Abstract
Glioblastoma, the most common primary brain tumor of adults, is characterized by poor survival rates. Programmed death ligand 1 (PD-L1, CD274) has been implicated in the immune escape of glioblastoma. The presence of human cytomegalovirus (HCMV) in glioblastoma multiforme (GBM) has sparked considerable interest and controversy. The exposure of toll-like receptor 3 (TLR3) to pathogens induces an antiviral state in cells or in animals. In the current study, the expression of PD-L1 and TLR3 in HCMV-infected glioma specimens was observed to be higher compared to the control. We therefore investigated if PD-L1 expression in glioblastoma is mediated by TLR3 triggering in HCMV infected glioblastoma. TLR3 siRNA transfections were utilized to identify the induction of PD-L1 via TLR3 triggering in HCMV infected cell lines. Also, IL-8 and TGF-β were detected by ELISA for the antitumor role of TLR3. Thus, we propose a novel immune treatment using a combination of PD-L1 blockade with TLR3 triggering against HCMV infected glioblastoma.
Keywords: PD-L1, HCMV, TLR3, glioma, immune treatment
Introduction
GBM is the most common adult primary brain tumor, which is characterized by highly invasive, infiltrative growth and has no clear etiology. Despite current treatments including chemotherapy and surgery, the median survival time is only about a year [1]. There is increased interest in elucidating that human cytomegalovirus (HCMV) is infected in a high percentage of GBM [2], even though the HCMV positive rate is controversial due to the sensitivity of the methods. There is no clear evidence that HCMV is an oncogenic virus, but some evidence has reminded us of its role in promoting the development of gliomas [3]. Programmed death 1 (PD-1) is an important immunosuppressive molecule which is expressed on the cell membrane. PD-1 and its ligand programmed death-ligand 1 (PD-L1) help cancer cells to evade the immune system and promote the tumor microenvironment [4]. Many studies have shown that an elevated expression of PD-L1 is found in glioma cells [5], and the expression of PD-L1 correlates with the degree of malignancy of gliomas [6]. In the anti-tumor treatment research of multiple tumors, it was found that the use of PD-L1 immunosuppressive agents can effectively improve the therapeutic effect and prolong the survival of patients compared with single-agent radiochemotherapy. toll-like receptors (TLRs) are type I transmembrane proteins containing the intracellular toll-interleukin-1 (IL-1) receptor (TIR) domain [7]. TLR3 belongs to the TLR family. It uses the distinct adaptor protein, TRIF (TIR domain-containing adaptor-inducing IFN-β) and is expressed in several subsets of immune cells, including dendritic cells and natural killer (NK) cells. TLR3 activation produces anti-inflammatory mediators [8]. The main function of TLR3 is to participate in the antiviral response and the production of type I interferon (IFN) [9]. Upon tumor antigen recognition by T cells, the released interferons trigger the inducible expression of PD-L1 by cancer cells, thereby inhibiting the antitumor immune response in a process known as adaptive immune resistance. HCMV resulting in the high expression of PD-L1 is of high importance for the clinical development of PD-1 blockade therapies for cancer. In the current study, the expressions of PD-L1 and TLR3 in HCMV-infected clinical glioma specimens were both detected at higher levels compared to the control groups. We therefore investigated if PD-L1 expression in glioblastoma is mediated by TLR3 triggering in HCMV infected glioblastoma. The exposure of TLR3, -4, -5, -7, and -9 to pathogens induces an antiviral state in cells and in animals. Thus, IL-8 and TGFβ are detected by ELISA because of the anti-tumor role of TLR3. Thus, we propose a novel immune treatment using the combination of PD-L1 blockade with TLR3 triggering against HCMV infected glioblastoma.
