Abstract
PC is one of the deadliest cancers, with unexpectedly high mortality. The main reason for poor prognosis is the high likelihood of invasion and metastasis of pancreatic cancer cells. Mechanism of exceptional protein phosphorylation that regulates cell invasion and metastasis in pancreatic cancer remain unclear. In our previous studies, we used high-throughput phosphorylation array to test two pancreatic cancer cell lines (PC-1 cells with a low potential, and PC-1.0 cells with a high potential, for invasion and metastasis). We noted that a total of 57 proteinsrevealed a differential expression (fold change 2.0). We supposed that insulin receptor substrate-1 (IRS-1) may play a significant role in pancreatic cancer invasion and metastasis. In this study, similar phosphorylation and protein expression levels together with morphological and functional characteristics were observed in PC-1.0 hamster pancreatic cancer cells and Aspc-1 human pancreatic cancer cells (similar to PC-1.0 in features) transiently transfected with IRS-1 siRNA. Our results indicated that proliferation, invasion and metastasis were reduced in both hamster and human pancreatic cancer cells. IRS-1 was found to regulate the target proteins involved in MAPK and PI3K signaling pathways, which include MEK1, MEK2 and AKT, at the protein and phosphorylation level. Low expression of IRS-1 in pancreatic cancer cells inhibited cell proliferation by targeting MEK1 and AKT, while inhibiting invasion and metastasis by targeting MEK2. Moreover, our results demonstrate that IRS-1 protein and phosphorylation expression levels are negatively controlled by LAR (protein tyrosine phosphatase, receptor type, F). LAR inhibited proliferation, invasion and metastasis of pancreatic cancer cells via a direct decrease of IRS-1 protein and phosphorylation expression levels. In summary, we demonstrate that IRS-1 regulates proliferation, invasion and metastasis of pancreatic cancer cells, and provides a new biomarker in an effort to develop novel therapeutic drug targets for pancreatic cancer treatment.
Keywords: IRS-1, proliferation, invasion, pancreatic cancer, MAPK, PI3K
Introduction
Pancreatic carcinoma is a highly lethal malignancy worldwide and has a very poor prognosis, with an overall 5-year survival rate of less than 5% after diagnosis [1]. It is characterized by rapid disease progression and absence of specific symptoms, largely precluding an early diagnosis and curative treatment, and is associated with a very poor prognosis [2]. By the time of diagnosis, the majority of patients are at an advanced stage of pancreatic cancer (PC), with invasion and/or metastasis present due to the highly aggressive nature [3]. However, only 10%-20% of patients are candidates for resection as approximately 50% of patients present with metastatic tumors and 35% present with locally advanced surgically unresectable disease [4]. The primary causes for a poor prognosis are local recurrences and/or distant metastasis after surgery. Pancreatic cancer remains a therapeutic challenge, and the cellular and molecular mechanisms of invasion/metastasis have not been elucidated clearly. PI3K/PTEN/AKT/mTORC1 and Raf/MEK/ERK are key pathways activated in PC [5]. Deregulation of these pathways can result in continuous cell growth, prevention of apoptosis and senescence, and chemotherapeutic drug resistance [6].
The MAPK signaling pathway is a highly conserved pathway that transfers extracellular signals to the nucleus. The MAPK pathway triggers a genetic signaling cascade, resulting in regulation of cell proliferation, differentiation, apoptosis, gene expression and cellular response to the external environment [7]. Targeting molecules in these pathways may be a therapeutic approach to treat pancreatic and other cancers [8].
Two hamster PC cell lines with different potentials for invasion and metastasis after intra-pancreatic transplantation, PC-1 (low potential) and PC-1.0 (high potential), were established from a pancreatic ductal carcinoma induced by N-nitrosobis (2-oxopropyl) amine (BOP) in a Syrian golden hamster [9-11].
Liquid chromatography-mass spectrometry (LC-MS) based on silac labeling was carried out on culture filtrate proteins to identify differentially expressed proteins between PC-1 and PC-1.0 cells (data not shown). LAR, also known as protein tyrosine phosphatase, receptor type, F (PTPRF), was identified as two-fold higher in PC-1 cells. Protein tyrosine phosphatase (PTP) issignaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. Cellular PTPases play a central role in the regulation of insulin action by dephosphorylating and inactivating the receptor kinase to terminate the insulin receptor signal [12]. The interactions among PTPRF, IRS-1, and MEK have been studied extensively [13], but their functions and interactions have not been elucidated exhaustively in PC cells.
