Abstract
Background: Obesity is a chronic metabolic disease characterized by excess fat accumulation. Disordered differentiation of preadipocytes is the leading cause of adipogenesis. Thus, a clarification of the molecular mechanisms that dominate adipocyte differentiation is imperative. MiR-30a-5p is reported to involve in the modulation of multiple cellular processes, including differentiation, whereas, the role of miR-30a-5p in adipocyte differentiation is still unclear. Methods: The abundances of miR-30a and Sirtuin 1 (SIRT1) mRNA were detected by RT-qPCR. SIRT1, PPARγ, C/EBPα, and FABP4 protein levels were assessed by western blot (WB). The accumulation of triglyceride (TG) was detected using Triglyceride Content Assay Kit. Cell proliferation activity was evaluated using the MTT assay. Bioinformatics software and the luciferase reporter assay were used to validate the true interaction between miR-30a-5p and SIRT1. Results: miR-30a-5p expression remains increased during adipocyte differentiation of 3T3-L1 cells. The overexpression of miR-30a-5p enforced adipocyte differentiation, reflected by the enrichment of PPARγ, C/EBPα, FABP4, and triglyceride, as well as the reduction of cell proliferation. SIRT1 was identified as a target of miR-30a-5p, and a supplement of SIRT1 suppressed 3T3-L1 cell differentiation. Conclusion: miR-30a-5p regulated 3T3-L1 cell differentiation by targeting STRT1, supporting the viewpoint that miR-30a-5p might function as a novel therapeutic target for obesity.
Keywords: Obesity, miR-30a-5p, Sirtuin 1, adipocyte differentiation
Introduction
Obesity, characterized by excessive fat accumulation, is defined as a body mass index (BMI) of 30 kg/m2 or greater [1,2]. With the alteration of people’s lifestyle and diet structure, obesity has reached an epidemic scale around the world and resulted in an increasing risk of life-threatening diseases, like type 2 diabetes, steatohepatitis, atherosclerosis, as well as specific types of cancer [3-6]. Statistically, the global incidence of obesity has roughly doubled from 1980 to 2014 [7]. Preadipocyte differentiation is at the core of obesity development. Currently, certain key factors dominating the terminal differentiation of preadipocytes into adipocytes have been identified. Peroxisome proliferator-activated receptors (PPARs) and CCAAT/enhancer-binding proteins (C/EBPs) are types of transcription factors that modulate adipocyte development, differentiation, and metabolism [8,9]. Fatty binding proteins (FABPs), widely known as the molecular chaperone of free fatty acid (FFA), are highly expressed in adipocytes and adipose tissue [10]. However, the exact mechanism controlling preadipocytes towards adipocyte differentiation remains to be clarified.
MicroRNAs (miRNAs) are a subclass of short, conservative, non-protein coding RNA, an estimated 21-23 nucleotides long [11]. In the human genome, up to 30% of genes can be negatively regulated by miRNAs through complementary binding sites between the miRNA seed sequence and the 3’ untranslated region (3’UTR) of target mRNA, leading to the degradation or translation inhibition of the functional gene [12,13]. A large number of studies have confirmed the involvement of miRNAs in various physiological and pathological processes, containing adipogenesis [14]. Expression profile analysis during preadipocyte differentiation shows the alterations of a series of miRNAs, including miR-10b, 101, 143, 34c, 98, and let-7b [15], indicating that adipocyte miRNAs may provide a possible method for the analysis of adipose tissue function. MiR-30a-5p, a widely known functional miRNA, is implicated in the occurrence and development of multiple biological contexts. In cancers, miR-30a-5p mainly functions as a tumor suppressor due to its alleviation of cell malignant phenotypes [16,17]. Moreover, miR-30a-5p is dysregulated in nervous system and renal diseases [18,19]. In recent years, the role of miR-30a-5p in cellular differentiation has been generally illuminated [20,21]. Here, we focus on the function and mechanism of miR-30a-5p in adipocyte proliferation and differentiation.
MiR-30a-5p levels increase progressively during the differentiation of preadipocytes. The overexpression of miR-30a-5p enforces the abundances of adipocyte markers PPARγ, C/EBPα, and FABP4, and induces the production of triglyceride, while it weakens cell proliferation. In preadipocytes, STAT1 is negatively regulated by miR-30a-5p. An enhanced abundance of SIRT1 attenuates adipocyte differentiation, which is just the opposite of the role of miR-30a-5p. These findings suggest that miR-30a-5p may serve as a promoter of adipogenesis, partly by directly targeting SIRT1.