Materials and methods
The detection of PD-L1 expression in clinical specimens by immunohistochemistry
Twenty patients with grade III glioma and grade IV glioma were randomly selected, and their paraffin-embedded brain tumor sections were stained. First, these specimens were dewaxed in xylene at a high to low concentration. Next, the samples underwent hydration using high to low concentrations of ethanol. The specimens were antigen-repaired by boiling with a sodium citrate buffer (pH 6.0) for two minutes and then cooled to room temperature. Next, we eliminated the endogenous peroxidase with 3% hydrogen peroxide. The specimens were washed with PBS 4 times and then incubated 2 hours with the primary antibody in the incubator at 37°C (PD-L1 antibody diluted at 1:1500, Proteintech, Inc.; IE2 antibody diluted at 1:100, Millipore, USA). After being washed with PBS, the specimens were incubated with a secondary antibody conjugated with horseradish peroxidase for 30 minutes. Then these specimens were exposed to DAB for 1 minute and hematoxylin for 2 seconds and observed after the application of a neutral gum seal.
Virus and cell lines
HCMV AD169 was donated by the Institut Pasteur in France and was routinely titrated (108 PFU/mL). Human embryonic lung fibroblasts (HELF) are deposited in our laboratory for HCMV virus amplification. Glioma cell U87 and U251 were purchased from the Shanghai cell bank and cultured and passaged in the routine manner for this experiment. Primary glioma cells from clinical patients and astrocytes are preserved in our lab.
Detection of PD-L1 mRNA expression by RT-qPCR
U87, U251, primary glioma cells and astrocyte were harvested at 0, 6, and 72 hours after the HCMV infection of these cells. At the same time, a control group (in which a DMEM medium containing 2% FBS was used instead of the virus solution) was harvested at the same time. RNA was extracted using an RNA extraction kit and then reverse transcribed. Real-time PCR used β-actin as an internal reference gene, and each of the reference and target genes had three parallel reaction tubes. The primers were synthesized by Shanghai Shenggong Biological Co., Ltd. (PD-L1 forward primer GGTGGTGCCGACTACAA; reversed primer TAGCCCTCAGCCTGACAT).
Detection of PD-L1 protein expression by immunofluorescence
U87, U251, primary glioma cells, and astrocyte were washed with PBS three times after infection with HCMV at 0, 24, and 72 h and fixed with 4% paraformaldehyde. Then we used the 0.1% TritonX-100 (diluted in PBS) permeabilized for 30 min. The samples were then blocked with 5% bovine albumin (BSA) and incubated overnight with the murine PD-L1 antibody which was provided by ProteintechTM. The next day, they were washed with PBS three times and then we added the fluorescein-conjugated Affinipure Goat Anti-Mouse IgG for two hours. Then we washed the samples three times with PBS, added DAPI to avoid light, incubated them for 10-15 min, washed them three times with PBS, and finally observed them with a confocal fluorescence microscope.
Down-regulation of TLR3 expression via cell transfection
The cells were cultured in a 35 mm Petri dish, the culture medium was discarded, and a serum-free medium was added after the cells with PBS and placing them in an incubator for 0.5 hour. Then we applied a Mix LipoFiterTM Liposomal Transfection Reagent with serum-free DMEM for 5 minutes. Next, we applied Mix siRNA (supplied by Shanghai GenePharma Co., Ltd., and the sequences are shown in Table 1) with serum-free DMEM for 5 minutes. We mixed the two reagents and let the solution stand for 20 minutes, then we added it to the petri dish and re-exchanged it to a new DMEM medium containing 10% serum after 4 hours. After 24 hours, the cell protein was extracted and Western-blot was used to detect whether TLR3 expression was decreased.
Table 1.