In our previous study, protein phosphorylation level differences between PC-1.0 and PC-1 cells were examined using the Phospho Explorer Antibody Array method [14]. The ratio of insulin receptor substrate-1 (IRS-1) phosphorylation at Ser636 in PC-1 cells compared to PC-1.0 cells was 0.43. This suggests that IRS-1 may play a significant role in signaling pathways in PC.
IRS-1 is a major member of the (IRS) family and acts as an important adaptor in insulin and insulin-like growth factor signaling [15]. It functions as a mediator molecule in signal transduction and is regulated by certain cytokines, hormones, and growth factor receptors [16]. IRS-1 also suppresses transforming growth factor-β induced epithelial-mesenchymal transition in lung cancer [17-20]. Serine phosphorylation of IRS-1 correlates closely with insulin resistance [19]. Patients with diabetes and obesity have a moderately increased relative risk of developing PC of 1.8 and 1.3 [22,23]. These studies indicate that a substantial number of patients with PC also suffer from diabetes [24]. The effects of IRS-1 on insulin resistance and diabetes are well studied, but the relationship between IRS-1 and PC is not fully elucidated.
In this study, we hypothesized that IRS-1 could be a key factor in PC signaling pathways and be involved in the migration and invasion of PC cells. Here, we examined the role of IRS-1 in proliferation, migration, and invasion of PC cells in vitro, and its effects on upstream and downstream pathways. In order to determine the function of IRS-1 in PC cells, the expression level of the IRS-1 gene was knocked down using IRS-1-specific small interfering RNA (siRNA). Subsequently, proliferation, apoptosis, invasion and migration of PC cells were analyzed. Additionally, the present study analyzed the association between these cellular functions and the MAPK and PI3K signaling pathways, in order to identify molecular mechanisms in the pathogenesis of PC. IRS-1 may serve as a useful predictor of the outcome of PC patients and could be a new target for clinical therapy.
Materials and methods
Cell lines and cell culture
Two hamster PC cell lines, the weakly invasive and metastatic cell line PC-1, and the highly invasive and metastatic cell line PC-1.0, were used. The PC-1 cell line was established from pancreatic ductal/ductal adenocarcinomas induced by BOP in a Syrian golden hamster [9]. The PC-1.0 cell line was established from a subcutaneous tumor produced after inoculation of PC-1 cells [10]. These two cell lines exhibited differential growth morphology in vitro. The PC-1 cells formed island-like cell colonies, and PC-1.0 cells mainly grew as single cells [11]. Human PC cells Aspc-1 and Capan-2, which have morphological and functional characteristics similar to PC-1.0 and PC-1 cells, respectively, were used to determine if the results from hamster cells coincide with human PC cells. These cell lines were incubated in RPMI-1640 (Gibco-BRL, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (Bioserum, Victoria, Australia), 100 U/ml penicillin G, and 100 he/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2.
Western blotting
Cells were grown in 90-mm dishes containing 10 ml of RPMI-1640 plus 10% fetal bovine serum. The cells were lysed in 1 ml ice-cold RIPA buffer (50 mM Tris, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, pH 7.5 with 1 mM phenylmethylsulfonyl fluoride, 1 mg/ml leupeptin, and 1 mg/ml aprotinin added immediately before use) on ice for 15 min. After centrifugation (5 min at 5000 rpm) at 4°C, the supernatants of cell lysates were collected and stored at -80°C. ß-actin was detected as a loading control.
Western blotting was performed as described previously (15). In brief, samples of equivalent total protein (20 μg) were run in a 5% polyacrylamide slab gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad, Anaheim, USA). The membranes were incubated with primary antibody diluted in 0.1% Tween-20/PBS overnight at 4°C. The blots were then incubated with horseradish peroxidase-conjugated secondary antibody (diluted 1:5000) in 0.1% Tween-20/PBS. Enhanced chemiluminescence (Santa Cruz Biotechnology) was used to detect the signals, which were developed on Kodak scientific imaging film (Eastman Kodak Company, Rochester, NY, USA).
The following antibodies were used: anti-IRS-1 anti-GAPDH and anti-β-actin antibodies (Cell signaling technology, Beverley, MA, USA). Blots were visualized using the ECL chemiluminescence detection system (GE healthcare Bio-science, Piscataway, NJ, USA).
siRNA transfection and real-time PCR
Aspc-1 and PC-1.0 cells were transfected using Lipofectamine 2000 reagent as instructed by the manufacturer (Invitrogen, Carlsbad, CA, USA). Total RNA was extracted from cells using TRIzol reagent (Invitrogen). IRS-1 and LAR expression levels were quantified by real-time PCR using the TaqMan MicroRNA assay system (Applied Biosystems, Foster City, CA, USA). GAPDH was used as an internal control. RNA expression was quantified using the 2-ΔΔCt method [23]. The primers used for IRS-1 were as follows: 5’-ACAGGGTGGGCCAAATTAAAC-3’ (forward) and 5’-ACCATGCATTGGTCTTTGTGTA-3’ (reverse). The primers used for LAR were as follows: 5’-ATCCGGTGGGCGTTTGTAAA-3’ (forward) and 5’-ACCGGATTCATTCCAAAGAGA-3’ (reverse).
