urine samples was positive for HPV16 in the cervical samples. None of the negative cases, either cervical or urine samples, were the same (Supplementary Table 1).
No CIN was compared against histologically confirmed CIN2/3 or CIN3 lesions. CIN1 was not considered in this analysis because grouping it with samples from normal epithelia is an error, due to it presenting an early cervical lesion; therefore, many of those cases were positive for HR-HPV infections (74.6% of the cervical and 70.1% of the urine samples), which affects the specificity of methodology with possible “false positives”. Compared to histologically confirmed CIN2/3, cervical-based detection of HR-HPV had a clinical sensitivity of 93.4% (95% CI: 84.1%-98.2%) and a clinical specificity of 80.4% (95% CI: 66.1%-90.6%). The corresponding sensitivity and specificity for urine specimens were 90.2% (95% CI: 79.8%-96.3%) and 71.7% (95% CI: 56.6%-84.0%), respectively. Moreover, the sensitivity estimated for CIN3 lesions compared to respective estimates for CIN2/3 was lower (cervical: 90.9%; 95% CI: 75.7%-98.1%; urine: 72.7%; 95% CI: 54.5%-86.7%); however, the specificity estimates were higher (cervical: 82.6%; 95% CI: 68.6%-92.2%; urine: 78.3%; 95% CI: 63.6%-89.1%) (Table 3).
Table 3.
Comparison of test agreement between carcinogenic HPV detection in cervical and urine samples for detection of histologically confirmed CIN2/3 and CIN3 lesions
| Cervical disease endpoint | No. of samples | % (95% CI) | Youden’s index (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Lesion present | Lesion absent | Sensitivity | Specificity | PPV | NPV | |||||
| CIN 2/3 | Total | TP | FN | FP | TN | |||||
| Cervical | 107/190 | 57 | 4 | 9 | 37 | 93.4 (84.1-98.2) | 80.4 (66.1-90.6) | 86.4 (75.7-93.6) | 90.2 (76.9-97.3) | 0.739 (0.625-0.853) |
| Urine | 107/190 | 55 | 6 | 13 | 33 | 90.2 (79.8-96.3) | 71.7 (56.6-84.0) | 80.9 (69.5-89.4) | 84.6 (69.5-94.1) | 0.619 (0.491-0.747) |
| CIN 3 | ||||||||||
| Cervical | 79/190 | 30 | 3 | 8 | 38 | 90.9 (75.7-98.1) | 82.6 (68.6-92.2) | 78.9 (62.7-90.5) | 92.7 (80.1-98.5) | 0.735 (0.577-0.893) |
| Urine | 79/190 | 24 | 9 | 10 | 36 | 72.7 (54.5-86.7) | 78.3 (63.6-89.1) | 70.6 (52.5-84.9) | 80.0 (65.4-90.4) | 0.51 (0.310-0.710) |
Note: TP: true positive; TN: true negative; FP: false positive; FN: false negative; PPV: positive predictive value; NPV: negative predictive value.
Discussion
Cervical cancer (CC) has among the highest prevalence and incidence of neoplasia in women worldwide, mostly in developing countries [31]. The main screening technique for cervical lesions is the Pap stain, which has a sensitivity that does not reach 60%. In recent years new HPV-based tests have emerged showing better sensitivity than the Pap test in CC diagnoses. It is known that almost all CCs are caused by HPV infections [32]. Consequently, in 2005, the IARC made recommendations that supported primary screening based on cytology and/or HPV-DNA testing [22]. The test for HR-HPV genotypes has recently been shown to be a better indicator of cervical cancer risk than cytology [33]. Co-testing of cytology and HPV detection at 5-year intervals is the preferred strategy for cervical cancer screening for women aged 30 to 64 years in the United States [34]. Nevertheless, the methods clinically validated for HPV detection, including the Pap test, usually require cervical brushing, a sampling method that is usually avoided in high-risk populations because of shame, religious or sociocultural background, or lack of access to health care centers. On the other hand, the urine-sampling method has some advantages over cervical scraping such as: non-invasive collection method, self-sampling method, and highly accepted among women [35], and a low amount of DNA can be detected [36]. Many reports indicates that HPV infections are very common and most women will clear HPV infections within 6-12 months, but in this sense, the presence of HR-HPV DNA does not mean that CC is present or will persist and progress to cancer [19,37,38]. Therefore, it is necessary for a HR-HPV test to be non-invasive, easy to use, and inexpensive, in order to increase the coverage of CC screening in high-risk populations, usually those in low-income regions.