The sequence of siRNAs that block TLR3 expression
| TLR3-homo-1757 | GUCCCAUUUAUUUCCUAAATT |
| UUUAGGAAAUAAAUGGGACTT | |
| TLR3-homo-2025 | GCGCUUUAAUCCCUUUGAUTT |
| AUCAAAGGGAUUAAAGCGCTT | |
| TLR3-homo-2658 | GGAGAUUCCAGAUUAUAAATT |
| UUUAUAAUCUGGAAUCUCCTT |
Detection of PD-L1 expression by Western-blot
After transfection of TLR siRNA in cell lines for 24 h, the cells were infected with HCMV. Total protein was extracted at 72 h. The proteins underwent electrophoresis by a precast-gel at a concentration of 10%. After that, the proteins were transferred to the nitrocellulose membrane. The nitrocellulose membranes were blocked with 5% nonfat milk powder for 1 hour at room temperature, then they were incubated with the PD-L1 and β-actin monoclonal antibodies overnight at 4°C. The next day, the membranes were washed by TBST and incubated with the horseradish peroxidase-conjugated secondary antibodies for 2 hours. The chemiluminescent signal was produced by the SuperSignal West Pico Kit (Thermo Fisher Scientific Inc.).
Cytokine measurements
The cells were transfected as described above and co-cultured with primary T cells (saved from the lab). The culture supernatants were collected and stored at -80°C. IL-8 and TGF-β levels were analyzed using an ELISA kit.
Statistical analysis
The data were analyzed using SPSS software version 24.0. Immunohistochemistry and Western-blot were analyzed using Student’s t test. P < 0.05 was considered statistically significant.
Results
The expression levels of PD-L1 and TLR3 in glioma sections of HCMV-infected glioma patients were elevated relative to those not infected with HCMV
We selected paraffin sections from 20 patients with grade III glioma and 20 patients with grade IV glioma for immunohistochemical staining. We used an immediate early (IE) protein to detect whether the patient’s glioma was infected with HCMV. IE protein was found in 70% of the grade III gliomas and 85% of the grade IV gliomas. Then we measured the expressions of PD-L1 and TLR3 in these specimens. We clearly found that in the IE-positive grade III and grade IV gliomas, the expression levels of PD-L1 and TLR3 (Figure 1Aa-c, 1Ba-c) were significantly higher than those of the IE-negative gliomas (Figure 1Ad-f, 1Bd-f). The expression levels of PD-L1 and TLR3 in the HCMV-infected gliomas were significantly higher than those in the uninfected groups (P < 0.05) (Figure 1C).