In vitro proliferation assay
The cells were seeded at a density of 5000 cells per well in 96-well plates in culture medium containing 10% FBS. After 3 days, the number of viable cells was counted using the Cell Counting Kit-8 (Dojindo Co., Kumamoto, Japan) according to the manufacturer’s instructions. The assay reagent is a tetrazolium compound (WST-x8) that is reduced by live cells into a colored formazan product that can be measured at 450 nm. The quantity of formazan product measured at 450 nm is directly proportional to the number of live cells in the culture. The experiments were repeated in triplicate.
In vitro invasion and migration assays
PC-1.0 and Aspc-1 cells were transiently transfected with siRNAs using Lipofectamine 2000 (Invitrogen, Grand Island, NY). For invasion Transwell assays, the transfected cells (5×104 cells/mL) in serum-free medium were added to the upper chambers, which was purchased from Costar (8 μm pore-size filters, New York, NY) and coated with Matrigel (dilution 1:4). The lower chambers were filled with RPMI-1640 containing 10% serum. After 24 h of incubation, the cells remaining in the upper chambers were removed,and the invasive cells in the lower chambers were fixed with 4% paraformaldehyde (SigmaAldrich), stained with crystal violet at room temperature, and counted under a microscope. For wound healing migration assay, the transfected cells were seeded onto 6-well plates for 24 h. A 1 mm-wide wound was made across the monolayer using the tip of a 200 μL pipette. Then, photos were taken at 0, 6, 12 and 24 h using a microscope. The PC-1.0 cells were performed as above described as a control. Each experiment was performed in duplicates and for three times. For confirming the level of IRS-1 protein in the knockdown cell, cells were subjected to real-time PCR and Western blot analysis using specific primers and antibodies as above-mentioned, respectively.
Statistical analysis
Statistical analysis and graphics were undertaken using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA). Results are presented as mean ± standard error of mean (SEM). Comparisons of quantitative data were analyzed by Student’s t-test or analysis of variance between two groups (two-tailed; P-values <0.05 were considered to be statistically significant).
Results
Migration and invasion of PC cells can be antagonized by inhibition of IRS-1
Since the results of the Phospho Explorer Antibody Array were from hamster PC cell lines, human PC cells Aspc-1 and Capan-2, which have morphological and functional characteristics similar to PC-1.0 and PC-1 cells, were used to determine if the results from hamster PC cells coincide with those from human PC cells. There was no significant difference in viability among the cell lines (Figure 1).
Figure 1.
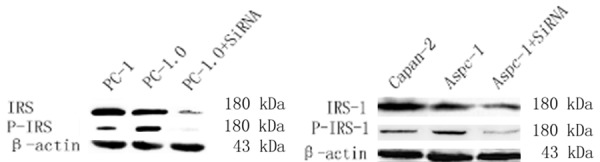
Western blot showed that protein expression and phosphorylation level were similar between Aspc-1 and PC-1.0 cells. Protein and phosphorylation expression levels were downregulated in cells transfected with si-IRS-1.
Western blotting (Figure 1) and real-time PCR (Figure 2) showed that IRS-1 was highly expressed in PC-1.0 and Aspc-1 cells, while IRS-1 expression was knocked down in cells transfected with si-IRS-1.
Figure 2.
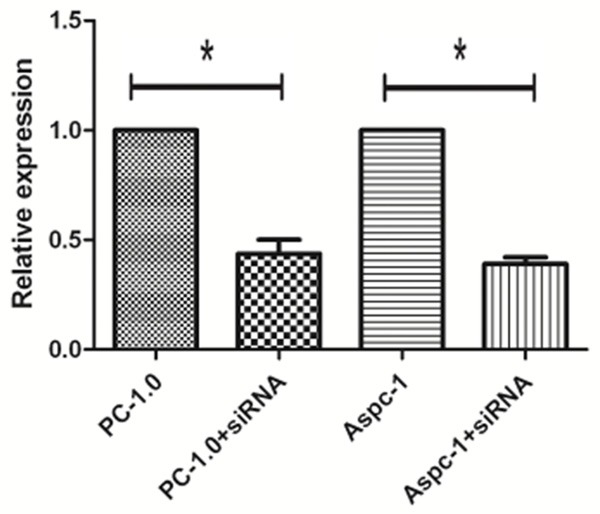
Real-time PCR showed that IRS-1 gene expression was downregulated in cells transfected with si-IRS-1.
