Abstract
Considered as true helper cells for B cells in antibody response, Tfh cells are associated with inflammation and immune abnormality. Acute pancreatitis is an acute abdominal disease characterized by inflammatory response and immune disorder. Thus, our objective was to study the frequency of circulating Tfh cells, together with the Tfh cell-related CD4+ T cells and inflammatory factors in patients with acute pancreatitis. We first examined the frequency of circulating Tfh cell subsets by detecting the expression of CXCR5, PD-1, ICOS and IL-21 by flow cytometry analysis. Then we investigated the abundance of helper T cells and Treg cells. In addition, the plasma level of IgA, IgM, and Tfh cell-related inflammatory factor were detected by cytometric bead array. We showed that the frequency of circulating Tfh cells increased significantly in patients of acute pancreatitis, including CD4+CXCR5+ cells, CD4+CXCR5+PD-1+ cells, CD4+CXCR5+ICOS+ cells, and CD4+CXCR5+IL-21+ cells. Also, increases in plasma IL-1, IL-6, IL-8, IL-17, IL-21 and IgA were observed in patients with acute pancreatitis compared to HCs. This finding indicates that Tfh cells play a vital role in the development and progression of acute pancreatitis that is dependent on IL-6 and IL-21.
Keywords: Acute pancreatitis, AP, Tfh cell, CXCR5
Introduction
Acute pancreatitis (AP) is an acute abdominal disease characterized by pancreatic enzyme activation and a localized inflammatory response. Risk factors for AP include gallstones, alcoholism, and hyperlipemia, while the clinical manifestations of pancreatitis vary with each individual. Most patients initially suffer from upper abdominal pain, followed by nausea, vomiting, fever, and other symptoms. Due to its various etiologies, clinical features, and potential to progress to severe acute pancreatitis (SAP), this condition presents high mortality and a poor prognosis [1,2]. Faced with the complex pathogenesis of pancreatitis, many studies have provided explanations for the condition that include NF-κB activation, IL-6 trans-signaling, hyperglycemia, and intestinal immune suppression [3-7]. In addition, CD4+ T cell-related immune responses are emerging as a possibility for the development of pancreatitis. Immunosuppression occurs during the early phase of SAP, and CD4+ lymphocyte counts fall below the normal range [8,9]. However, Zheng et al. [10] found that nicotine reduces the mortality of people with AP by upregulating the number and suppressive capacity of CD4+CD25+ Treg cells.
Follicular B-helper T cells or T-follicular helper (Tfh) cells, localize within active germinal centers (GCs) and the peripheral blood circulation. Unlike other effector CD4+ T cells, Tfh cells express Bcl-6 and CXCR5. Also, Tfh cells highly express programmed death-1 (PD-1), Inducible costimulatory (ICOS), and IL-21. ICOS is vital important for the initial stage of Tfh cell differentiation, providing a critical early signal to induce the transcription factor Bcl-6. Then, Bcl-6 subsequently induces high CXCR5 expression, which functionally drives Tfh cell migration into B cell follicles in a CXCL13-dependent manner [11-13].
Tfh cells are associated with multiple autoimmune diseases. In patients with immunoglobulin G4-related ophthalmic disease (IgG4-ROD), the expression of Bcl-6 and ICOS is significantly higher and is mainly detected in GCs. Likewise, CXCR5+ Tfh cells were detected in the GCs and tissues in patients with IgG4-ROD and IgG4-related lymphadenopathy (IgG4-RL) [14]. In the peripheral blood of patients with ankylosing spondylitis, CD4+ICOS+ T cells were more prevalent in active cases compared to inactive cases. Indeed, there was a positive correlation between the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the percentage of CD4+CXCR5+ICOS+ T cells [15].
Trypsin activation in the pancreas used to be considered a crucial factor in AP development, but it is now clear that subsequent infection, fluid loss, inflammation, and immune responses may all play important parts in the progression of AP. However, views on what constitutes most to the exacerbation of AP differ. Tfh cells and their secreted factor (IL-21) are vital for B cell differentiation. Also, IL-21 potently induces Th17 differentiation and promotes or sustains Th17 lineage commitment [16,17]. Therefore, we speculated that Tfh cells may play a significant role in the abnormal immunity of SAP patients. However, lymphoid tissues from patients with AP are difficult to obtain, and Morita et al. [18] has shown that CD4+CXCR5+ T cells from peripheral blood are functionally similar to Tfh cells from secondary lymphoid organs. Therefore, our objective was to study the frequency of circulating Tfh cells, together with the Tfh cell-related CD4+ T cells in patients with AP.