Materials and methods
Cell culture and differentiation
3T3-L1 cells obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% Newborn calf serum (NCS) (Gibco, Grand Island, NY, USA), 2 mM L-Glutamax (Gibco), and antibiotics (Solarbio, Beijing, China). To stimulate adipogenesis, the cells were further incubated, allowing them to reach confluence in the designated 2 days. At this point (Day 0), the confluent cells were switched into a differentiation medium (DM), consisting of DMEM with 10% newborn calf serum, 10 µg/ml insulin, 0.5 mM 3-isobutyl-l-methylxanthine (IBMX), and 2.5 µM Dexamethasone (DEX). About 2 days after incubation, IBMX and DEM in the DM medium were omitted and the cells were grown in an insulin-containing medium for an additional 2 days. Then, the insulin was further removed, and the cells were cultured for an additional 4 days. 293T cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and cultured in DMEM medium in the presence of 10% fetal bovine serum (FBS; Gibco) and antibiotics.
Plasmid and cell transfection
miR-30a-5p mimics (miR-30a-5p) and a negative control (NC), an miR-30a-5p inhibitor (Anti-miR-30a-5p) and an inhibitor control (anti-NC), Sirtuin 1 (SIRT1)-overexpression lentivirus plasmid (SIRT1), short hairpin RNA for SIRT1 (shSIRT1) and a scrambled control (Scramble) were obtained from Genepharma Co., Ltd (Shanghai, China). 3T3-L1 cells were seeded into 6-well plates and transiently transfected with miRNAs or plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) referring to the manufacturer’s protocols. About 2 days after transfection, the medium was switched to induce adipogenesis.
Reverse-transcription quantification PCR (RT-qPCR)
Total RNA was isolated from 3T3-L1 cells using Trizol reagent (Thermo Fisher Scientific, Wilmington, DE, USA) in accordance with the manufacturer’s instructions, followed by an analysis of the RNA’s purity using Nanodrop 2000 (Thermo Fisher Scientific). 1 µg RNA was reversely transcribed into cDNA for miR-30a-5p or SIRT1 using a TaqManTM MicroRNA Reverse Transcription kit (Thermo Fisher Scientific) or a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Then, a qPCR reaction was performed using a SYBR Green Real-Time PCR Master Mixes (Thermo Fisher Scientific) and run on an ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The results were evaluated by the 2-ΔΔCt method independently in triplicate with U6 small nuclear RNA (snRNA) or GAPDH as a housekeeping gene for miR-30-5p or SIRT1. All the primers were obtained from Invitrogen: the RT primer for miR-30a-5p (5’-CTCAACTGGTGTCGTGGAGTCG GCAATTCAGTTGAGCTTCCAGT-3’). The qPCR primers: miR-30a-5p (Forward, 5’-ACTCAGCTGGTGTAAACATCCTCGAC-3’; Reverse, 5’-TGGTGTCGTGGAGTCG-3’), SIRT1 (Forward, 5’-CAAAGGAGCAGATTAGTAGGCG-3’; Reverse, 5’-CTCTGGCATGTCCCACTATCAC-3’), U6 snRNA (Forward, 5’-CTCGCTTCGGCAGCACA-3’; Reverse, 5’-AACGCTTCACGAATTTGCGT-3’), GAPDH (Forward, 5’-TATGATGATATCAAGAGGGTAGT-3’; Reverse, 5’-TGTATCCAAACTCATTGTCATAC-3’).
Western blot (WB)
The 3T3-L1 cells were lysed in a RIPA lysis buffer (Thermo Fisher Scientific) containing proteinase inhibitor (Solarbio). Equal amounts of protein extracts were loaded on the SDS-PAGE gel and electro-transferred onto polyvinylidene fluoride (PVDF) membranes. After being blocked with 5% nonfat milk, the membranes were incubated with primary antibodies against PPARγ (1:1000; Abcam, Cambridge, MA, USA), C/EBPα (1:1000; Abcam), FABP4 (1:2000; Abcam), and SIRT1 (1:1000; Abcam) overnight at 4°C, with anti-GAPDH (1:2500; Abcam) as a loading control. Then the membranes were further hatched with a horseradish peroxidase (HRP)-conjugated secondary antibody (1:5000; Abcam) for 1 h at 37°C. The protein blots were visualized using the enhanced chemiluminescence system (Roche, Basel, Switzerland), and quantified by densitometry using the Image-Pro Plus software (Bio-Rad, Hercules, CA, USA).
Triglyceride (TG) assay
The transfected 3T3-L1 cells were stimulated for differentiation for 8 days in a DM medium. Then the cells were harvested using a lysis buffer and homogenized by sonication. The quantification of TG was determined using a Triglyceride Content Assay Kit (Solarbio) in accordance with the protocol provided by manufacturer. The results were normalized to the total protein determined by the BCA Assay.
MTT assay
Cell proliferation viability was evaluated using the MTT assay. 3T3-L1 cells transfected with NC, miR-30-5p, or anti-miR-30-5p were seeded into 96-well plates at a density of 5 × 103 cells/well. At different periods after transfection, the medium was removed and 0.5 mg/mL of MTT reagent (Sigma, St. Louis, MO, USA) was introduced for an additional 4 h, followed by the addition of dimethyl sulfoxide (DMSO) to dissolve formazan. The absorbance at 490 nm was measured with a microplate reader (Bio-Rad, Hercules, CA, USA).