A transwell assay showed that invasion of cells transfected with si-IRS-1 was markedly depressed compared with untreated controls (56.04%, PC1.0 cells, and 64.01%, Aspc-1 cells; P<0.05) (Figure 3). A wound healing assay consistently showed a similar migration pattern in si-IRS-1 cells compared to controls (Figure 4).
Figure 3.

Quantification of transwell assay for treated group and control group. Cells were counted in triplicate wells and in three identical experiments, *Compared with the PC-1.0, P-value <0.01 (n = 3).
Figure 4.

Effect of IRS-1 on the migration after using siRNA in highly metastatic PC-1.0 and Aspc-1 cells. The cells were incubated for 24 h. The percentage migration was calculated and graphed. *Compared with the PC-1.0, P-value <0.01 (n = 3).
PC-1.0 and Aspc-1 cells grow in a sporadic way. IRS-1 knockdown PC-1.0 and Aspc-1 cells grew in an aggregated or clumped pattern in culture meanwhile cellular pseudopod were attenuated or inexistent (Figure 5).
Figure 5.
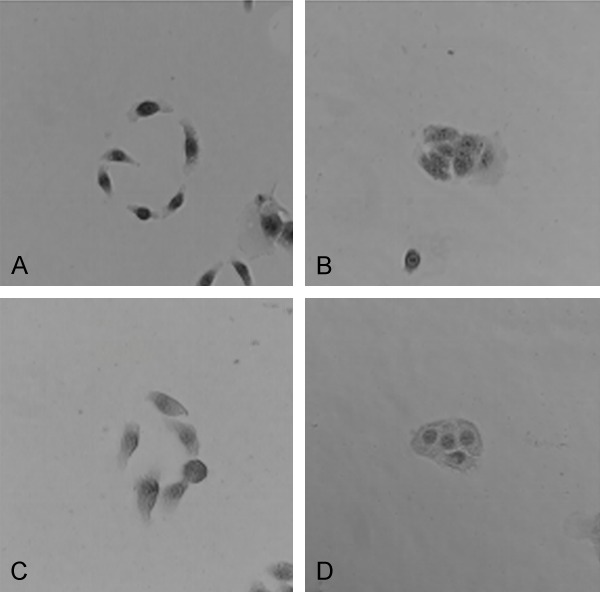
IRS-1-knockdown PC-1.0 (A and B) and Aspc-1 (C and D) cells grew in an aggregated or clumped pattern in culture meanwhile cellular pseudopod were attenuated or inexistent.
The effects of IRS-1 knockdown on cell proliferation in PC-1.0 and Aspc-1 PC cells were also investigated. We found that downregulation of IRS-1 inhibited proliferation and growth of PC-1.0 and Aspc-1 cells (Figure 6).
Figure 6.

Aspc-1 and PC-1.0 cells were transfected with si-IRS-1 and incubated for 24 h. Cell proliferation was decreased. Experiments were repeated in triplicate. *Compared with the PC-1.0, P-value <0.01 (n = 3).
Our observations indicated that IRS-1 regulates the migration and invasion of PC-1.0 and Aspc-1 cells, and IRS-1 knockdown inhibits proliferation and growth simultaneously alters cellular morphology and growth patterns.
Effect of IRS-1 on PC cells and interactions with MAPK and PI3K pathway molecules
A previous study indicated that inhibition of MEK1 was an effective and specific method of inhibiting cell proliferation and inducing G0/G1 arrest while MEK2 specifically disrupted cell morphology and reduced the invasive ability of cultured PC-1.0 cells [24]. However, the mechanism of these actions is unknown. Similar patterns observed in the current study reveal that there may be a relationship between IRS-1 and MEK1/2.
First, the efficiency of IRS-1 transfection was evaluated by real-time PCR, and there was no significant difference between PC-1.0 and Aspc-1 cells in knockdown efficiency (data not shown). Western blotting was carried out to identify if IRS-1 regulates MEK1/2; results showed that the protein expression level of MEK1/2 downregulated when IRS-1 was knocked down by siRNA. Protein expression levels of IRS-1 in MEK1 and MEK2-knockdown PC-1.0 cells [24] and si-MEK1 and si-MEK2 Aspc-1 cell did not differ remarkably (Figure 7).
Figure 7.