Materials and methods
Patients and controls
Patients with AP (n=35) were enrolled from the Department of Emergency or Intensive Care Unit of our hospital during 2017. All patients fulfilled the criteria of acute pancreatitis (2012 Atlanta classification) [1]. Healthy controls (HCs) (n=20) without autoimmune diseases [including but not limited to, systemic lupus erythematosus (SLE), rheumatoid arthritis, and ankylosing spondylitis], benign, and malignant tumors were included. Peripheral blood samples were obtained from all participants and stored at 4°C for at most 4 hours. Plasma was centrifuged and stored at 80°C to measure inflammatory factors. Peripheral blood mononuclear cells (PBMCs) were isolated with density gradient medium for further use.
Ethics approval and consent to participate
All the procedures were implemented based on the principles of the Declaration of Helsinki, and the design of the work was reviewed and approved by the Ethics Committee of our Hospital. All subjects agreed to participate in this study and provided their written informed consents.
Chemicals and reagent
Density gradient medium were purchased from Stemcell technologies. All Cytometric Bead Array (CBA) reagent: (IgA, IgM, IL-1, IL-4, IL-6, IL-8, IL-17, IL-21, IFN-γ) were purchased from BD Pharmingen. Rat anti-human CXCR5, mouse anti-human PD1, mouse anti-human IL-17A, mouse Anti-human IFN-γ were purchased from BD Pharmingen. The CD3 monoclonal antibody, CD4 monoclonal antibody, ICOS monoclonal antibody, fixable viability dye, IL-21 monoclonal antibody, IL-4 monoclonal antibody, FOXP3 monoclonal antibody were eBioscience products purchased from Thermo Fisher Scientific. All corresponding isotype reagents were purchased from the respective companies.
Flow cytometry analysis
PBMCs were washed twice and stained for 30 minutes at 4°C in the dark with the surface marker antibodies or isotype-matched controls. Meanwhile, for the detection of Foxp3, the cells were reacted with fixation/permeabilization solution for 30 minutes at room temperature, washed twice the with permeabilization buffer, then stained with Foxp3 antibody for 30 minutes at room temperature in the dark. In order to detect inflammatory interleukin, the PBMCs were preincubated with Cell Stimulation Cocktail for 4.5 hours in the cell incubator (37°C, 5% CO2). Analyses were performed with the BD LSRFortessaTM X-20. Due to the limited number of PBMC, only 14 AP samples underwent intracellular staining.
Cytometric bead array
The standard materials were prepared in assay diluent. Capture beads and PE fluorescence detection reagent were diluted according to the manufacturer’s instructions. Mixed capture beads and samples were incubated at room temperature in the dark. PE detection reagent was then added; the samples were gently mixed and incubated at room temperature. Then, 1 mL wash buffer was added, and samples were centrifuged for 5 minutes. Finally, the supernatant was discarded, and 300 μL wash buffer was added to the pellet. The results were detected with a BD LSRFortessaTM X-20 and expressed as median fluorescent intensity (MFI).
Statistical analysis
All data are expressed as the mean ± SEM and were compared with Student’s t-tests or one-way ANOVA. P values less than 0.05 were considered significant. Analyses were undertaken with IBM SPSS Statistics (version 19, IBM Corp., USA) and Prism (version 5.0, GraphPad Software, Inc., USA).
Results
Increased frequency of circulating Tfh cells subsets
We first examined the expression of CXCR5, PD-1 and ICOS (the surface markers of Tfh cells) (Figure 1). In AP patients, the CXCR5 was expressed by 8.68±0.60% of CD3+CD4+ cells; and to a lesser extent in healthy adult blood, CXCR5 was expressed by 6.24±0.40% (P < 0.01). Furthermore, an increase of ICOS and PD-1 in CD3+CD4+CXCR5+ cells was observed (0.17±0.01% vs 0.37±0.04%, P < 0.0001; 1.87±0.15% vs 2.53±0.25%, P < 0.05, respectively). Taken together, these results suggested that the frequency of circulating Tfh cells increased in patients of AP.