In this framework, we evaluated the clinical performance of urine-based HPV DNA testing and its different genotypes detected in 190 paired cervical and urine samples of women referred from a public health care center (Temuco, Chile). The prevalence of HR-HPV infection was increased with the grade of CIN disease. This prevalence was higher in urine than cervical samples for CIN1, CIN2, and SCC. Nevertheless, the prevalence in CIN3 was higher in the cervical than in the urine samples. Several reports indicate that HR-HPV detection in urine samples could be increased as the cervical lesion progresses due to the exfoliation of cervical cells infected with HPV DNA [24,39-41].
Also, we found an OPA of 73.7% with a moderate concordance rate between HR-HPV infections detected in tissue samples from cervical lesions and urine. These results are similar to those reported by Sahasrabuddhe et al., (OPA = 79.2%; k = 0.55) [24], Mendez et al., (OPA = 76.0%) [39], and Bisset et al., (k = 0.58). These three studies used the Linear Array HPV Genotyping Test. On the other hand, the results obtained in this study were slightly lower than those documented by Tanzi et al., with a Cohen’s kappa of 0.80 obtained in a population of women infected with HIV using an in-house PCR [15], and Bernal et al. (OPA = 88.0%; k = 0.76) using a Cobas 4800 HPV test in a population of women with an abnormal PAP test [25]. One possible explanation of this variation could be the presence of DNA HPV from urethral and vulvar epithelial tissues, because these tissues are susceptible to different HPV genotype infections than cervical tissue, usually non-carcinogenic genotypes [15,35]. The method we used detects both oncogenic and non-oncogenic HPV types, unlike the COBAS 4800 methods detect only carcinogenic HPV types; this could likely affect the OPA and Cohen’s kappa value. Nevertheless, we demonstrated genotype-level similarities between sampling approaches due to infection with HR-HPV, LR-HPV and/or HPV16 being uniform across either the cervical or the urine sampling methods. This is quite interesting because it shows that urine samples as well as cervical samples can be used with this method.
The clinical sensitivity and specificity of urinary detection shows that 90.2% and 71.7% (cervical: 93.4% and 80.4%, respectively) of women with CIN2/3 could be accurately detected with this type of sampling. In women with CIN3, the sensibility decreased slightly to 72.7% with a specificity of 78.3% (cervical: 90.9% and 82.6%, respectively) for the urine samples. Sahasrabuddhe et al., [24] reported a sensitivity and specificity of 80.8% and 53.3% for women with CIN2/3 and 90.0% and 45.9% for women with CIN3 in urine samples. Another study conducted by Bernal et al. [25] showed a sensitivity and specificity in women with CIN2/3 of 95.0% and 51.7% and for CIN3 100% and 54.1%, respectively. Sellors et al. [35] obtained a sensitivity and specificity of 44.8% and 69.7% in women with HSIL (CIN2/3).
A recent meta-analysis reported that urine-based testing is an alternative to cervical sampling [42]. In this regard, our study supports the use of urine-based sampling as a viable method for screening and genotyping HPV infection in women who lack access to CC screening or that avoid pelvic examinations. Moreover, in Chile, especially in non-urbanized areas, a large number of women do not undergo CC screening due to the invasiveness of the sample collection. Therefore, this method represents an important alternative to increase coverage for those populations that are difficult to include in CC screening programs.