Luciferase reporter assay
Partial cDNA sequences corresponding to the 3’-UTR of SIRT1 were amplified and inserted into the psiCHECK-2 luciferase reporter vector (Promega, Madison, WI, USA), also called a wide-type SIRT1 (SIRT1-wt) reporter. Then, the putative binding sites in SIRT1-wt reporter were mutated using a KOD-plus-mutagenesis kit (Toyobo, Osaka, Japan) to generate a mutant SIRT1 (SIRT1-mut) reporter. Then a Wt or mut luciferase reporter plasmid was transfected into miR-30a-5p-overexpressed 293T cells. At 48 h post-transfection, the cells were harvested to detect the luciferase reporter activity using the Dual-Luciferase reporter assay system (Promega).
Statistical analysis
The significant differences between the groups were assessed using Student’s t-test or ANOVA using SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). The quantitative data among the groups were expressed as the mean ± standard deviation (SD) from three independent experiments. P < 0.05 was considered statistically significant.
Results
miR-30a-5p showed a significant upregulation during adipocyte differentiation
To investigate the role of miR-30a-5p in preadipocyte differentiation, we initially identified the changes of miR-30a-5p in 3T3-L1 cells during adipogenesis. By RT-qPCR, we demonstrated that miR-30a-5p levels increased progressively until the end point of differentiation (Figure 1). Thus, we hypothesized that miR-30a-5p might be a major modulator of adipogenesis.
Figure 1.
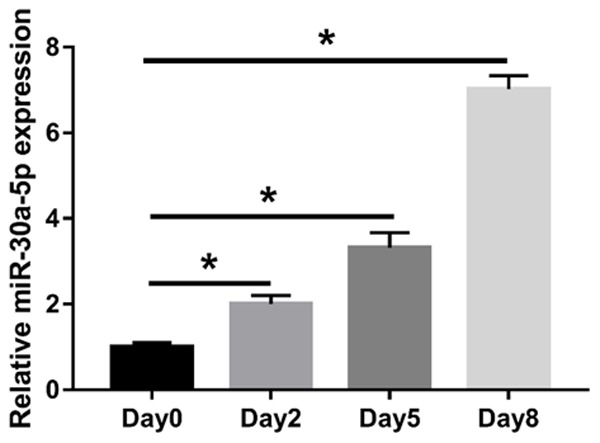
Enhanced abundance of miR-30a-5p during adipocyte differentiation of 3T3-L1 cells. 3T3-L1 cells were stimulated to differentiate two days post confluence (Day 0). RT-qPCR was performed to quantify miR-30a-5p at the designated time points of the differentiation. *P < 0.05, compared with Day 0 group.
miR-30a-5p stimulated the adipocyte differentiation of the 3T3-L1 cells
To further explore the role of miR-30a-5p in the adipocyte differentiation of 3T3-L1 cells, miR-30a-5p was transfected into 3T3-L1 cells to overexpress miR-30a-5p (Figure 2A). After about 8 days of differentiation, the cells were harvested for the detection of PPARγ, C/EBPα, and FABP4 proteins by WB. The results revealed that the expressions of these three proteins were obviously elevated in miR-30a-5p-overexpressed 3T3-L1 cells compared to the NC group (Figure 2B-E). Likewise, the accumulation of TG in the 3T3-L1 cells transfected with miR-30a-5p was also increased relative to the NC group (Figure 2F). These findings indicated that miR-30a-5p might function as an activator of adipogenesis in 3T3-L1 cells.
Figure 2.
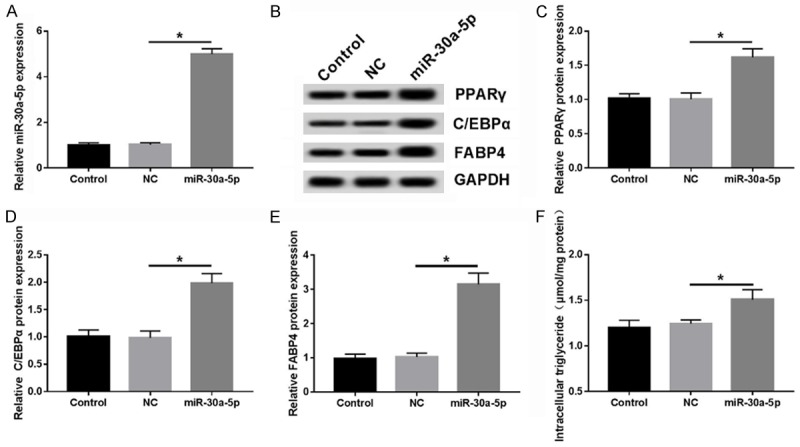
miR-30a-5p promotes adipocyte differentiation of 3T3-L1 cells. A. The abundance of miR-30a-5p in 3T3-L1 cells transfected with NC or miR-30a-5p was measured by RT-qPCR. B-E. The expressions of the PPARγ, C/EBPα, and FABP4 proteins were determined by WB at the end points of differentiation (Day 8). F. The accumulation of TG was determined using the TG Content Assay Kit and relative to the total protein. *P < 0.05, compared with NC group.