Figure 1.
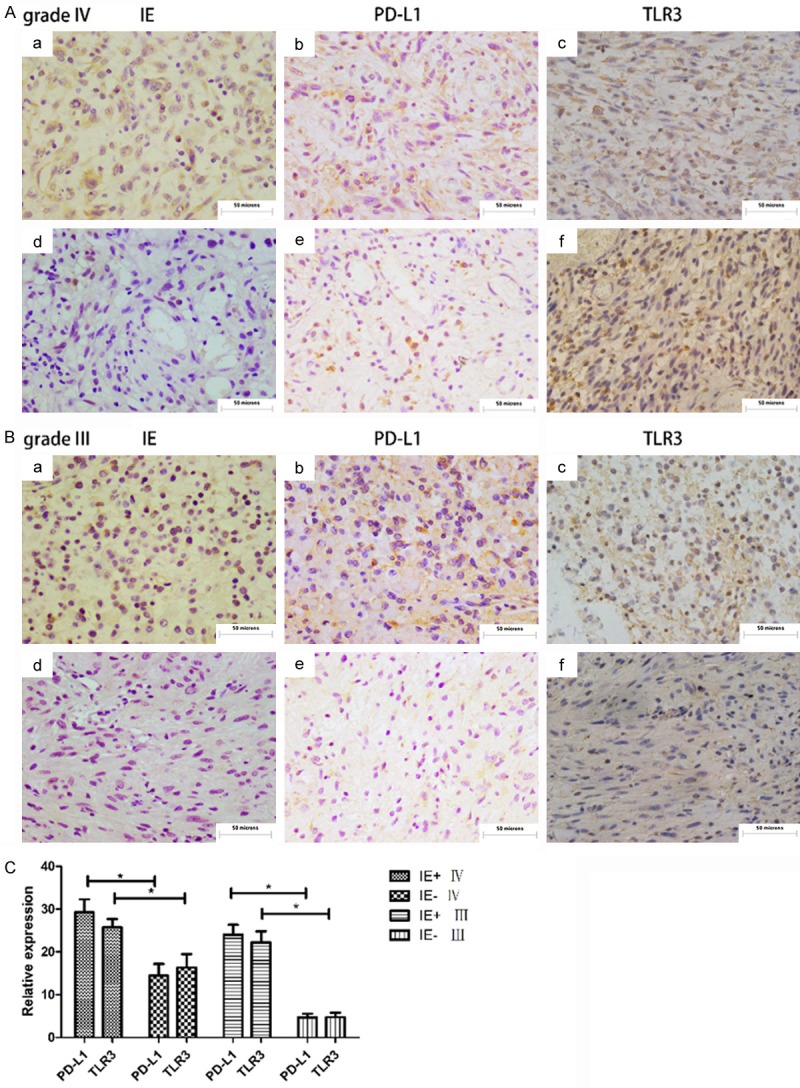
(Aa-c) are the immunohistochemical staining results of IE, PD-L1, and TLR3 in the same grade IV glioma patient, (d-f) are from another patient, respectively. (B) The same settling of the two grade III glioma patients as described above. (C) The staining information of the 40 patient specimens was summarized and analyzed by IMAGE J and the results were statistically analyzed using SPSS. Patients were divided into an HCMV-infected group and an HCMV-uninfected group by IE staining. In the grade IV glioma HCMV-infected group and in the HCMV-uninfected group, the expression levels of PD-L1 and TLR3 were different, which was statistically significant, P < 0.05. In the grade III glioma HCMV-infected group and the HCMV-uninfected group, the expression levels of PD-L1 and TLR3 were different, which was statistically significant, P < 0.05.
In U87, the primary glioma cell line, the PD-L1 mRNA and protein expressions increased post HCMV infection
The mRNA expression of PD-L1 in the U87 cell line and in the primary glioma cells both significantly increased at 6 h and 72 h post infection with HCMV compared with the 0 h group as a negative control (P < 0.05) (Figure 2A). In immunofluorescence staining, the expression levels of PD-L1 in U87 (Figure 2Cc, 2Ce), primary glioma (Figure 2Dc, 2De) and astrocytes (Figure 2Ec, 2Ee) at 24 hours and 72 hours post infection with HCMV were significantly higher compared to the uninfected group (Figure 2Ca, 2Da, 2Ea), and the results were statistically significant (Figure 2B) (P < 0.05).
Figure 2.
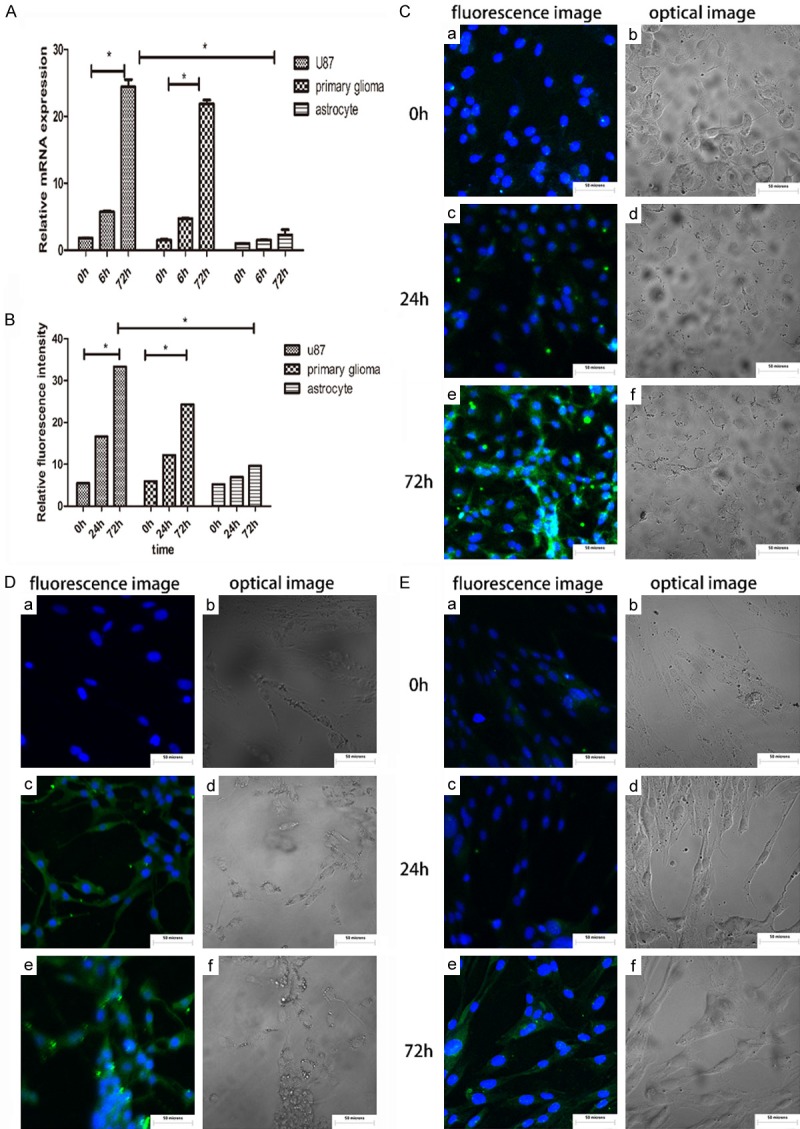