Acknowledgements
We would like to thank everyone from the participating healthcare institutions who collaborated in this study. This work was supported by the National Fund for Scientific and Technological Development of Chile (FONDECYT) [11150802 to P.B., 11150622 to C.G.I., 3180550 to K.B.]; the Corporation for Production Development (CORFO) [12IDL2-18157, 09CN14-5960 to CEGIN]; Dirección de Investigación, Universidad de La Frontera (DIUFRO) [DI14-0072 to A.A and J.L., DI17-0079 to K.B.]; the National Commission for Scientific and Technological Research - the Fund for Research Center in Priority Areas (CONICYT-FONDAP) [15130011 to JCR] and The Millennium Institute on Immunology and Immunotherapy [Nº P09-016-F to JCR]. The authors alone are responsible for the content and writing of this article.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Wheeler CM. The natural history of cervical human papillomavirus infections and cervical cancer. Obstet Gynecol Clin North Am. 2013;40:165–76. doi: 10.1016/j.ogc.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Alemany L, Saunier M, Alvarado-Cabrero I, Quirõs B, Salmeron J, Shin HR, Pirog EC, Guimerà N, Hernandez-Suarez G, Felix A, Clavero O, Lloveras B, Kasamatsu E, Goodman MT, Hernandez BY, Laco J, Tinoco L, Geraets DT, Lynch CF, Mandys V, Poljak M, Jach R, Verge J, Clavel C, Ndiaye C, Klaustermeier J, Cubilla A, Castellsagué X, Bravo IG, Pawlita M, Quint WG, Muñoz N, Bosch FX, De Sanjosé S. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136:98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alemany L, Cubilla A, Halec G, Kasamatsu E, Quirós B, Masferrer E, Tous S, Lloveras B, Hernández-Suarez G, Lonsdale R, Tinoco L, Alejo M, Alvarado-Cabrero I, Laco J, Guimerà N, Poblet E, Lombardi LE, Bergeron C, Clavero O, Shin HR, Ferrera A, Felix A, Germar J, Mandys V, Clavel C, Tzardi M, Pons LE, Wain V, Cruz E, Molina C, Mota JD, Jach R, Velasco J, Carrilho C, López-Revilla R, Goodman MT, Quint WG, Castellsagué X, Bravo I, Pawlita M, Muñoz N, Bosch FX, De Sanjosé S. Role of human papillomavirus in penile carcinomas worldwide. Eur Urol. 2016;69:953–61. doi: 10.1016/j.eururo.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 4.De Sanjosé S, Alemany L, Ordi J, Tous S, Alejo M, Bigby SM, Joura EA, Maldonado P, Laco J, Bravo IG, Vidal A, Guimerà N, Cross P, Wain GV, Petry KU, Mariani L, Bergeron C, Mandys V, Sica AR, Félix A, Usubutun A, Seoud M, Hernández-Suárez G, Nowakowski AM, Wilson G, Dalstein V, Hampl M, Kasamatsu ES, Lombardi LE, Tinoco L, Alvarado-Cabrero I, Perrotta M, Bhatla N, Agorastos T, Lynch CF, Goodman MT, Shin HR, Viarheichyk H, Jach R, Cruz MO, Velasco J, Molina C, Bornstein J, Ferrera A, Domingo EJ, Chou CY, Banjo AF, Castellsagué X, Pawlita M, Lloveras B, Quint WG, Muñoz N, Bosch FX HPV VVAP study group. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49:3450–61. doi: 10.1016/j.ejca.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Brunner AH, Grimm C, Polterauer S, Hefler L, Stani J, Heinze G, Horvat R. The prognostic role of human papillomavirus in patients with vaginal cancer. Int J Gynecol Cancer. 2011;21:923–9. doi: 10.1097/IGC.0b013e31821bc615. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, editors. No 11 [Internet] Vol. 11. Lyon, France: International Agency for Research on Cancer; 2013. Globocan 2012 v1.0, cancer incidence and mortality worldwide: IARC cancerbase. http://globocan.iarc.f. [Google Scholar]
- 7.MINSAL. Series y Gráficos de Mortalidad [Internet] 2017. Available from: http://www.deis.cl/series-y-graficos-de-mortalidad/
- 8.Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461–84. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 9.Peto PJ, Gilham PC, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364:249–56. doi: 10.1016/S0140-6736(04)16674-9. [DOI] [PubMed] [Google Scholar]
- 10.Safaeian M, Solomon D, Castle PE. Cervical cancer prevention-cervical screening: science in evolution. Obstet Gynecol Clin North Am. 2007;34:739–60. doi: 10.1016/j.ogc.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cronjé HS. Cervical screening strategies in resourced and resource-constrained countries. Best Pract Res Clin Obstet Gynaecol. 2011;25:575–84. doi: 10.1016/j.bpobgyn.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Sherwani R, Khan T, Akhtar K, Zeba A. Conventional pap smear and liquid based cytology for cervical cancer screening-a comparative study. Journal of Cytology. 2007;24:167–172. [Google Scholar]