miR-30a-5p inhibition attenuated the adipocyte differentiation of the 3T3-L1 cells
Here, we further explored the role of miR-30a-5p inhibition in preadipocyte differentiation. The introduction of the miR-30a-5p inhibitor in the 3T3-L1 cells markedly lowered the expression of miR-30-5p in the 3T3-L1 cells (Figure 3A). Following the loss-of-function analysis that described the abundances of the PPARγ, C/EBPα, and FABP4 proteins, we found that the accumulation of TG was decreased in the miR-30a-5p-reduced 3T3-L1 cells compared to the anti-NC group (Figure 3B-F), pointing out that knockdown of miR-30a-5p inhibited the adipocyte differentiation of the 3T3-L1 cells, which is fully the inverse of the impacts of miR-30a-5p overexpression.
Figure 3.
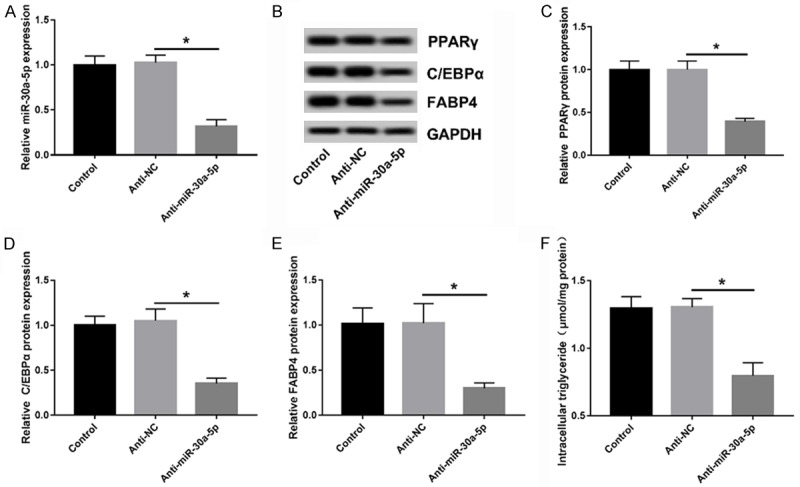
Reduction of miR-30-5p suppresses the adipocyte differentiation of 3T3-L1 cells. A. miR-30a-5p expression in 3T3-L1 cells transfected with anti-NC or anti-miR-30a-5p was measured by RT-qPCR. B-E. Analyses of PPARγ, C/EBPα, and FABP4 protein levels by WB at 8 days after the beginning time of differentiation. F. The quantification of TG was assessed using a TG Content Assay Kit. *P < 0.05, compared with the anti-NC group.
miR-30a-5p receded the 3T3-L1 cell proliferation
The proliferation and differentiation of adipocytes is the basis for the accumulation of lipids in adipose tissues [21]. To probe whether miR-30a-5p affects adipocyte proliferation, 3T3-L1 cells were transiently transfected with miR-30a-5p mimics or an inhibitor. The results showed that miR-30a-5p expression increased about 5-fold in the mimics group, while it decreased about 3-fold in the inhibitor group, as compared to the negative controls (Figure 4A). An MTT assay showed that the proliferation activity kept increasing in a time-dependent manner (Figure 4B). Compared with the NC and the anti-NC groups, miR-30a-5p overexpression was inhibited, while the knockdown induced cell proliferation in the 3T3-L1 cells (Figure 4B). In sum, miR-30a-5p might be a suppressor of 3T3-L1 cell proliferation.
Figure 4.
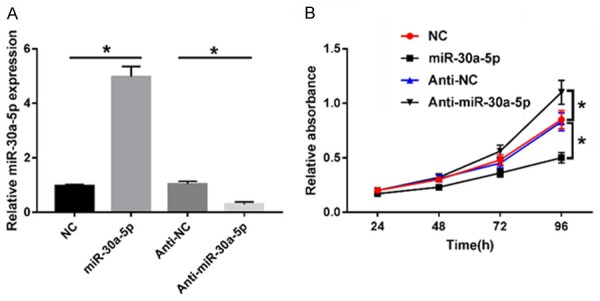
miR-30a-5p promoted the adipocyte proliferation of 3T3-L1 cells. A. Alteration of miR-30a-5p in 3T3-L1 cells transfected with NC, miR-30a-5p, anti-NC, Anti-miR-30a-5p was determined by RT-qPCR. B. Proliferation activity of 3T3-L1 cells in each group was evaluated by MTT assay. *P < 0.05, compared with the NC or the anti-NC group.