Figure 1.
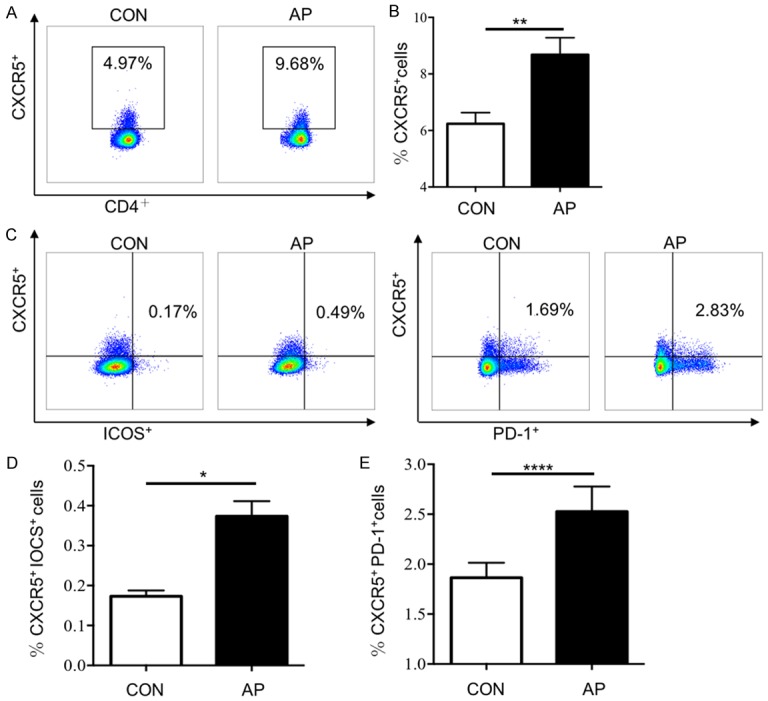
The proportion of Tfh cells in AP patients increased. The ratios of Tfh cells in peripheral blood of AP patients (n=35) and HCs (n=20) were detected by flow cytometry. A. Representative flow cytometry figure of the ratio of CXCR5+ cells in CD3, CD4 double positive cells; B. Statistical analysis of ratio of CXCR5+ cells in CD3, CD4 double positive cells; C. Representative flow cytometry figure of the ratio of CXCR5+ICOS+ cells and CXCR5+ PD-1+ cells in CD3, CD4 double positive cells; D. Statistical analysis of ratio of CXCR5+ICOS+ cells in CD3, CD4 double positive cells; E. Statistical analysis of the ratio of CXCR5+PD-1+ cells in CD3, CD4 double positive cells. Results are expressed as mean ± SEM, NS: no significant difference, *, P < 0.05; **, P < 0.01; ***, P < 0.001, ****, P < 0.0001.
Frequency of IL-21+ circulating Tfh cells
One of the most distinctive features of Tfh cells is the secretion of IL-21, an cytokine that is essential for the differentiation of Tfh cells and B cells [18,19]. Hence, we examined the frequency of IL-21+ circulating Tfh cells and the plasma-level expression of IL-21 in AP (Figure 2A, 2B). Compared to HCs, AP patients had a significant high level of circulating CD3+CD4+CXCR5+IL-21+ cells (median: 0.32±0.05% vs 0.64±0.12% P < 0.05). Notably, the level of plasma IL-21 was also higher in people with AP than in the HCs (median MFI: 71.42±0.71% vs 76.91±1.17%, P < 0.01) (Figure 2C). IL-21 secreted by Tfh cells is important for B cell differentiation and immunoglobulin production regulation [18]. Thus, we next measure the expression level of immunoglobulin in patients with AP.
Figure 2.