(A) mRNA expression of PD-L1 at 6 h and 72 h after infection with HCMV in U87, primary glioma and astrocytes. U87, primary glioma 72 hours after infection with HCMV, the expression of PD-L1 mRNA increased significantly, and was statistically significant, P < 0.05. The astrocytes did not change much. (B) Fluorescence images were analyzed using IMAGE J and the statistical analysis was performed using SPSS. The protein expression of PD-L1 was significantly increased in U87 and in primary glioma after HCMV infection for 72 H, with statistical significance, P < 0.05, but the astrocyte changes were not obvious. (C-E) Cellular immunofluorescence was used to detect the expression of PD-L1 in U87, primary glioma, and astrocytes after HCMV infection. (a, c, e) are fluorescent images and (b, d, f) are optical images, respectively.
By blocking the expression of TLR3 , the expression of PD-L1 in glioma cells after HCMV infection was reduced compared with the unblocked group
The siRNA down-regulation of the TLR3 expression was loaded with liposome and transfected into cells for 24 hours, and the protein expression level of TLR3 was detected by Western blot. Using ImageJ to analyze the results (Figure 3A), the expression of TLR3 decreased by more than 70%. The results showed that the expression level of PD-L1 in the TLR3 blocking group (TLR3-group) decreased compared to the TLR3 unblocked group (TLR3+ group) (Figure 3B). Using ImageJ to compare the gray values of the Western blot, the results were statistically significant (Figure 3C) (P < 0.05).
Figure 3.
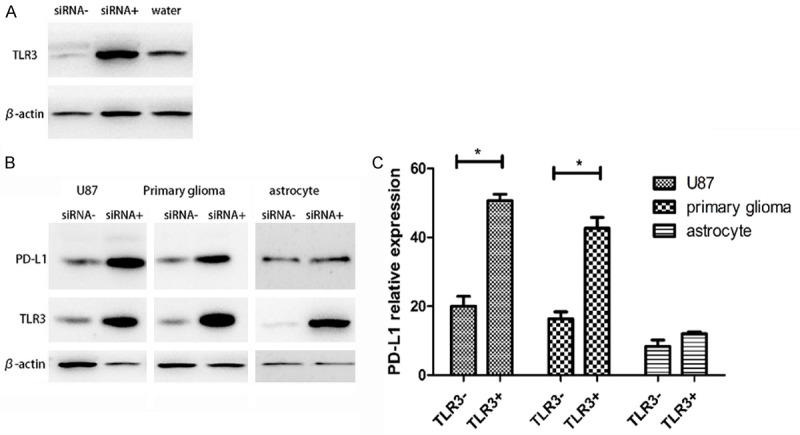
A. siRNA- is an siRNA that blocks TLR3 from being transfected into cells, and siRNA+ is a random protein siRNA transfected into cells, and water is transfected into cells with water instead of siRNA. Using software analysis, the expression level of TLR3 in siRNA+ was reduced by more than 70% relative to the level in siRNA- and water. B. The Western blot result of the expression level of PD-L1 after 72 hours of HCMV infection in U87, primary glioma, and astrocytes. C. The result of first analyzing the Western blot image using IMAGE J, and the obtained result was statistically analyzed using SPSS. In the U87 and primary glioma groups, the expression levels of PD-L1 in the TLR3+ and TLR3-groups were statistically significant, P < 0.05. In the astrocyte group, this difference was not significant.