SIRT1 targetedly bound to miR-30a-5p
To clarify the potential mechanism underlying how miR-30a-5p is involved in the modulation of adipocyte differentiation in 3T3-L1 cells, the online software program Targetscan was used to predict the potential target genes of miR-30a-5p. Among the predicted target genes, SIRT1, containing the complementary sites of miR-30a-5p in 3’UTR, was selected due to its indicated function in lipometabolism [22] (Figure 5A). To confirm the true interaction between miR-30a-5p and SIRT1, a SIRT1 luciferase reporter (SIRT1-wt or SIRT1-mut) containing the wild-type or mutant miR-30a-5p binding sites, was transfected into 293T cells along with miR-30a-5p or NC. Indeed, the miR-30a-5p introduction resulted in a lowered luciferase activity of SIRT1-wt construct relative to the NC group, but there were no significant changes in the SIRT1-mut reporter (Figure 5B). Moreover, the abundances of SIRT1 mRNA and protein in the 3T3-L1 cells under the action of the miR-30a-5p mimics or the inhibitor were measured by RT-qPCR or WB. As the results show in Figure 5C and 5D, miR-30a-5p negatively regulated the expression of SIRT1 at the mRNA or protein level. These results suggest that SIRT1 is a potential target of miR-30a-5p.
Figure 5.
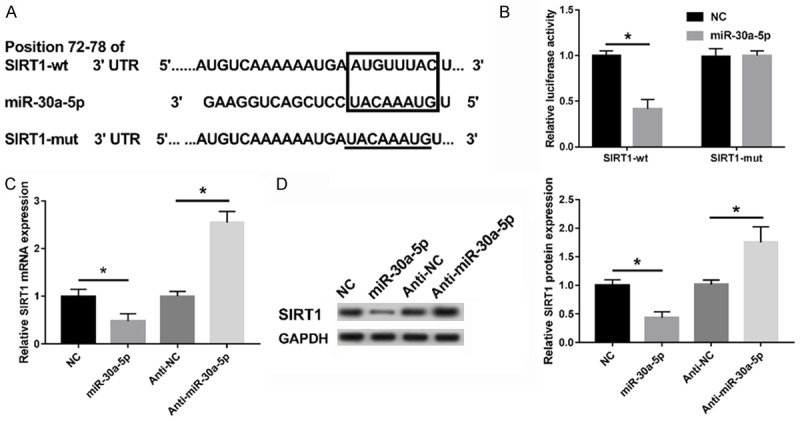
SIRT1 was targeted by miR-30a-5p. A. The putative binding sites of miR-30a-5p in the 3’UTR of SIRT1 was predicted using the online software program Targetscan, as well as the mutant in SIRT1 3’UTR. B. Luciferase reporter assay revealed the inhibitory effect of miR-30a-5p on the activity of SIRT1. C, D. The expressions of SIRT1 mRNA and protein in the miR-30a-5p-overexpressed or inhibited 3T3-L1 cells were detected by RT-qPCR or WB assay. *P < 0.05, compared with the NC or the anti-NC group.
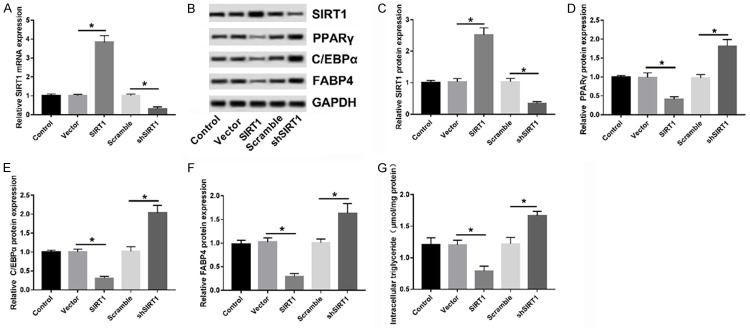
SIRT1 repressed the differentiation of the 3T3-L1 adipocytes
In the present study, a gain and loss-of function assay was performed to investigate the role of SIRT1 in adipocyte differentiation. 3T3-L1 cells were introduced with SIRT1 or shSIRT1 to overexpress or inhibit SIRT1, with a vector or scramble-transfection group as a control (Figure 6A). Next, the protein levels of PPARγ, C/EBPα, and FABP4 in each group were detected at the end stage of differentiation by WB. Expectedly, the expressions of these three proteins were notably downregulated in SIRT1-overexpressed 3T3-L1 cells but upregulated in SIRT1-inhibited 3T3-L1 cells compared to relative controls (Figure 6B-F). Similarly, the overexpression or knockdown of SIRT1 also reduced or promoted TG production (Figure 6G), indicating the negative regulation of SIRT1 in 3T3-L1 cell differentiation.
