Abstract
Gastrodin (GAS) is an active constituent of Chinese herbal medicine tianma (Gastrodia elata), which is commonly used to extinguish wind (TCM term). Tianma is also widely used to treat various neurological diseases such as stroke, dizziness, epilepsy, etc. Its clinical effect is quite satisfactory. However, the underlying mechanism has not been fully explored. In the present study, we choose a permanent cerebral occlusion model, MCAO, and used multiple methods to investigate the medicine. Our results show a significant improvement in neurological score after 3 days of GAS treatment. In addition, neurons in the hippocampus were rescued within after 7 days GAS treatment. Then we explore the drug’s mechanism in the acute phase of stroke. CRP and IL-1β are common inflammatory factors. Elisa showed GAS can reduce these inflammatory factors in serum in the acute phase of stroke. What’s more, GAS can up-regulate the expression of Bcl-2 and down-regulate the expression of BAX in the ischemic hemisphere, and the same result is observed in the protein level. The expression of Caspase-3 is also suppressed, indicating GAS has ability to inhibit apoptosis during the acute phase of stroke. On the other hand, GAS can up-regulate the expression of VEGF, thusly promoting micro-vacsular regeneration. In conclusion, our results demonstrate that GAS can alleviate the symptoms of stroke through various mechanisms. GAS might also serve as a potential candidate to treat acute cerebral infarction.
Keywords: Gastrodin, cerebral infarction, inflammation, apoptosis, revascularization
Introduction
Cerebral infarction is a common disease worldwide and has a high fatality and disability rate [1]. The incidence of cerebral infarction has increased drastically during the past 4 decades [2]. Recombinant thrombolytic agent tissue plasminogen activator (rtPA) is widely used to treat ischemic stroke [3], but, it has many shortcomings, such as a potential risk of hemorrhage, a limited therapeutic time window, etc. [4,5]. As a result, novel and effective medicine is still urgently needed.
The pathological characteristics of cerebral infarction in the acute phase includes a series of subsequent biochemical events, such as oxidative stress, inflammatory responses, programmed cell death, etc. [6,7]. Hypothetically, any agent that suppresses inflammation and inhibits apoptosis can be beneficial to treat stroke.
Increasing clinical evidence has proven that using the ‘Extinguish Wind method’ in the acute phase of stroke could achieve satisfactory results. The Chinese herbal medicine tianma (Gastrodia elata) is the key factor, and gastrodin (GAS) is the main extract of tianma [8,9]. It has been widely used in the clinical treatment of neurologic diseases such as ischemic stroke and dizziness. Pharmacological research revealed it has anti-oxidant and anti-inflammatory effects.
In the present study, middle cerebral artery occlusion (MCAO) was applied to establish an eternal rat ischemic cerebral infarction model. Inflammation factors and apoptosis relative proteins were measured to determine the mechanisms of GAS on ischemic stroke.
Materials and methods
Reagents
Gastrodin was purchased from the Shanghai Tongtian Pharmaceutical Corporation. GAS was dissolved in saline and administered intraperitoneally once a day. The GAS dose was 100 mg/kg. After MCAO intervention, the GAS was immediately administered intraperitoneally.
Animals
SPF male Sprague-Dawley rats (250-300 g body weight) were purchased from Vital River Laboratory Animal Technology Co. Ltd., Beijing, China. The animals were kept under a 12 h/12 h light/dark cycle at a controlled temperature and humidity and given food and water ad libitum. All of the animal procedures were performed according to China’s animal welfare laws and approved by the Sichuan University Committee on the Care and Use of Laboratory Animals.
6 to 8 week old male Wistar rats were purchased from the Shanghai Experimental Animal Center (Shanghai, China). The experimental procedures were approved and performed according to the guidelines of laboratory animal care and use. All efforts were made to reduce the number of animals tested and their suffering.