Blocking the expression of TLR3 induced TGF-β secretion and inhibited IL-8 release in the glioma cells, but was ineffective against the astrocytes
To access the role of TLR3 triggering in the HCMV-infected glioma cells on T-cell stimulatory properties, we co-cultured glioma cells or astrocytes with primary T cells. We divided the glioma cells and astrocytes into two groups: the TLR3-(TLR3-blocked) group and the TLR3+ (TLR3-unblocked) group. In U87 and primary glioma, the secretion of TGF-β in the TLR3-group was significantly higher than it was in TLR3+ group. In the astrocytes, the TLR3 blocking and unblocking had no significant effect on the secretion of TGF-β (Figure 4A). The presence of TLR3 had a significant effect on the release of IL-8. In the case of TLR3 blocking, the IL-8 release could not be detected in each cell line, but a large amount of IL-8 release could be detected in TLR3 unblocked gliomas. In the astrocytes, the unblocked group had only a small amount of IL-8 that could be measured (Figure 4B). In summary, when TLR3 is blocked, it can promote the secretion of TGF-β and inhibit the release of IL-8.
Figure 4.
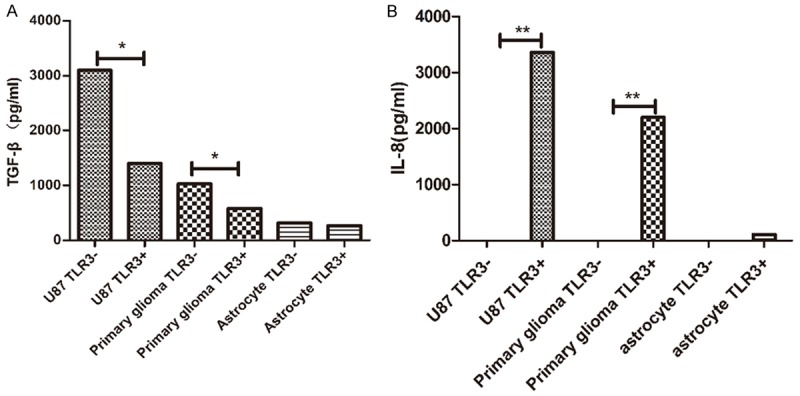
Co-culture glioma cells or astrocytes with primary T cells in 96-well plates. The HCMV virus was infected after the transfection of TRL3 siRNA for 24 h, and the supernatant was taken 24 hours later to detect TGF-β and IL-8. The TLR3-group was transfected with TLR3 SIRNA and the TLR3+ group was replaced with liposomes. A. In U87 and primary glioma, the expression of TGF-β was increased after blocking TLR3, P < 0.05, but this phenomenon was not obvious in the astrocytes. B. In the three cell lines, IL-8 was almost undetectable after blocking TLR3, but in the unblocked group, the expression level was high, P < 0.01. In the astrocytes and in the unblocked group, only a small amount of IL-8 can be measured.
Discussion
HCMV genomes are associated with GBM tumors [2]. Glioma cells exhibit HCMV gene products, significantly altering the host immune response to tumors [10] and contributing to the generation and maintenance of the immunosuppressive microenvironment [11]. PD-1/PD-L1 has been suggested as a mechanism to assist tumors against autoimmunity and to promote self-tolerance [12]. To date, there is no definite answer due to the use of different tissue sampling strategies, different antibodies and staining protocols, and different evaluation schemes for the staining patterns.