Figure 6.
SIRT1 inhibited adipocyte differentiation of 3T3-L1 cells. A. The expression levels of SIRT1 mRNA in 3T3-L1 cells transfected with Vector, SIRT1, Scramble, and shSIRT1 were examined by RT-qPCR. B-F. Analyses of the PPARγ, C/EBPα, and FABP4 proteins by WB at 8 days after the start of differentiation. G. The quantification of TG was performed using the TG Content Assay Kit. *P < 0.05, compared with Vector or Scramble group.
Discussion
Obesity is a common chronic disease worldwide and leads to a grievous threat to public health. Thus, clarifying the mechanism and proposing novel molecular events that are associated with adipocyte differentiation is essential for the development of anti-obesity drugs. In the present study, we demonstrated that miR-30a-5p is kept highly expressed in 3T3-L1 cells during adipogenesis. Gain and loss-of function analyses revealed that miR-30a-5p stimulated cell differentiation but inhibited the proliferation in 3T3-L1 cells. Further study of this mechanism indicated a targeted interaction between miR-30a-5p and SIRT1 by the complementary binding sites. In contrast with miR-30a-5p, SIRT1 exhibited an inhibitory effect on adipocyte differentiation.
Differentiation is considered to be a key process in the transformation of preadipocytes to mature adipocytes. A series of alterations in gene expression are required for adipocyte differentiation in preadipocytes [23]. A growing number of studies have indicated that miRNAs play a key role in the regulation of adipogenesis. As reported by Chen et al., highly expressed miR-146 in mature adipocytes can inhibit cell proliferation and induce differentiation in visceral preadipocytes by directly targeting the Kruppel-like transcription factor KLF7 [24]. Zhang et al. suggested that the activation of miR-140-5p by C/EBPα induces the converting of stromal cells and preadipocyte into mature adipocytes by targetedly binding the transforming growth factor-β receptor I (Tgfbr1) [25]. Moreover, lysine-specific demethylase 6b (Kdm6b) targeted by miR-20a inhibited adipocyte differentiation in 3T3-L1, ST2 and C3H10T1/2 cells [26]. In contrast, miR-139-5p was identified to be a suppressor of adipocyte differentiation in 3T3-L1 cells, which was reflected by the decreased expression of the adipogenesis-related genes PPARγ, aP2, and FAS [27]. Enforced expression of miR-195a retarded the accumulation of lipid and reduced the expression of the adipocyte markers PPARγ and aP2 both in 3T3-L1 and C3H10T1/2 cells by interacting with Zfp423 [28].
MiR-30a-5p, a member of the miR-30 family with 22 bp in length, is highly conserved in humans. Up to now, a large number of studies have proposed the role of the miR-30 family in several types of cancers, including glioma [29], liver caner [30], non-small cell lung cancer (NSCLC) [31], and breast cancer [32]. In recent years, a possible involvement of the miR-30 family in adipogenesis has also been documented. MiR-30c may function as a promoter of adipogenesis by enforcing the expression of adipocyte markers and inducing lipid accumulation [33]. This hypothesis is further demonstrated by Karbiener, who proposed that miR-30c stimulates adipocyte differentiation by co-interacting with PAI-1 and ALK2 [34]. Additionally, the miR-30 family exerts a gradual upregulation during adipogenic differentiation, and the enforced expression of miR-30a and miR-30d contributes to the differentiation of adipose tissue-derived stem cells towards mature adipocytes [35]. In line with the above studies, we also observed an elevated expression of miR-30a-5p and further confirmed the stimulatory effect of miR-30a-5p on adipocyte differentiation in 3T3-L1 cells, which was reflected by the enrichment of PPARγ, C/EBPα, FABP4, as well as the increased accumulation of TG.
SIRT1 is a mammalian homologue of the silent information regulator 2 (Sir2), mainly localized in the nucleus. It is convincing that SIRT1 seems to be a major mediator of cellular metabolism by catalyzing NAD-dependent protein deacetylation. In differentiated fat cells, a supplement of Sirt1 derives lipodieresis and fat loss, and the reduction of fat is enough to prolong the murine life-span [36]. SIRT1 is downregulated during preadipocyte differentiation, and the knockdown of SIRT1 stimulates adipogenesis and insulin sensitivity in obesity by the induction of the activity of transcription factor PPARγ [37]. Enhanced Sirt1 activity in Sirt7-/- mice weakens adipocyte differentiation, thereby reducing lipid accumulation [38]. In view of the implication of SIAT1 in adipogenesis, we hypothesized that miR-30a-5p might trigger adipocyte differentiation by inactivating SIRT1. A bioinformatic prediction and luciferase reporter assay validated the true interaction between miR-30a-5p and SIRT1 by direct binding. A gain and loss-of function assay revealed that overexpression of SIRT1 impaired adipocyte differentiation, and SIRT1 interference enhanced it.