- 13.Park IA, Lee SN, Chae SW, Park KH, Kim JW, Lee HP. Comparing the accuracy of thinprep pap tests and conventional papanicolaou smears on the basis of the histologic diagnosis: a clinical study of women with cervical abnormalities. Acta Cytol. 2000;45:525–31. doi: 10.1159/000327859. [DOI] [PubMed] [Google Scholar]
- 14.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Palma PD, Del Mistro A, Gillio-Tos A, Minucci D, Naldoni C, Rizzolo R, Schincaglia P, Volante R, Zappa M, Zorzi M, Cuzick J, Segnan N. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492–501. doi: 10.1093/jnci/djn065. [DOI] [PubMed] [Google Scholar]
- 15.Tanzi E, Bianchi S, Fasolo MM, Frati ER, Mazza F, Martinelli M, Colzani D, Beretta R, Zappa A, Orlando G. High performance of a new PCRbased urine assay for HPV-DNA detection and genotyping. J Med Virol. 2013;85:91–8. doi: 10.1002/jmv.23434. [DOI] [PubMed] [Google Scholar]
- 16.Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJF, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi P, Berkhof J, Peto J, Meijer CJLM, Cuzick J, Zappa M, Carozzi F, Confortini M, Dalla Palma P, Zorzi M, Del Mistro A, Gillio-Tos A, Naldoni C, Rijkaart D, Van Kemenade F, Bulkmans N, Heideman D, Rozendaal R, Kenter G, Almonte M, Roberts C, Desai M, Sargent A, Ryd W, Naucler P. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 17.Paavonen J. Human papillomavirus infection and the development of cervical cancer and related genital neoplasias. Int J Infect Dis. 2007;11(Suppl 2):S3–9. doi: 10.1016/S1201-9712(07)60015-0. [DOI] [PubMed] [Google Scholar]
- 18.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 19.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 20.Castle PE, Stoler MH, Wright TC, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011;12:880–90. doi: 10.1016/S1470-2045(11)70188-7. [DOI] [PubMed] [Google Scholar]
- 21.Agorastos T, Chatzistamatiou K, Katsamagkas T, Koliopoulos G, Daponte A, Constantinidis T, Constantinidis TC HERMES study group. Primary screening for cervical cancer based on high-risk human papillomavirus (HPV) detection and HPV 16 and HPV 18 genotyping, in comparison to cytology. PLoS One. 2015;10:e0119755. doi: 10.1371/journal.pone.0119755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.IARC Working Group on the Evaluation of Cancer. Cervix Cancer Screening. Lyon: IARC; 2005. Cervix Cancer Screening (IARC Handbooks of Cancer Prevention, 10) pp. 201–12. [Google Scholar]
- 23.MINSAL. Guías Clínicas AUGE Cáncer Cérvico Uterino [Internet] Santiago: 2015 [cited 2017 Mar 20]. p. 102. Available from: http://www.bibliotecaminsal.cl/wp/wp-content/uploads/2016/04/GPC-CaCU-Final.PLdocx. [Google Scholar]
- 24.Sahasrabuddhe VV, Gravitt PE, Dunn ST, Brown D, Allen RA, Eby YJ, Smith K, Zuna RE, Zhang RR, Gold MA, Schiffman M, Walker JL, Castle PE, Wentzensen N. Comparison of human papillomavirus detections in urine, vulvar, and cervical samples from women attending a colposcopy clinic. J Clin Microbiol. 2014;52:187–92. doi: 10.1128/JCM.01623-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernal S, Palomares JC, Artura A, Parra M, Cabezas JL, Robles A, Martín Mazuelos E. Comparison of urine and cervical samples for detecting human papillomavirus (HPV) with the cobas 4800 HPV test. J Clin Virol. 2014;61:548–52. doi: 10.1016/j.jcv.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Sahasrabuddhe VV, Gravitt PE, Dunn ST, Robbins D, Brown D, Allen RA, Eby YJ, Smith KM, Zuna RE, Zhang RR, Gold MA, Schiffman M, Walker JL, Castle PE, Wentzensen N. Evaluation of clinical performance of a novel urinebased HPV detection assay among women attending a colposcopy clinic. J Clin Virol. 2014;60:414–7. doi: 10.1016/j.jcv.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.INE. Compendio estadístico regional La Araucanía, informe anual 2015 [Internet] Compendio Estadístico Regional 2015- Región de La Araucanía. 2015 [cited 2017 Sep 15] . pp. 1–92. Available from: http://www.inearaucania.cl/archivos/files/pdf/SistemaEstadisticoRegional/Compendio Estadístico Regional 2015 - La Araucanía.