Induction of focal cerebral ischemia-reperfusion
Permanent cerebral infarction was induced by middle cerebral artery occlusion (MCAO) in the rats using the intraluminal filament technique [10]. The left common carotid artery, internal carotid artery (ICA), and external carotid artery (ECA) were exposed through a midline incision in the neck, and a monofilament nylon suture (Xinong Technology Corporation.) with a silicone-coated tip was plugged into the ICA, 16-18 mm from the bifurcation, through the ECA stump and was gently advanced to cause middle cerebral artery occlusion (MCAO). The sham group underwent similar surgical procedures without the occlusion of the middle cerebral artery.
Neurological deficit evaluation
Neurological deficits were monitored after the MCAO surgery and on 1, 3, 5, 7 day after MCAO. The Bederson method was applied to monitor neurological deficits every other day after the MCAO surgery. There were 4 parts: 1) Performance of right forelimb. One: Adduction not adjacent to the skin; Two: Adduction adjacent to the skin; Three: Adduction, adjacent to the skin and curled up; Four: The whole body is turned around to the right. 2) Muscular tension of both forelimbs. Zero: Both forelimbs can grasp, myodynamia is normal; One: The right forelimb is weak but can still grasp; Two: The right forelimb can’t grasp. 3) Placed on a smooth surface, observing resistance when pushing. Zero: No difference in resistance when pushing either the right or left side; One: Less resistance when pushing the right side; Two: Can be pushed over when pushing the right side. 4) Left upper eyelid. Zero: Normal; Two: Left blepharoptosis, left eye has more excretion.
Western blot analysis
A Western blot analysis was performed as described previously [11]. Briefly, Equal amounts of protein were loaded for immunoblotting. An SDS-PAGE was performed to separate the different sizes of the proteins. After electrophoresis, the protein was transferred to nitrocellulose membranes using a wet transfer system (BIO-RAD). Then the membrane was blocked with 5% nonfat milk (BIORAD) for 1 h at room temperature. Next, the primary antibody was incubated at 4°C overnight. After the membranes were washed with TBST, they were incubated with a secondary antibody (1:3000) at room temperature for 1 hour. Then the membranes were washed 3 times with TBST. Finally, protein signals were detected using an ECL kit (BioRad) and the UVP GelDoc image system (UVP). The images were analyzed by Image J (NIH, Bethesda, MD, USA).
Total RNA extraction and qPCR
Total RNA was isolated from the brain tissue using a Trizol reagent (GibcoBRL, Grand Island, NY) according to the manufacturer’s instructions. Then 200 ng of total RNA was reverse-transcribed in a 10 μl reaction using a reverse-transcription system. The sequence of primers is listed in Table 1. Amplification and detection were performed under the following conditions: an initial hold at 95°C for 10 s followed by 40 cycles at 95°C for 30 s and 62°C for 30 s, and then 72°C for 1 min. PCR products with 1.2% agarose gel electrophoresis were visualized by EB staining under UV illumination. The gel was scanned and the band intensity was measured by densitometry. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Table 1.
Sequence of primers used in the study
| Gene | Primer F | Primer R |
|---|---|---|
| GAPDH | GTTACCAGGGCTGCCTTCTC | GGGTTTCCCGTTGATGACC |
| Bcl-2 | GCGTCAACAGGGAGATGTCA | GTTCCACAAAGGCATCCCAG |
| Bax | TTGCTACAGGGTTTCATCCAGG | CACTCGCTCAGCTTCTTGGT |
| Caspase-3 | GGAGCTTGGAACGCGAAGAA | GTCCATCGACTTGCTTCCAT |
| AKT | CGCTTCTTTGCCAACATCGT | ACACTCCATGCTGTCATCTTGA |
| VEGF | CGGTTCCAGAAGGGAGAGGA | CTGGGACCACTTGGCATGG |
Results
GAS can improve the neurological score in MCAO rats time-dependently
To detect effect of GAS in cerebral infarction patients, we chose MCAO rats as a cerebral infarction model. The general process of the experiment is shown in Figure 1A.