In our study, the expression of PD-L1 in the HCMV-infected group was higher compared with HCMV-uninfected group in clinical glioma specimens with immunohistochemical staining. In the study by Ding, et al., the expression of immediate early protein 2 of HCMV was detected in 76.1% of the specimens [13]. In our study, the number of HCMV negative specimens was not enough due to the HCMV infection rate in the gliomas being more than 85%, which might have affected the experimental results.
In glioma cell line U87 and in the primary glioma cell line, the expression of PD-L1 both increased in the HCMV infected group compared to the control group as shown by RT-qPCR and Western blot, which is consistent with the results in the study by Peng, et al., an increase in the expression of PD-L1 in CD8 T cells after HBV infection [14]. HCMV infection also led to the increased expression of PD-L1 in glioma cells. However there was no significant difference in PD-L1 expression between the infected and uninfected astrocytes, which suggests that the upregulation of PD-L1 with HCMV occurs not in the normal cells but in the glioma cells. In a study by Zheng, et al., it is suggested that in glioblastoma, higher PD-L1 level indicates a significantly shorter survival [15]. This suggests that a higher PD-L1 level is one of the factors that enables HCMV to promote an increase in the malignancy of glioma.
Combaz-Lair, et al., and Boes, et al. confirmed that TLR3 regulates the upregulation of PD-L1 expression in malignant pleural mesothelioma [16] and neuroblastoma cells [17], just as HBV can regulate the immune characteristics of infected cells through the TLR pathway [18]. After the immunohistochemical staining of the clinical specimens, the expression of TLR3 increased in the HCMV-infected group compared with the HCMV-uninfected group. Therefore, we propose that HCMV induced PD-L1 upregulation is mediated via TLR3 triggering.
Therefore, we customized an siRNA that antagonizes TLR3 and transfected it into cell lines. After HCMV infection, the expression of PD-L1 increased compared with the uninfected group, and the expression of PD-L1 showed no difference between the TLR3 siRNA treated HCMV infected group and the uninfected group, which suggests that HCMV regulated the expression of PD-L1 in gliomas via TLR3. A similar finding in Boes, et al.’s study found that TLR3 triggers a strong up-regulation of PD-L1 on neuroblastoma cells [17]. Therefore, when TLR3 is expressed due to an antagonism, the expression level of PD-L1 also decreases.
Next, we studied how TLR3-blocked the effects of the cytokine profile of glioma cells. IL-8 is considered to be an adaptive response of cancer cells to the environment, and it can help cancer cell migration [19]. When TLR3 was blocked, the release of IL-8 decreased from 3000 mg/ml to a level almost too small to measure. At the same time, the released amount of TGF-β as an immunosuppressive cytokine was increased by about 50%.
Nowadays, the median survival time of patients with glioma is still less than one year [20], and the effective treatment of glioma is a big challenge. To date, PD-1 monoclonal antibody therapy has improved survival and has significant clinical benefits. More importantly, PD-1 monoclonal antibody treatment lasts for a long time, is low in toxicity, and is suitable for a wide range of cancer types, especially with solid tumors [21,22]. However, the median survival time of glioma patients treated with PD-1 monoclonal antibody increased by only a few months [23,24]. As we know, multiple-pronged strategies are the key to success in immunotherapy, such as the combination of PD-L1 blockade with TLR3 triggering against HCMV infected glioblastoma. Perhaps this will serve as a new therapy in the future for the treatment of gliomas.