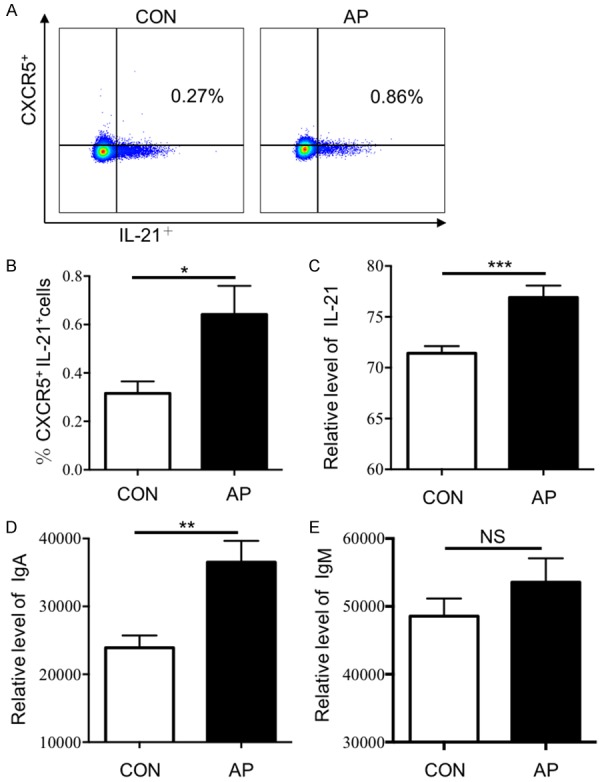
The expression levels of IL-21 and IgA in AP patients increased. The ratio of CXCR5+IL-21+ cells in peripheral blood of AP patients (n=14) and HCs (n=7) were detected by flow cytometry and the expression levels of IL-21, IgA and IgM were detected by CBA in AP patients (n=35) and HCs (n=20). A. Representative flow cytometry figure of the ratio of CXCR5+IL-21+ cells in CD3, CD4 double positive cells; B-E. Statistical analysis of: B. The ratio of CXCR5+IL-21+ cells in CD3, CD4 double positive cells; C. Expression levels of plasma IL-21 (MFI); D. Expression levels of plasma IgA (MFI); E. expression levels of plasma IgM (MFI); Results are expressed as mean ± SEM, NS: no significant difference, *, P < 0.05; **, P < 0.01; ***, P < 0.001, ****, P < 0.0001.
Level of plasma IgA and IgM
We currently have showed the frequency of circulating Tfh cells and plasma IL-21 increased in AP patients, so we assayed the levels of plasma IgA and IgM (Figure 2D, 2E). CBA detection revealed that, compared to HCs, the level of plasma IgA was significantly higher in AP patients (median MFI: 23919±1793 vs 36520±3145, P < 0.01). While IgM was slightly increased in AP patients (median MFI: 48552±2609 vs 53558±3526, P > 0.05). IgA plays an important role in mucosal defense and is the first line of defense against pathogens, while excessive levels of IgA may deposit in the glomerular mesangial and aggravate kidney damage.
Frequency of helper T cells and Treg cells
Previous research confirmed there was an interaction between Tfh cells and Th17 cells [20], thus we next examined the frequency of Th1, Th2 and Th17 cells (Figure 3A-C, 3E-G). All three subsets increased slightly in AP patients, but not significantly (median: Th1 cells, 20.78±3.99% vs 23.23±3.20%, P > 0.05; Th2 cells, 2.77±0.34% vs 3.39±0.41%, P > 0.05; Th17 cells, 0.84±0.12% vs 1.08±0.14%, P > 0.05). It has been confirmed that there was an connection between Tfh cells and Treg cells, depending on the molecule, PTEN [21]. The absence of PTEN in Treg cells increases the number of Tfh cells and GCs. Also, there was a significant change in Treg cells abundance between the AP patients and HCs (median: Treg cells, 4.68±0.35% vs 5.88±0.28%, P < 0.05) (Figure 3D, 3H).
Figure 3.