Figure 1.
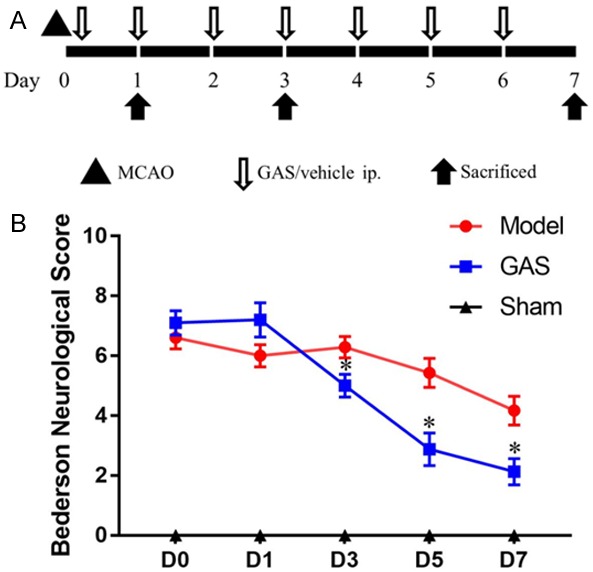
GAS can improve the neurological score in MCAO rats time-dependently. A. A schematic diagram of the design for molding, drug treatment and sample collection. B. Quantitative evaluation of the Bederson neurological score, GAS can significantly improve the neurobehavioral scores in MCAO rats 3 days after infarction. *P<0.05 vs. model group.
We adopted Bederson’s score to evaluate neurological deficits [12]. The higher the Bederson’s score, the worse condition the rat was in. The sham group suffered no neurological damage, so, the Bederson’s scores for those rats were recorded as 0. As shown in Figure 1B, after 3 days’ GAS treatment, a significant difference was observed between the model group and the GAS group (P<0.05). The neurological damage was gradually alleviated day by day during the 7-day GAS treatment.
GAS has a protective effect on neurons in hippocampus area of MCAO rats
To record the protective effects of GAS on neurons in an ischemic area of MCAO rats’ brains, we chose NeuN as a neuron biomarker. Immunohistochemistry was applied to determine neuron damage in the hippocampus area after the infarction. The results showed a significant difference between the MCAO group and the GAS group in day 7 (Figure S1), indicating that GAS has a protective effect on neurons from ischemic strikes.
GAS can inhibit cerebral ischemia induced inflammation in the ischemic hemisphere
To investigate the inflammatory reactions of MCAO rats after the ischemic strike of the brain, we tested an inflammatory factor in the serum. An ELISA test revealed that the concentration of CRP and IL-1β increased after the infarction of MCA. Compared with the model group, GAS reduced the CRP level on day 1 and day 3 significantly, with no significantly difference on day 7 (Figure 2A). The concentration of IL-1β in both groups increased in the first 7 days, and the IL-1β in the GAS group was lower than model group on day 1 (Figure 2B). Indicating GAS can reduce the concentration of CRP and IL-1β in serum at the acute phase.
Figure 2.