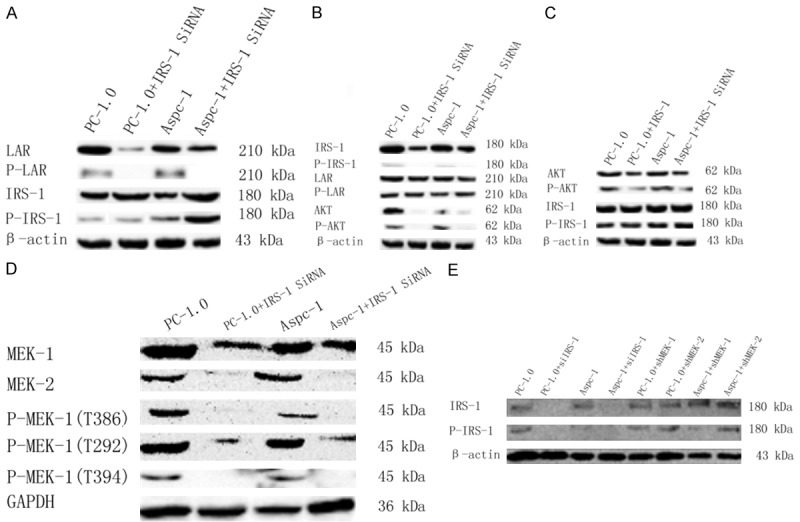
Western blot showed protein and phosphorylation levels of multiple proteins. A. LAR protein and phosphorylation levels were downregulated when treated with LAR siRNA, while IRS-1 protein and phosphorylation levels were downregulated; B. IRS-1 protein and phosphorylation levels were downregulated when treated with IRS-1 siRNA, while LAR protein and phosphorylation levels were not regulated obviously; C. AKT protein and phosphorylation levels were downregulated when treated with AKT inhibitor, while IRS-1 protein and phosphorylation levels were not regulated obviously; D. MEK1 and MEK2 total protein levels and MEK1 (T386), MEK1 (T292) and MEK2 (t394) phosphorylation levels were downregulated when treated with IRS-1 siRNA; E. IRS-1 protein and phosphorylation levels were not regulated obviously in MEK1 and MEK2 stably transfected PC-1.0 cells.
LAR, initially screened out by LC-MS based on silac labeling (data not shown), was two-fold greater in PC-1 cells than in PC-1.0 cells. Since PC-1 cells are conservative compared to PC-1.0 cells, we supposed that LAR downregulates phosphorylation levels of IRS-1 and further regulates invasion and metastasis of pancreatic cancer cells. Western blotting showed that LAR was highly expressed in PC-1.0 and Aspc-1 cells, and LAR expression was knocked down in si-LAR transfected cells. Interestingly, IRS-1 expression and phosphorylation levels were increased in si-LAR expressing cells. However, LAR expression and phosphorylation levels were not reduced in PC-1.0 and Aspc-1 cells expressing si-IRS-1 (Figure 7).
Discussion
Pancreatic carcinoma is the most aggressive form of malignancy and warrants more treatment options owing to its poor prognosis and the fact that there is currently only a single drug therapy, which faces the challenge of drug resistance [27]. Presently, most research studies on the molecular mechanisms of PC invasion and metastasis focus on the differences between heterologous highly and weakly invasive and metastatic PC cell lines. These cell lines have considerable disadvantages in that they may include several different cell types. The homologous PC cell lines PC-1 and PC-1.0, which were established from an experimental PC model in our previous study [9,10], show different potentials for invasion and metastasis [28,29].
LAR, identified by LC-MS based on silac labeling in PC-1 and PC-1.0 cells in the present study (data not shown), is widely expressed in insulin-sensitive tissues [30,31]. It is a transmembrane protein that is localized to the membrane fraction of the cell [32,33], and its cytoplasmic domain has a catalytic preference for the regulatory phosphotyrosines of the insulin receptor kinase domain in vitro [34,35]. Decreased LAR expression results in an augmentation of additional post-receptor events, including IRS-1 tyrosine phosphorylation, IRS-1 complexing with the p85 subunit of PI3-kinase, IRS-1 associated PI3-kinase activity, and the activation of both MAP kinase kinase (MEK) and MAP kinase (MAPK) [36,37]. The physiological role of LAR in the regulation of reversible tyrosine phosphorylation of the insulin receptor and post-receptor substrates in the insulin action pathway have been well studied; however, its role in PC and the exact molecular mechanism in the MAPK and PI3K pathways were not fully known. This study showed that LAR negatively regulates protein and phosphorylation levels of IRS-1 and AKT in PC-1.0 and Aspc-1 cells. Post-translational modifications, which occur at multiple stages after translation, are transient in nature, and target proteins constantly flux between the functionally active and inactive states. Phosphorylation involves the addition of a phosphate group to serine (Ser), threonine (Thr) or tyrosine (Tyr) residues on proteins. These reversible events are the most common form of modifying biological activity, and influence different cellular processes including proliferation, apoptosis, invasion and migration.