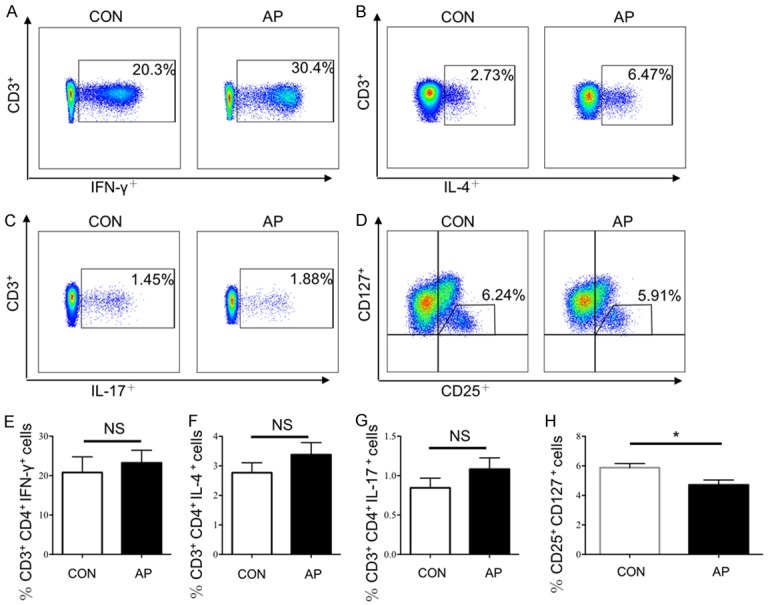
The proportion of T help cells and Treg cells in AP patients. The proportion of T help cells and Treg cells in AP patients (n=14) and HCs (n=7) were detected by flow cytometry. A. Representative flow cytometry figure of the proportion of Th1 cells; B. Representative flow cytometry figure of the proportion of Th2 cells; C. Representative flow cytometry figure of the proportion of Th17 cells; The proportion of Treg cells and Treg cells in AP patients (n=35) and HCs (n=20) were detected by flow cytometry. D. Representative flow cytometry figure of the proportion of Treg cells; E-H. Statistical analysis of: E. Proportion of Th1 cells; F. Proportion of Th2 cells; G. Proportion of Th17 cells; H. Proportion of Treg cells; Results are expressed as mean ± SEM, NS: no significant difference, *, P < 0.05; **, P < 0.01; ***, P < 0.001, ****, P < 0.0001.
Expression of Tfh cell-related inflammatory factor
Tfh cells are correlated with IL-6, IL-17, and IFN-γ [22,23], and Treg cell stability was associated with the repression of Tfh and Th1 cells [21]. So, we next investigated the plasma level of IL-1, IL-4, IL-6, IL-8, IL-17 and IFN-γ (Figure 4). Increased in plasma IL-1, IL-6, IL-8 and IL-17 were observed in AP patients compared to HCs, and these increases, except for IL-17, were statistically significant (medians MFI: IL-1, 93.54±4.71 vs 137.60±10.67, P < 0.001; IL-6, 133.80±5.84 vs 3067.00±678.00, P < 0.001; IL-8, 411.60±53.31 vs 2162.00±240.20, P < 0.0001; and IL-17, 79.06±2.24 vs 91.63±3.33, P < 0.01, Figure 4A, 4C-E). For IL-4 and IFN-γ, there was no change in the levels of inflammatory factors between patients with AP and the HCs. Systemic inflammatory response syndrome (SIRS) is one of the serious complication in AP, involving an overwhelming release of cytokines, including pro-inflammatory and anti-inflammatory components. Currently research showed many Tfh cell-related inflammatory factors increased significantly in patient with AP. Therefore, we speculate Tfh cells may be involved in SIRS.
Figure 4.
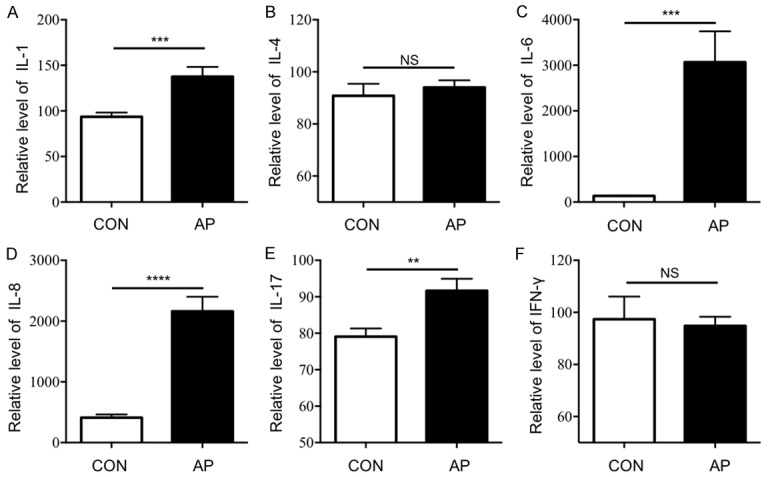
The expression levels of Tfh cell-associated inflammatory cytokines in AP patients increased. The expression levels of Tfh cell-associated inflammatory cytokines in AP patients (n=35) and HCs (n=20) were detected, including IL-1, IL-4, IL-6, IL-8, IL-17 and IFN-γ. Statistical analysis of: A. Expression levels of plasma IL-1 (MFI); B. Expression levels of plasma IL-4 (MFI); C. Expression levels of plasma IL-6 (MFI); D. Expression levels of plasma IL-8 (MFI); E. Expression levels of plasma IL-17 (MFI); F. Expression levels of plasma IFN-γ (MFI); Results are expressed as mean ± SEM, NS: no significant difference, *, P < 0.05; **, P < 0.01; ***, P < 0.001, ****, P < 0.0001.