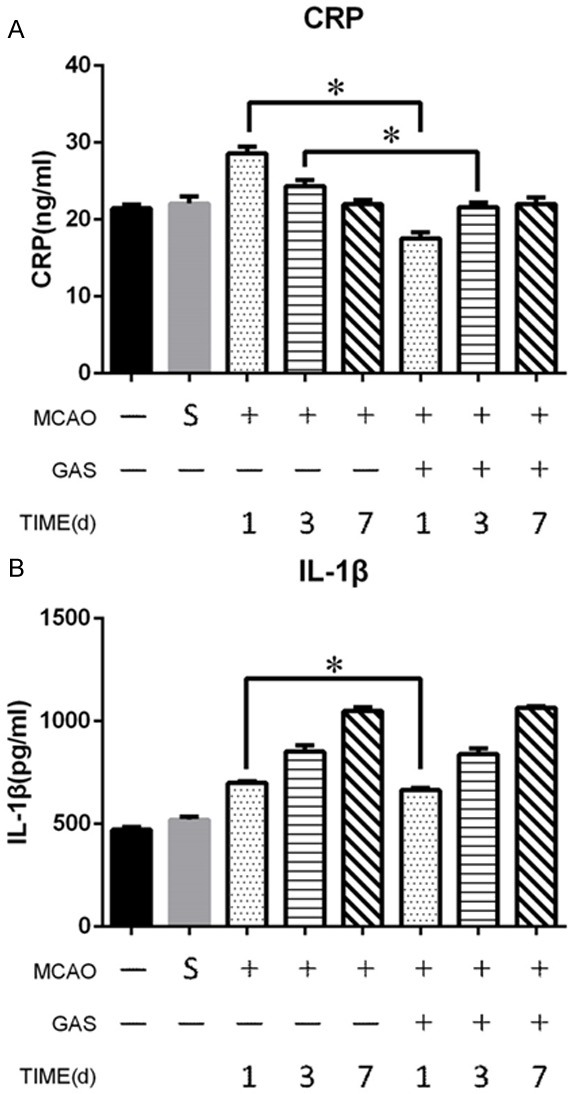
GAS can inhibit cerebral ischemia induced inflammation in serum. A. GAS can reduce the serum level of CRP after 1 and 3 days compared with the MCAO group. B. GAS can reduce the serum level of IL-1β after 1 day compared with the MCAO group. *P<0.05.
GAS can inhibit the intrinsic apoptosis pathway in the ischemic hemispheres
To investigate the effect of GAS on the apoptosis pathway of ischemic hemisphere, we tested the concentration of the Bcl-2 family, BAX and Bcl-2, in the ischemic hemisphere. During the first 7 days after infarction, BAX consistently increased in both the MCAO and GAS groups. Additionally, GAS significantly reduced the amount of BAX protein in the acute phase (Figure 3A). Whereas the concentration of Bcl-2 consistently decreased after the infarction, the Bcl-2 protein in the GAS group was slightly higher than it was in the MCAO group, but there was no significant difference (Figure 3B). Next, we performed immunohistochemistry to confirm the results. The results also showed that the GAS group had less BAX expression and more Bcl-2 expression in the acute phase of infarction (Figure S2).
Figure 3.
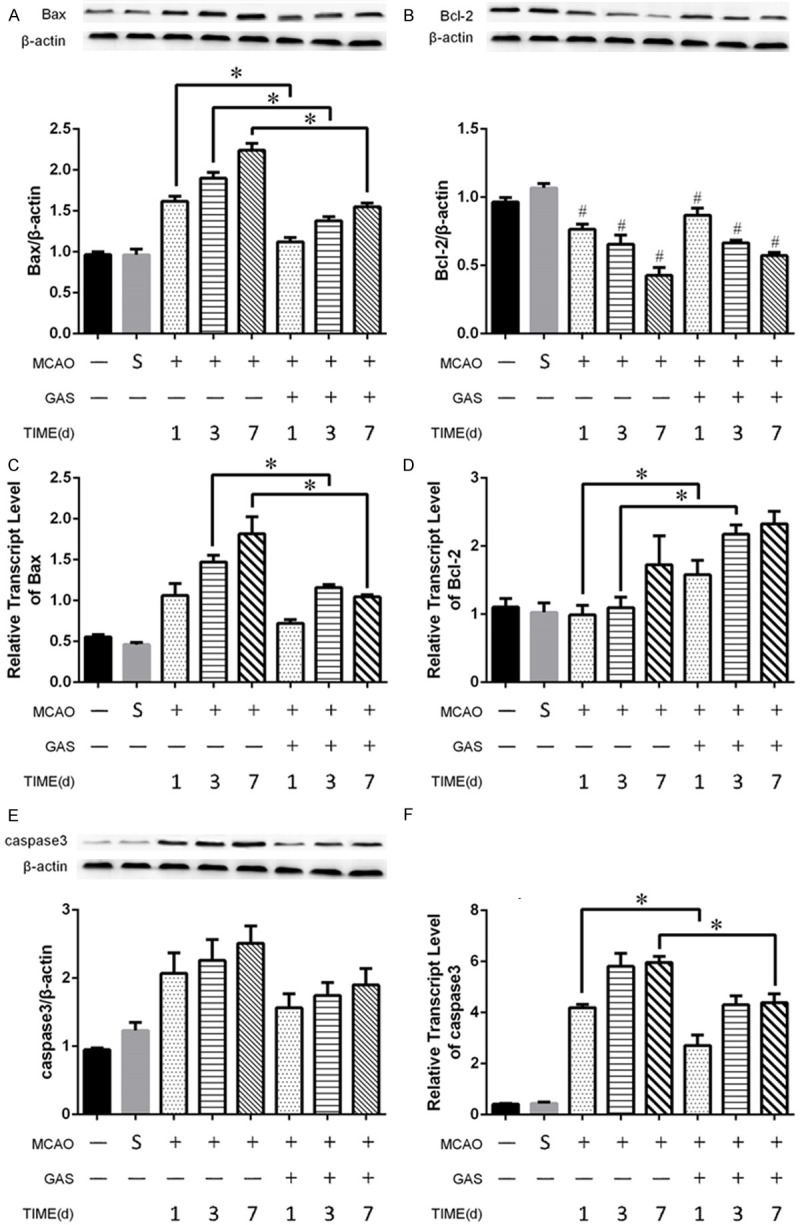
GAS can inhibit the intrinsic apoptosis pathway in the ischemic hemispheres. A, C. GAS can reduce both the expression and transcription levels of BAX in the acute phase of ischemic strike. B, D. GAS can increase the transcription level of Bcl-2 in the acute phase of ischemic strike, and no significant difference was observed in the protein level between the MACO group and the GAS group. E, F. GAS can reduce the transcription level of caspase 3 in the acute phase of ischemic strike, and no significant difference was observed in the protein levels between the two groups. *P<0.05. #P<0.05 compared with control and sham group.
Then we further explored the expression levels of the two signal molecules in the Bcl-2 family in the ischemic hemisphere. Bax mRNA increased gradually in the first week after the infarction in the MCAO group, and GAS can reverse this trend, and there was a significant difference between the MCAO group and the GAS group on days 3 and 7 (Figure 3C). As for the Bcl-2, the result showed that GAS significantly increased the expression level in the ischemic hemisphere on days 1 and 3 (Figure 3D).
Then we investigates the effects of GAS on the classic apoptotic molecule caspase 3. In terms of protein levels, caspase 3 was slightly increased in the ischemic hemisphere during the first week after infarction. GAS can reduce the concentration of caspase 3, but there was no significant difference between the two groups (Figure 3E). As for the mRNA level, they shared the same tendency, and there were significant differences in days 1 and 7 (Figure 3F).
GAS boosts the growth of blood vessels in the ischemic hemisphere to rescue neurons
To investigate the effects of GAS on blood vessels, we chose vascular endothelial growth factor (VEGF) as our target. The concentration of VEGF in the serum revealed an increasing trend in the MCAO group during the first week after the ischemic strike, as GAS has an tendency to enhance this trend. There was a significant increase in the GAS group compared with the MCAO group at each time point (Figure 4A).
Figure 4.