Tan X. reported that the ratio of IRS-1 phosphorylation levels at Ser636 in PC-1 cells compared to PC-1.0 cells was 0.43 using the Phospho Explorer Antibody Array method [14]. Recent studies revealed that IRS-1 could induce invasion and migration in non-small cell lung cancer (NSCLC) cells and that expression levels correlated with NSCLC patient survival [38] and regulated myoblast cell proliferation [39]. Since morphological and functional characteristics of PC-1 and PC-1.0 cells are different, we speculated that IRS-1 plays an important role in invasion and metastasis in PC. Experiments showed that IRS-1 induced proliferation, invasion and migration of PC cells. However, these proliferation, invasion and migration effects were largely reversed in PC-1.0 and Aspc-1 cells transfected with LAR siRNA. IRS-1 is negatively regulated by LAR by protein expression and phosphorylation levels. Transmission of extracellular stimuli into the nucleus largely depends on reversible protein phosphorylation. MAPK is one of the signaling pathways known to mediate cell proliferation, apoptosis, and metastasis that activate the serine/threonine kinases which belong to the MAPK superfamily [40,41].
The MEK family, which only has two isoforms MEK1 and MEK2, is a highlight of the MAPK superfamily. A previous study [26] demonstrated that MEK1 and MEK2 mediate different biological functions in PC cells. The results showed that inhibition of MEK1, but not MEK2, was an effective and specific method of inhibiting cell proliferation and inducing G0/G1 arrest. Moreover, targeting MEK2 mRNA specifically disrupted cell morphology and reduced the invasive ability of cultured PC cells [26]. The study is in agreement with our observations that proliferation, invasion and metastasis are reduced in diverse si-IRS-1 cells. We found that protein expression and phosphorylation of MEK1 and MEK2 were reduced in the PC-1.0 and Aspc-1 cell lines. Combined with our previous [26] study, we suggest that MEK1 and MEK2 regulate proliferation and invasion of pancreatic cells, respectively and are induced by IRS-1. Furthermore, analyses in our study showed that IRS-1 regulates AKT protein expression and phosphorylation simultaneously. AKT is a significant chemokine in the PI3K/AKT signaling pathway usually involved in regulating cell proliferation and cell cycle dynamics [42]. A CCK-8 assay showed that proliferation of PC-1.0 and Aspc-1 cells was decreased when AKT was blocked. This finding could indicate that cooperation exists between MEK1 and AKT to regulate proliferation of PC cells, and that there may be crosstalk between MAPK and PI3K signaling pathways through IRS-1.
In conclusion, IRS-1 plays a significant role in regulating proliferation, invasion and migration of Aspc-1 human PC cells and PC-1.O hamster PC cells. LAR could negatively regulate protein expression and phosphorylation of IRS-1. The downregulation of IRS-1 expression and phosphorylation may inhibit the proliferation, invasion and migration of PC cells, and this function may be achieved through MEK1, MEK2 and AKT, members of the MAPK and PI3K signaling pathways. There may also be cooperation between MAPK and PI3K signaling pathways to regulate proliferation of PC cells. These findings provide new scientific evidence to facilitate the clinical treatment of PC. In the future, we should pay more attention to the signal passing functions of the phosphoproteome, particularly specific amino acid residues.
Taken together, IRS-1 suppressed by LAR targeted and positive regulated MEK-1 and MEK-2 which are related to invasion and metastasis, may allow us to identify new biomarkers for the early detection of PC.