Discussion
For a benign disease, AP (especially SAP) is associated with high mortality and significant morbidity, posing a great social and economic burden [24,25]. The annual incidence of AP ranges from 33 to 45/100,000 persons [26,27]. A 10-year study [28] showed that the incidence of first time AP has increased among those younger than 15 years-old and older than 65 years. The mortality rates also differ based on country, being 1.9% in Japan [27], 4.3%-3.3% in Taiwan [28], and 7.8% in European countries. The causes of death related to AP vary, ranging from septic shock and cardiac arrest and multiple organ failure and internal bleeding.
Here, we showed that the frequency of circulating Tfh cells increased significantly in patients of AP, including CD3+CD4+CXCR5+ cells, CD3+CD4+CXCR5+PD-1+ cells and CD3+CD4+CXCR5+ICOS+ cells. Such Tfh cells are considered true helper cells for B cells in antibody response. Previous work showed that Tfh cells are associated with multiple autoimmune diseases. Yang et al. [14] demonstrated that ICOS increased in patients with IgG4-RD and CD4+CXCR5+ cells were mainly observed in GCs and T-B junctions in lymph nodes form patients with IgG4-RL. Xu et al. [11] used flow cytometry to monitor a circulating Tfh cell subset and found that the frequency of CD4+CXCR5+ICOS+ increased significantly in patients with SLE patients; furthermore, a higher frequency of circulating Tfh cells might have more SLE activity. A similar result was published in patients with AS [15]. Taken together, we speculate in AP patients, the body’s immune response may be overloaded in a process initiated or supported by Tfh cells.
Previous research confirmed there is an interaction between Tfh cells, Treg cells, and Th17 cells in AS. After anti-TNF-α therapy, Th17 cells significantly decreased in responders, but were notably enhanced in non-responders, and Treg cells demonstrated exactly the opposite phenomenon [20]. Depending on a crucial protein PTEN, active maintenance of Treg cell stability was associated with the repression of Th1 and Tfh responses [21]. Hence, we measured the abundance of Treg and T helper cells. Surprisingly, unlike in autoimmune diseases, the frequency of Treg cells seemed decreased in AP samples compared to HC samples. In addition, Th1, Th2, and Th17 cells increased in the enrolled AP patients (though the increase was not significant). We propose that further research with larger-scale samples is needed.
IL-21 is essential for the generation and differentiation of Tfh cells and the function of B cells [18,19]. Also, IL-21 is sufficient and necessary for Th17 differentiation [16]. Previous research showed that IL-21 was associated with autoimmune diseases. In patients with AS, an increase in IL-21 is associated with an increase in plasma immunoglobulin [15]. Yang et al. [14] revealed the conjunction of highly expressed IL-21 and CXCR5 in samples of diseased tissue from patients with IgG4-RD. Here, we demonstrated that AP patients had a significant high level of circulating CD3+CD4+CXCR5+IL-21+ cells and a significant increase in plasma IL-21. Then we assayed the plasma immunoglobulin levels and found that IgA was remarkably increased in AP patients. IgA plays an important role in mucosal defense and is the first line of defense against pathogens, while excessive level of IgA may deposit in the glomerular mesangial and aggravated kidney damage. These results suggested that IL-21 may be a regulatory element of AP, mediating B cell differentiation and regulating the secretion of immunoglobulins. Excessive IgA may lead to kidney damage and even renal failure, which is considered an important component of multiple organ dysfunction syndrome (MODS).