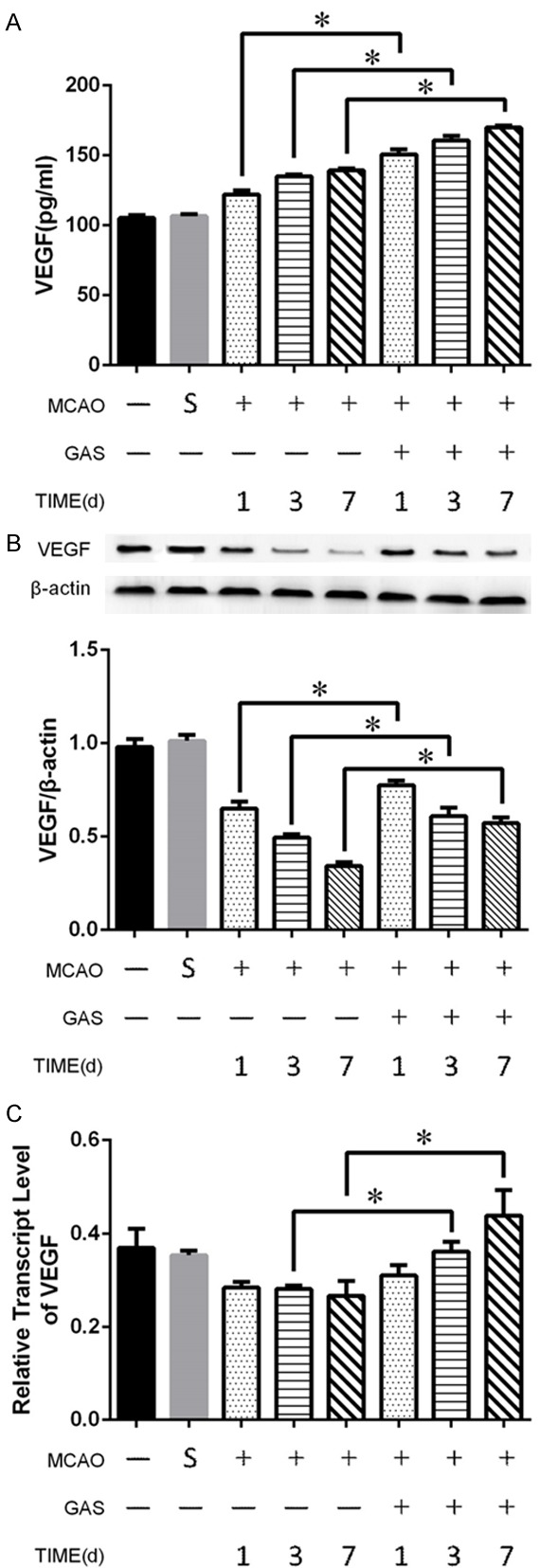
GAS boosts the growth of blood vessels in the ischemic hemisphere to rescue neurons. A. GAS increased the serum level of VEGF in the acute phase of infarction. B. GAS increased the protein level of VEGF in ischemic hemisphere tissue. C. GAS increased the transcription level of VEGF in ischemic hemisphere tissue. *P<0.05.
The Western blot showed that the concentration of VEGF in the ischemic hemisphere consistently decreased after the infarction during the first week. However, GAS can inhibit this process significantly (Figure 4B). On the transcription level, GAS even can boost the mRNA of VEGF. There were significant differences between the MCAO group and the GAS group on day 3 and day 7 (Figure 4C).
Discussion
Middle cerebral artery occlusion (MCAO) is a commonly-used animal model stroke research. In this study, we chose MCAO rats as our target to explore pathogenesis during ischemic strikes and the effects of GAS on it. The result was that GAS has multiple advantages: it works quickly, it promotes remarkable improvement, and it is effective on multiple targets.
We demonstrated that GAS could improve neurological score of MCAO rats in the acute phase as soon as 3 days after infarction. There was no significant difference in the number of neurons in the hippocampus area during the 3 days after the stroke, but on the 7th day there were more surviving neurons in the GAS group than in MCAO group, an indication that GAS can rescue neurons in ischemia and with hypoxia status and therefore improve the neurological score. In the meantime, our study also revealed the crucial role of hippocampal neurons in neurological recovery.
In order to explore the underlying mechanisms of GAS in improving neurological score, we did further study.