Acknowledgements
This work was supported by National Nature Science Foundation of China (NO. 30973501).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer stat. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Anastasios S, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–72. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 3.Tuveson D, Neoptolemos J. Understanding metastasis in pancreatic cancer: a call for new clinical approaches. Cell. 2012;148:21–3. doi: 10.1016/j.cell.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Thilo H, Büchler MW. Pancreatic cancer: advances in treatment, results and limitations. Dig Dis. 2013;31:51–6. doi: 10.1159/000347178. [DOI] [PubMed] [Google Scholar]
- 5.Tyagi N, Bhardwaj A, Singh AP, McClellan S, Carter JE, Singh S. p-21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT- and ERK-dependent activation of NF-κB pathway. Oncotarget. 2014;5:8778–89. doi: 10.18632/oncotarget.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maertens O, Cichowski K. An expanding role for RAS GTPase activating proteins (RAS GAPs) in cancer. Adv Biol Regul. 2014;55:1–14. doi: 10.1016/j.jbior.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK (MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38 (SAPK) Cancer Res. 2003;63:1684–95. [PubMed] [Google Scholar]
- 8.Chiarini F, Evangelisti C, McCubrey JA, Martelli AM. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci. 2015;36:124–35. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Egami H, Takiyama Y, Cano M, Houser WH, Pour PM. Establishment of hamster pancreatic ductal carcinoma cell line (PC-1) producing blood group-related antigens. Carcinogenesis. 1989;64:349–353. doi: 10.1093/carcin/10.5.861. [DOI] [PubMed] [Google Scholar]
- 10.Egami H, Tomioka T, Tempero M, Kay D, Pour PM. Development of intrapancreatic transplantable model of pancreatic duct adenocarcinoma in Syrian golden hamsters. Am J Pathol. 1991;138:557–61. [PMC free article] [PubMed] [Google Scholar]
- 11.Pour PM, Egami H, Takiyama Y. Patterns of growth and metastases of induced pancreatic cancer in relation to the prognosis and its clinical implications. Gastroenterology. 1991;100:529–36. doi: 10.1016/0016-5085(91)90226-b. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein BJ. Regulation of insulin receptor signaling by protein-tyrosine dephosphorylation. Receptor. 1993;3:1–15. [PubMed] [Google Scholar]
- 13.Kulas DT, Goldstein BJ, Mooney RA. The transmembrane protein-tyrosine phosphatase LAR modulates signaling by multiple receptor tyrosine kinases. J Biol Chem. 1996;271:748–54. doi: 10.1074/jbc.271.2.748. [DOI] [PubMed] [Google Scholar]
- 14.Tan X, Liu P, Huang Y, Zhou L, Yang Y, Wang H, Yu B, Meng X, Zhang X, Gao F. Phosphoproteome analysis of invasion andmetastasis-related factors in pancreatic cancer cells. PLoS One. 2016;11:e0152280. doi: 10.1371/journal.pone.0152280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White MF. Insulin signaling in health and disease. Science. 2003;302:144–147. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 16.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Gibson SL, Zhefu M, Shaw LM. Divergent roles for IRS-1 and IRS-2 in breast cancer metastasis. Cell Cycle. 2007;6:631–7. doi: 10.4161/cc.6.6.3987. [DOI] [PubMed] [Google Scholar]
- 18.Ma Z, Gibson SL, Byrne MA, Zhang J, White MF, Shaw LM. Suppression of insulin receptor substrate 1 (IRS-1) promotes mammary tumor metastasis. Mol Cell Biol. 2006;26:9338–51. doi: 10.1128/MCB.01032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi J, Wang DM, Wang CM, Hu Y, Liu AH, Zhang YL, Sun B, Song JG. Insulin receptor substrate-1 suppresses transforming growth factor-beta1-mediated epithelial-mesenchymal transition. Cancer Res. 2009;69:7180–7. doi: 10.1158/0008-5472.CAN-08-4470. [DOI] [PubMed] [Google Scholar]
- 20.Reiss K, Wang JY, Romano G, Furnari FB, Cavenee WK, Morrione A, Tu X, Baserga R. IGF-I receptor signaling in a prostatic cancer cell line with a PTEN mutation. Oncogene. 2000;19:2687–94. doi: 10.1038/sj.onc.1203587. [DOI] [PubMed] [Google Scholar]
- 21.Araújo EP, De Souza CT, Gasparetti AL, Ueno M, Boschero AC, Saad MJ, Velloso LA. Shortterm in vivo inhibition of insulin receptor substrate-1 expression leads to insulin resistance, hyperinsulinemia, and increased adiposity. Endocrinology. 2005;146:1428–37. doi: 10.1210/en.2004-0778. [DOI] [PubMed] [Google Scholar]