MODS is considered the most serious complication of AP. The presence of organ failure indicates severe disease, and 30% of patients who develop organ failure die [29]. Considering the cause of MODS, SIRS plays a vital role, and a significant number of AP patients with SIRS develop MODS. Hence, the American College of Gastroenterology Guideline proposed that AP patients with either organ failure or SIRS should be admitted to an intensive care unit whenever possible [2]. The views on the cause of SIRS differ, while the result is clear, involving an overwhelming release of cytokines, including pro-inflammatory and anti-inflammatory components. Here we showed that AP patients had a significant increase in plasma Tfh cell-relevant inflammatory factors, with the increase in IL-6 being most prominent. Tfh cell generation depends on IL-6, IL-21, and STAT-3 [30]. These data suggested that IL-6 correlated with the impact of Tfh cells on AP. However, further research is needed to determine whether excess IL-6 enhances Tfh cell generation in patients with AP.
In conclusion, our study showed that CXCR5+ Tfh cells and plasma IL-6 and IL-21 were increased in patients with AP. Given the effects of Tfh cells on B cells and T helper cells, as well as the phenomena of SIRS and upregulated plasma immunoglobulin levels, we speculate that Tfh cells play a vital role in the development and progression of AP that is dependent upon IL-6 and IL-21. These data revealed a new target for the treatment of AP.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81670581, 81671901, 81600501, 81470941). All the authors listed have approved the manuscript for publication, and declared no conflict of interest. RX and YC operated the experiment and drafted the manuscript. MQ, HX, YY and HN provided help for the experiment. ZZ, JZh, ZX, EM and EC provided suggestions form clinical practice. JF and TZ design the experiment and revising the manuscript. All authors read and approved the final manuscript.
Disclosure of conflict of interest
None.
References
- 1.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 2.Tenner S, Baillie J, DeWitt J, Vege SS American College of Gastroenterology. the American journal of gastroenterology. Vol. 108. Nature Publishing Group; 2013. American College of Gastroenterology guideline: management of acute pancreatitis. 1400-15-1416. [DOI] [PubMed] [Google Scholar]
- 3.Gukovskaya AS, Hosseini S, Satoh A, Cheng JH, Nam KJ, Gukovsky I, Pandol SJ. Ethanol differentially regulates NF-kappaB activation in pancreatic acinar cells through calcium and protein kinase C pathways. Am J Physiol Gastrointest Liver Physiol. 2004;286:G204–13. doi: 10.1152/ajpgi.00088.2003. [DOI] [PubMed] [Google Scholar]
- 4.Felderbauer P, Müller C, Bulut K, Belyaev O, Schmitz F, Uhl W, Schmidt WE. Pathophysiology and treatment of acute pancreatitis: new therapeutic targets--a ray of hope? Basic Clin Pharmacol Toxicol Basic Clin Pharmacol Toxicol. 2005;97:342–50. doi: 10.1111/j.1742-7843.2005.pto_274.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Neuhöfer P, Song L, Rabe B, Lesina M, Kurkowski MU, Treiber M, Wartmann T, Regnér S, Thorlacius H, Saur D, Weirich G, Yoshimura A, Halangk W, Mizgerd JP, Schmid RM, Rose-John S, Algül H. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest. 2013;123:1019–31. doi: 10.1172/JCI64931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh JH, Jeon H, Park SM, Choi E, Lee GS, Kim JW, Lee KJ. Diabetes mellitus is associated with mortality in acute pancreatitis. J Clin Gastroenterol. 2018;52:178–83. doi: 10.1097/MCG.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 7.Qiao SF, Lu TJ, Sun JB, Li F. Alterations of intestinal immune function and regulatory effects of L-arginine in experimental severe acute pancreatitis rats. World J Gastroenterol. 2005;11:6216–8. doi: 10.3748/wjg.v11.i39.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Immunosuppression in patients with severe acute pancreatitis. J Gastroenterol. 2006;41:779–84. doi: 10.1007/s00535-006-1852-8. [DOI] [PubMed] [Google Scholar]