The pathogenesis process after a stroke is quite complicated and is associated with various mechanisms. However, more and more evidence shows that inflammatory responses play a crucial role in it [13]. A growing number of studies has demonstrated that inflammation is the crucial pathogenesis after infarction. CRP and IL-1β were chosen as classic inflammatory factors, and our results showed a notable reduction of these inflammatory factors in the GAS group, indicating that GAS can suppress the systemic inflammatory response.
Several studies found that GAS could penetrate through the blood-brain barrier [14,15]. However, whether GAS can suppress the inflammatory response in ischemic brain tissue remains unclear and deserves further study.
Apoptosis is another important pathogenesis in the ischemic hemisphere after stroke. Proteins in the Bcl-2 family play a key role in the pathogenesis of apoptosis. Generally, there are two kinds of protein in the Bcl-2 family: pro-apoptosis and anti-apoptosis. We choose Bcl-2 and Bax as representatives to elucidate role of GAS in the acute stroke phase. The results showed a significant reduction of Bax in the GAS group compared to the MCAO group. On the mRNA level, GAS still has the effect of bringing down the Bax level after day 3. As for Bcl-2, there was no significant difference between the two groups. However, on the mRNA level, GAS can drastically increase Bcl-2 mRNA in the very early stages of infarction. In addition, we evaluated the expressions of Bcl-2 and Bax in the hippocampus area by mean of immunohistochemistry. The result supported our previous work, as there was a significant increase of Bcl-2 and a reduction of Bax. The result revealed that the neuro-protective effect of GAS may have something to do with inhibiting apoptosis in the hippocampus area.
To further clarify our findings, we explored the expression and transcription levels of caspase 3 in the ischemic hemisphere as well. Caspase 3 is a protease that can digest certain substrates and inhibit DNA repair, which leads to cell apoptosis [16,17]. Our research demonstrated that GAS also could decrease the protein and mRNA levels of caspase-3 in a permanent infarction model. In this way, it inhibits the progression of apoptosis and increases the neurological scores of MCAO rats.
Several studies have shown that GAS has anti-apoptotic and anti-inflammation properties in a transient cerebral model [18,19], and our research also demonstrated that GAS also has the same effect in MCAO rats.
More and more studies have demonstrated that revascularization is a key pathophysiological process after cerebral infarction, in which VEGF plays a crucial role [20,21]. Our study then confirmed that GAS also could increase the serum level of VEGF as well as the level in the ischemic hemisphere, and the transcription level is also up-regulated in the acute phase of infarction. That implies that GAS can promote revascularization in damaged brain tissue.
In conclusion, the present study demonstrated that GAS was able to improve neurological scores and rescue neurons in a permanent infarction model. The mechanism may be associated with the suppression of the inflammatory response, inhibiting apoptosis, and improving revascularization in the ischemic hemisphere. However, whether the neuro-protective effect of GAS is associated with the hippocampus area remains unclear. Further research is still needed to determine how GAS suppresses inflammation and the specific pathway involved in inhibiting apoptosis after GAS treatment.