- 28.Aedo AS, Melo AA, García P, Guzmán GP, Capurro VI, Roa S JC. Detection and typification of human papilloma virus in pre cancerous cervical lesions. Rev Med Chil. 2007;135:167–73. doi: 10.4067/s0034-98872007000200004. [DOI] [PubMed] [Google Scholar]
- 29.Brebi P, Ili CG, Andana A, Menzel D, Lopez J, Guzman P, Melo A, Buchegger K, Roa JC. Frequency of human papillomavirus in women attending cervical cancer screening program in Chile. BMC Cancer. 2017;17:518. doi: 10.1186/s12885-017-3496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Brule AJ, Pol R, Fransen-Daalmeijer N, Schouls LM, Meijer CJ, Snijders PJ. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol. 2002;40:779–87. doi: 10.1128/JCM.40.3.779-787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 32.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gage JC, Schiffman M, Katki HA, Castle PE, Fetterman B, Wentzensen N, Poitras NE, Lorey T, Cheung LC, Kinney WK. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerrero-Preston R, Valle BL, Jedlicka A, Turaga N, Folawiyo O, Pirini F, Lawson F, Vergura A, Noordhuis M, Dziedzic A, Perez G, Renehan M, Guerrero-Diaz C, De Jesus Rodriguez E, Diaz-Montes T, Rodriguez Orengo J, Mendez K, Romaguera J, Trock BJ, Florea L, Sidransky D. Molecular triage of premalignant lesions in liquid-based cervical cytology and circulating cell-free dna from urine, using a panel of methylated human papilloma virus and host genes. Cancer Prev Res (Phila) 2016;9:915–24. doi: 10.1158/1940-6207.CAPR-16-0138. [DOI] [PubMed] [Google Scholar]
- 35.Sellors JW, Lorincz AT, Mahony JB, Mielzynska I, Lytwyn A, Roth P, Howard M, Chong S, Daya D, Chapman W, Chernesky M. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ. 2000;163:513–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Enerly E, Olofsson C, Nygård M. Monitoring human papillomavirus prevalence in urine samples: a review. Clin Epidemiol. 2013:67–79. doi: 10.2147/CLEP.S39799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bulkmans NW, Berkhof J, Bulk S, Bleeker MC, van Kemenade FJ, Rozendaal L, Snijders PJ, Meijer CJ POBASCAM Study Group. High-risk HPV type-specific clearance rates in cervical screening. Br J Cancer. 2007;96:1419–24. doi: 10.1038/sj.bjc.6603653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiffman M, Wentzensen N. Human papillomavirus (HPV) infection and the multi-stage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:553–60. doi: 10.1158/1055-9965.EPI-12-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendez K, Romaguera J, Ortiz AP, Lopez M, Steinau M, Unger ER. Urine-based human papillomavirus DNA testing as a screening tool for cervical cancer in high-risk women. Int J Gynecol Obstet. 2014;124:151–5. doi: 10.1016/j.ijgo.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta A, Arora R, Gupta S, Prusty BK, Kailash U, Batra S, Das BC. Human papillomavirus DNA in urine samples of women with or without cervical cancer and their male partners compared with simultaneously collected cervical/penile smear or biopsy specimens. J Clin Virol. 2006;37:190–4. doi: 10.1016/j.jcv.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Brinkman JA, Jones WE, Gaffga AM, Sanders JA, Chaturvedi AK, Slavinsky IJ, Clayton JL, Dumestre J, Hagensee ME. Detection of human papillomavirus DNA in urine specimens from human immunodeficiency virus-positive women. J Clin Microbiol. 2002;40:3155–61. doi: 10.1128/JCM.40.9.3155-3161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pathak N, Dodds J, Zamora J, Khan K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. BMJ. 2014;349:g5264. doi: 10.1136/bmj.g5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