- 9.Lee B, Zhao Q, Habtezion A. Immunology of pancreatitis and environmental factors. Curr Opin Gastroenterol. 2017;33:383–9. doi: 10.1097/MOG.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 10.Zheng YS, Wu ZS, Zhang LY, Ke L, Li WQ, Li N, Li JS. Nicotine ameliorates experimental severe acute pancreatitis via enhancing immunoregulation of CD4+ CD25+ regulatory T cells. Pancreas. 2015;44:500–6. doi: 10.1097/MPA.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, Liu J, Cui X, Zuo Y, Zhang Z, Li Y, Tao R, Li Y, Pang J. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell Immunol. 2015;295:46–51. doi: 10.1016/j.cellimm.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Ramiscal RR, Vinuesa CG. T-cell subsets in the germinal center. Immunol Rev. 2013;252:146–55. doi: 10.1111/imr.12031. [DOI] [PubMed] [Google Scholar]
- 13.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–46. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Wei R, Liu Q, Shi Y, Li J. Frequency and distribution of CD4+CXCR5+ follicular B helper T cells within involved tissues in IgG4related ophthalmic disease. Mol Med Rep. 2017;16:9512–20. doi: 10.3892/mmr.2017.7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu S, Yang T, Pan F, Xia G, Hu Y, Liu L, Fan D, Duan Z, Ding N, Xu S, Cai G, Wang L. Increased frequency of circulating follicular helper T cells in patients with ankylosing spondylitis. Mod Rheumatol. 2015;25:110–5. doi: 10.3109/14397595.2014.902149. [DOI] [PubMed] [Google Scholar]
- 16.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 17.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–10. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M, Guo Z, Ju W, Ryffel B, He X, Zheng SG. The development and function of follicular helper T cells in immune responses. Cell Mol Immunol. 2012;9:375–9. doi: 10.1038/cmi.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xueyi L, Lina C, Zhenbiao W, Qing H, Qiang L, Zhu P. Levels of circulating Th17 cells and regulatory T cells in ankylosing spondylitis patients with an inadequate response to anti-TNF-α therapy. J Clin Immunol. 2013;33:151–61. doi: 10.1007/s10875-012-9774-0. [DOI] [PubMed] [Google Scholar]
- 21.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178–87. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jogdand GM, Mohanty S, Devadas S. Regulators of Tfh cell differentiation. Front Immunol. 2016;7:520. doi: 10.3389/fimmu.2016.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Wu H, Qiu H, Yang H, Deng Y, Zhao M, Luo H, Zhou X, Xie Y, Chan V, Lau CS, Lu Q. The expression of Bcl-6 in circulating follicular helper-like T cells positively correlates with the disease activity in systemic lupus erythematosus. Clin Immunol. 2016;173:161–70. doi: 10.1016/j.clim.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 24.McNabb-Baltar J, Ravi P, Isabwe GA, Suleiman SL, Yaghoobi M, Trinh QD, Banks PA. A population-based assessment of the burden of acute pancreatitis in the United States. Pancreas. 2014;43:687–91. doi: 10.1097/MPA.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 25.Hornung L, Szabo FK, Kalkwarf HJ, Abu-El-Haija M. Increased burden of pediatric acute pancreatitis on the health care system. Pancreas. 2017;46:1111–4. doi: 10.1097/MPA.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey CF, Zhou H, Harvey DJ, White RH. The incidence and case-fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994-2001. Pancreas. 2006;33:336–44. doi: 10.1097/01.mpa.0000236727.16370.99. [DOI] [PubMed] [Google Scholar]
- 27.Satoh K, Shimosegawa T, Masamune A, Hirota M, Kikuta K, Kihara Y, Kuriyama S, Tsuji I, Satoh A, Hamada S Research Committee of Intractable Diseases of the Pancreas. Nationwide epidemiological survey of acute pancreatitis in Japan. Pancreas. 2011;40:503–7. doi: 10.1097/MPA.0b013e318214812b. [DOI] [PubMed] [Google Scholar]
- 28.Shen HN, Lu CL, Li CY. Epidemiology of first-attack acute pancreatitis in Taiwan from 2000 through 2009: a nationwide population-based study. Pancreas. 2012;41:696–702. doi: 10.1097/MPA.0b013e31823db941. [DOI] [PubMed] [Google Scholar]
- 29.Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813–20. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–49. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