Acknowledgements
This study was funded by a Beijing Municipal Natural Science Foundation grant awarded to Shaoqing Wang (grant No. 7174299) and by the Beijing Key Laboratory Opening Project (grant No. TMCD201601) awarded to Yongping Fan.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Tommasina R, Giorgio F, Carmine M. Stroke in the very old: a systematic review of studies on incidence, outcome, and resource use. J Aging Res. 2011;2011:108785. doi: 10.4061/2011/108785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 populationbased studies: a systematic review. Lancet Neurol. 2009;8:306–307. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang B, Sun XJ, Ju CH. Thrombolysis with alteplase 4.5-6 hours after acute ischemic stroke. Eur Neurol. 2011;65:170. doi: 10.1159/000324291. [DOI] [PubMed] [Google Scholar]
- 4.Tan Z, Li X, Turner RC, Logsdon AF, Lucke-Wold B, DiPasquale K, Jeong SS, Chen R, Huber JD, Rosen CL. Combination treatment of r-tPA and an optimized human apyrase reduces mortality rate and hemorrhagic transformation 6 h after ischemic stroke in aged female rats. Eur J Pharmacol. 2014;738:368–373. doi: 10.1016/j.ejphar.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin X, Liu J, Liu W. Early ischemic blood brain barrier damage: a potential indicator for hemorrhagic transformation following tissue plasminogen activator (tpa) thrombolysis? Curr Neurovasc Res. 2014;11:254–262. doi: 10.2174/1567202611666140530145643. [DOI] [PubMed] [Google Scholar]
- 6.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi YK, Cho GS, Hwang S, Kim BW, Lim JH, Lee JC, Kim HC, Kim WK, Kim YS. Methyleugenol reduces cerebral ischemic injury by suppression of oxidative injury and inflammation. Free Radic Res. 2010;44:925–935. doi: 10.3109/10715762.2010.490837. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Moon KD, Oh SY, Kim SP, Lee SR. Ether fraction of methanol extracts of Gastrodia elata, a traditional medicinal herb, protects against kainic acid-induced neuronal damage in the mouse hippocampus. Neurosci Lett. 2001;314:65–68. doi: 10.1016/s0304-3940(01)02296-0. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Lee SR, Moon KD. Ether fraction of methanol extracts of Gastrodia elata, medicinal herb protects against neuronal cell damage after transient global ischemia in gerbils. Phytother Res. 2010;17:909–912. doi: 10.1002/ptr.1246. [DOI] [PubMed] [Google Scholar]
- 10.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 11.Nan Y, Wang S, Jia W. Caspase independent cleavages of TDP-43 generates 35kD fragment that cause apoptosis of breast cancer cells. Biochem Biophys Res Commun. 2018;497:51–57. doi: 10.1016/j.bbrc.2018.01.190. [DOI] [PubMed] [Google Scholar]
- 12.Tan J, Li CY, Li T, et al. Identification of a rat model of focal cerebral infarction by relative body weights and Bederson’s scale scores. Journal of Clinical Rehabilitative Tissue Engineering Research. 2011;15:6875–6878. [Google Scholar]
- 13.Muir KW, Tyrrell P, Sattar N, Warburton E. Inflammation and ischaemic stroke. Curr Opin Neurol. 2007;20:334–342. doi: 10.1097/WCO.0b013e32813ba151. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Chen G, Zeng S. Distribution and metabolism of gastrodin in rat brain. J Pharm Biomed Anal. 2008;46:399–404. doi: 10.1016/j.jpba.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Lin LC, Chen YF, Lee WC, Wu YT, Tsai TH. Pharmacokinetics of gastrodin and its metabolite p-hydroxybenzyl alcohol in rat blood, brain and bile by microdialysis coupled to LC-MS/MS. J Pharm Biomed Anal. 2008;48:909–917. doi: 10.1016/j.jpba.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 17.Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–51. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng Z, Wang S, Chen G, Cai M, Liu R, Deng J, Liu J, Zhang T, Tan Q, Hai C. Gastrodin alleviates cerebral ischemic damage in mice by improving anti-oxidant and anti-inflammation activities and inhibiting apoptosis pathway. Neurochem Res. 2015;40:661–673. doi: 10.1007/s11064-015-1513-5. [DOI] [PubMed] [Google Scholar]
- 19.Liu B, Li F, Shi J, Yang D, Deng Y, Gong Q. Gastrodin ameliorates subacute phase cerebral ischemia-reperfusion injury by inhibiting inflammation and apoptosis in rats. Mol Med Rep. 2016;14:4144–4152. doi: 10.3892/mmr.2016.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim OJ, Hong SH, Oh SH, Kim TG, Min KT, Oh D, Kim NK. Association between VEGF polymorphisms and homocysteine levels in patients with ischemic stroke and silent brain infarction. Stroke. 2011;42:2393–2402. doi: 10.1161/STROKEAHA.110.607739. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