Conclusion
In conclusion, miR-30a-5p is gradually upregulated during adipocyte differentiation. The high abundance of miR-30a-5p derives cell differentiation and diminishes proliferation in preadipocytes by directly targeting SIRT1. These findings indicate that targeting the miR-30a/SIRT1 axis might be an effective therapeutic avenue for obesity and obesity-related diseases.
Acknowledgements
This study was supported by the National Natural Science Foundation of China, Youth Foundation (No. 81502828).
Disclosure of conflict of interest
None.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Raatikainen K, Heiskanen N, Heinonen S. Transition from overweight to obesity worsens pregnancy outcome in a BMI-dependent manner. Obesity (Silver Spring) 2006;14:165–171. doi: 10.1038/oby.2006.20. [DOI] [PubMed] [Google Scholar]
- 3.Bril F, Ortiz-Lopez C, Lomonaco R, Orsak B, Freckleton M, Chintapalli K, Hardies J, Lai S, Solano F, Tio F. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Intl. 2015;35:2139–2146. doi: 10.1111/liv.12840. [DOI] [PubMed] [Google Scholar]
- 4.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw PT, Monda KL, Stevens J. Metabolic syndrome in healthy obese, overweight, and normal weight individuals: the atherosclerosis risk in communities study. Obesity. 2013;21:203–209. doi: 10.1002/oby.20248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendis S, Davis S, Norrving B. Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Storke. 2015;46:e121–122. doi: 10.1161/STROKEAHA.115.008097. [DOI] [PubMed] [Google Scholar]
- 8.Christodoulides C, Vidalpuig A, Stephens J, Harris R. PPARs and adipocyte function. Mol Cellular Endocrinol. 2010;318:61–68. doi: 10.1016/j.mce.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Cho YW, Hong SH, Jin Q, Wang L, Lee JE, Gavrilova O, Ge K. Histone methylation regulator PTIP is required for PPARγ and C/EBPα expression and adipogenesis. Cell Metab. 2009;10:27–39. doi: 10.1016/j.cmet.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang TS, Todorčević M, Ruyter B, Torstensen BE. Altered expression of CCAAT/enhancer binding protein and FABP11 genes during adipogenesis in vitro in atlantic salmon (salmo salar) Aquaculture Nutrition. 2010;16:72–80. [Google Scholar]
- 11.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 12.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, Lander ES, Kellis M. Systematic discovery of regulatory motifs in human promoters and 3’ UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guixian S, Guangfeng X, Chenbo J, Chunmei S, Yahui S, Ling C, Lijun Z, Lei Y, Yaping Z, Xirong G. The role of microRNA-26b in human adipocyte differentiation and proliferation. Gene. 2014;533:481–487. doi: 10.1016/j.gene.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Kajimoto K, Naraba H, Iwai N. MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA. 2006;12:1626–1632. doi: 10.1261/rna.7228806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baraniskin A, Birkenkampdemtroder K, Maghnouj A, Zöllner H, Munding J, Kleinscory S, Reinacherschick A, Schwartewaldhoff I, Schmiegel W, Hahn SA. MiR-30a-5p suppresses tumor growth in colon carcinoma by targeting DTL. Carcinogenesis. 2012;33:732–739. doi: 10.1093/carcin/bgs020. [DOI] [PubMed] [Google Scholar]
- 17.He R, Yang L, Lin X, Chen X, Lin X, Wei F, Liang X, Luo Y, Wu Y, Gan T. MiR-30a-5p suppresses cell growth and enhances apoptosis of hepatocellular carcinoma cells via targeting AEG-1. Int J Clin Exp Pathol. 2015;8:15632–15641. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Zhang C, Chen H, Li L, Tu Y, Liu C, Shi S, Zen K, Liu Z. Evaluation of microRNAs miR-196a, miR-30a-5P, and miR-490 as biomarkers of disease activity among patients with FSGS. Clin J Am Soc Nephrol. 2014;9:1545–1552. doi: 10.2215/CJN.11561113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croce N, Gelfo F, Ciotti MT, Federici G, Caltagirone C, Bernardini S, Angelucci F. NPY modulates miR-30a-5p and BDNF in opposite direction in an in vitro model of Alzheimer disease: a possible role in neuroprotection? Mol Cell Biochem. 2013;376:189–195. doi: 10.1007/s11010-013-1567-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang BW, Cai HF, Wei XF, Sun JJ, Lan XY, Lei CZ, Lin FP, Qi XL, Martin P, Hong C. miR-30-5p regulates muscle differentiation and alternative splicing of muscle-related genes by targeting MBNL. Int J Mol Sci. 2016;17:182. doi: 10.3390/ijms17020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Zhang F, Shugart YY, Yang L, Li X, Liu Z, Sun N, Yang C, Guo X, Shi J. The early growth response protein 1-miR-30a-5p-neurogenic differentiation factor 1 axis as a novel biomarker for schizophrenia diagnosis and treatment monitoring. Transl Psychiatry. 