Acknowledgements
The authors are grateful to the individuals who helped to make this study possible. This work was supported by the National Natural Science Foundation of China (81471958).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Ranganathan P, Clark PA, Kuo JS, Salamat MS, Kalejta RF. Significant association of multiple human cytomegalovirus genomic Loci with glioblastoma multiforme samples. J Virol. 2012;86:854–864. doi: 10.1128/JVI.06097-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soroceanu L, Cobbs CS. Is HCMV a tumor promoter? Virus Res. 2011;157:193–203. doi: 10.1016/j.virusres.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straub M, Drecoll E, Pfarr N, Weichert W, Langer R, Hapfelmeier A, Gotz C, Wolff KD, Kolk A, Specht K. CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget. 2016;7:12024–12034. doi: 10.18632/oncotarget.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, Gabrusiewicz K, Ling X, Zhou S, Ivan C, Chen JQ, Burks JK, Fuller GN, Calin GA, Conrad CA, Creasy C, Ritthipichai K, Radvanyi L, Heimberger AB. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18:195–205. doi: 10.1093/neuonc/nov172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao Y, Tao R, Wang X, Wang Y, Mao Y, Zhou LF. B7-H1 is correlated with malignancy-grade gliomas but is not expressed exclusively on tumor stem-like cells. Neuro Oncol. 2009;11:757–766. doi: 10.1215/15228517-2009-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 8.Hanke ML, Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci (Lond) 2011;121:367–387. doi: 10.1042/CS20110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen XL, Xu YY, Chen L, Wang GL, Shen Y. TLR3 plays significant roles against HBV-Associated HCC. Gastroenterol Res Pract. 2015;2015:572171. doi: 10.1155/2015/572171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobbs CS. Evolving evidence implicates cytomegalovirus as a promoter of malignant glioma pathogenesis. Herpesviridae. 2011;2:10. doi: 10.1186/2042-4280-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S, Consortium NC. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi L, Chen S, Yang L, Li Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6:74. doi: 10.1186/1756-8722-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding D, Han S, Wang Z, Guo Z, Wu A. Does the existence of HCMV components predict poor prognosis in glioma? J Neurooncol. 2014;116:515–522. doi: 10.1007/s11060-013-1350-9. [DOI] [PubMed] [Google Scholar]
- 14.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Zhang C, Liu X, Wang Z, Sun L, Li G, Liang J, Hu H, Liu Y, Zhang W, Jiang T. Molecular and clinical characterization of PD-L1 expression at transcriptional level via 976 samples of brain glioma. Oncoimmunology. 2016;5:e1196310. doi: 10.1080/2162402X.2016.1196310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combaz-Lair C, Galateau-Salle F, McLeer-Florin A, Le Stang N, David-Boudet L, Duruisseaux M, Ferretti GR, Brambilla E, Lebecque S, Lantuejoul S. Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas. Hum Pathol. 2016;52:9–18. doi: 10.1016/j.humpath.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Boes M, Meyer-Wentrup F. TLR3 triggering regulates PD-L1 (CD274) expression in human neuroblastoma cells. Cancer Lett. 2015;361:49–56. doi: 10.1016/j.canlet.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Lucifora J, Bonnin M, Aillot L, Fusil F, Maadadi S, Dimier L, Michelet M, Floriot O, Ollivier A, Rivoire M, Ait-Goughoulte M, Daffis S, Fletcher SP, Salvetti A, Cosset FL, Zoulim F, Durantel D. Direct antiviral properties of TLR ligands against HBV replication in immune-competent hepatocytes. Sci Rep. 2018;8:5390. doi: 10.1038/s41598-018-23525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 20.Stark AM, van de Bergh J, Hedderich J, Mehdorn HM, Nabavi A. Glioblastoma: clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg. 2012;114:840–845. doi: 10.1016/j.clineuro.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764–782. doi: 10.1016/j.clinthera.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro MG, Baker GJ, Lowenstein PR. Blocking immunosuppressive checkpoints for glioma therapy: the more the merrier! Clin Cancer Res. 2014;20:5147–5149. doi: 10.1158/1078-0432.CCR-14-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mann J, Ramakrishna R, Magge R, Wernicke AG. Advances in radiotherapy for glioblastoma. Front Neurol. 2017;8:748. doi: 10.3389/fneur.2017.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]