- 22.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog. 2012;51:53–63. doi: 10.1002/mc.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012;51:64–74. doi: 10.1002/mc.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen DK, Andren-Sandberg A, Duell EJ, Goggins M, Korc M, Petersen GM, Smith JP, Whitcomb DC. Pancreatitis-diabetes-pancreatic cancer: summary of an NIDDK-NCI workshop. Pancreas. 2013;42:1227–37. doi: 10.1097/MPA.0b013e3182a9ad9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–93. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 26.Zhou L, Tan X, Kamohara H, Wang W, Wang B, Liu J, Egami H, Baba H, Dai X. MEK1 and MEK2 isoforms regulate distinct functions in pancreatic cancer cells. Oncol Rep. 2010;24:251–5. doi: 10.3892/or_00000853. [DOI] [PubMed] [Google Scholar]
- 27.Kim MP, Evans DB, Vu TM, Fleming JB. The recognition and surgical management of heritable lesions of the pancreas. Surg Oncol Clin N Am. 2009;18:99–119. ix. doi: 10.1016/j.soc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Pour PM, Egami H, Takiyama Y. Patterns of growth and metastases of induced pancreatic cancer in relation to the prognosis and its clinical implications. Gastroenterology. 1991;100:529–36. doi: 10.1016/0016-5085(91)90226-b. [DOI] [PubMed] [Google Scholar]
- 29.Kurizaki T, Egami H, Hirota M, Akagi J, Ohmachi H, Yamamoto S, Ogawa M. Characterization of cancer cell dissociation factor in a highly invasive pancreatic cancer cell line. Cancer. 1995;75(Suppl):1554–61. doi: 10.1002/1097-0142(19950315)75:6+<1554::aid-cncr2820751528>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein BJ, Meyerovitch J, Zhang WR, et al. Hepatic protein-tyrosine phosphatases and their regulation in diabetes. Adv Prot Phosphatases. 1991;6:1–17. [Google Scholar]
- 31.Longo FM, Martignetti JA, Le Beau JM, Zhang JS, Barnes JP, Brosius J. Leukocyte common antigen-related receptor-linked tyrosine phosphatase. Regulation of mRNA expression. J Biol Chem. 1993;268:26503–11. [PubMed] [Google Scholar]
- 32.Ahmad F, Goldstein BJ. Purification, identification and subcellular distribution of three predominant protein-tyrosine phosphatase enzymes in skeletal muscle tissue. Biochim Biophys Acta. 1995;1248:57–69. doi: 10.1016/0167-4838(95)00003-d. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad F, Goldstein BJ. Alterations in specific protein-tyrosine phosphatases accompany insulin resistance of streptozotocin diabetes. Am J Physiol. 1995;268:932–40. doi: 10.1152/ajpendo.1995.268.5.E932. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto N, Zhang WR, Goldstein BJ. Insulin receptor and epidermal growth factor receptor dephosphorylation by three major rat liver protein-tyrosine phosphatases expressed in a recombinant bacterial system. Biochem J. 1992;284:569–76. doi: 10.1042/bj2840569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto N, Feener EP, Zhang WR, Goldstein BJ. Insulin receptor protein-tyrosine phosphatases. Leukocyte common antigen-related phosphatase rapidly deactivates the insulin receptor kinase by preferential dephosphorylation of the receptor regulatory domain. J Biol Chem. 1992;267:13811–4. [PubMed] [Google Scholar]
- 36.Kulas DT, Zhang WR, Goldstein BJ, Furlanetto RW, Mooney RA. Insulin receptor signaling is augmented by antisense inhibition of the protein tyrosine phosphatase LAR. J Biol Chem. 1995;270:2435–8. doi: 10.1074/jbc.270.6.2435. [DOI] [PubMed] [Google Scholar]
- 37.Kulas DT, Goldstein BJ, Mooney RA. The transmembrane protein-tyrosine phosphatase LAR modulates signaling by multiple receptor tyrosine kinases. J Biol Chem. 1996;271:748–54. doi: 10.1074/jbc.271.2.748. [DOI] [PubMed] [Google Scholar]
- 38.Cao M, Li Y, Lu H, Meng Q, Wang L, Cai L, Dong X. miR-23a-mediated migration/invasion is rescued by its target, IRS-1, in non-small cell lung cancer cells. J Cancer Res Clin Oncol. 2014;140:1661–70. doi: 10.1007/s00432-014-1725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motohashi N, Alexander MS, Shimizu-Motohashi Y, Myers JA, Kawahara G, Kunkel LM. Regulation of IRS1/Akt insulin signaling by microRNA-128a during myogenesis. J Cell Sci. 2013;126:2678–91. doi: 10.1242/jcs.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shih YW, Shieh JM, Wu PF, Lee YC, Chen YZ, Chiang TA. Alpha-tomatine inactivates PI3K/Akt and ERK signaling pathways in human lung adenocarcinoma A549 cells: effect on metastasis. Food Chem Toxicol. 2009;47:1985–95. doi: 10.1016/j.fct.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Chen PN, Hsieh YS, Chiou HL, Chu SC. Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chem Biol Interact. 2005;156:141–50. doi: 10.1016/j.cbi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28. doi: 10.1146/annurev-med-062913-051343. [DOI] [PubMed] [Google Scholar]