2017;7:e998. doi: 10.1038/tp.2016.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Ann Med. 2011;43:198–211. doi: 10.3109/07853890.2010.547211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Dai YM, Ji CB, Yang L, Shi CM, Xu GF, Pang LX, Huang FY, Zhang CM, Guo XR. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol Cell Endocrinol. 2014;393:65–74. doi: 10.1016/j.mce.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Chang A, Li Y, Gao Y, Wang H, Ma Z, Li X, Wang B. miR-140-5p regulates adipocyte differentiation by targeting transforming growth factor-β signaling. Sci Rep. 2015;5:18118. doi: 10.1038/srep18118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Guo F, Wang G, Wang J, Zheng F, Guan X, Chang A, Zhang X, Dai C, Li S, Li X, Wang B. miR-20a regulates adipocyte differentiation by targeting lysine-specific demethylase 6b and transforming growth factor-β signaling. Int J Obes. 2015;39:1282–1291. doi: 10.1038/ijo.2015.43. [DOI] [PubMed] [Google Scholar]
- 27.Mi L, Chen Y, Zheng X, Li Y, Zhang Q, Mo D, Yang G. MicroRNA-139-5p suppresses 3T3-L1 preadipocyte differentiation through notch and IRS1/PI3K/Akt insulin signaling pathways. J Cell Biochem. 2015;116:1195–1204. doi: 10.1002/jcb.25065. [DOI] [PubMed] [Google Scholar]
- 28.Yun UJ, Song NJ, Yang DK, Kwon SM, Kim K, Kim S, Jo DG, Park WJ, Park KW, Kang H. MiR-195a inhibits adipocyte differentiation by targeting the preadipogenic determinator Zfp423. J Cell Biochem. 2015;116:2589–2597. doi: 10.1002/jcb.25204. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Wang K, Han L, Zhang A, Shi Z, Zhang K, Zhang H, Yang S, Pu P, Shen C. PRDM1 is directly targeted by miR-30a-5p and modulates the Wnt/β-catenin pathway in a Dkk1-dependent manner during glioma growth. Cancer Lett. 2013;331:211–219. doi: 10.1016/j.canlet.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Li WF, Dai H, Ou Q, Zuo GQ, Liu CA. Overexpression of microRNA-30a-5p inhibits liver cancer cell proliferation and induces apoptosis by targeting MTDH/PTEN/AKT pathway. Tumour Biol. 2015;37:5885–5895. doi: 10.1007/s13277-015-4456-1. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, Zeng Y, Li W, Qin H, Lei Z, Shen D, Gu D, Huang JA, Liu Z. CD73/NT5E is a target of miR-30a-5p and plays an important role in the pathogenesis of non-small cell lung cancer. Mol Cancer. 2017;16:34. doi: 10.1186/s12943-017-0591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Kang L, Zhao W, Feng Y, Liu W, Wang T, Mai H, Huang J, Chen S, Liang Y. miR-30a-5p suppresses breast tumor growth and metastasis through inhibition of LDHA-mediated Warburg effect. Cancer Lett. 2017;400:89–98. doi: 10.1016/j.canlet.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 33.Irani S, Hussain MM. Role of microRNA-30c in lipid metabolism, adipogenesis, cardiac remodeling and cancer. Curr Opin Lipidol. 2015;26:139–146. doi: 10.1097/MOL.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 34.Karbiener M, Neuhold C, Opriessnig P, Prokesch A, Bognerstrauss JG, Scheideler M. MicroRNA-30c promotes human adipocyte differentiation and co-represses PAI-1 and ALK2. RNA Biol. 2011;8:850–860. doi: 10.4161/rna.8.5.16153. [DOI] [PubMed] [Google Scholar]
- 35.Zaragosi LE. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011;12:R64. doi: 10.1186/gb-2011-12-7-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picard F, Kurtev M, Chung N, Toparkngarm A, Senawong T, Oliveira RMD, Leid M, Mcburney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayoral R, Osborn O, Mcnelis J, Johnson AM, Oh DY, Izquierdo CL, Chung H, Li P, Traves PG, Bandyopadhyay G. Adipocyte SIRT1 knockout promotes PPARγ activity, adipogenesis and insulin sensitivity in chronic-HFD and obesity. Mol Metab. 2015;4:378–391. doi: 10.1016/j.molmet.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang J, Ianni A, Smolka C, Vakhrusheva O, Nolte H, Krüger M, Wietelmann A, Simonet NG, Adriansegarra JM, Vaquero A. Sirt7 promotes adipogenesis in the mouse by inhibiting autocatalytic activation of Sirt1. Proc Natl Acad Sci U S A. 2017;114:E8352–E8361. doi: 10.1073/pnas.1706945114. [DOI] [PMC free article] [PubMed] [Google Scholar]