Abstract
Objective: To determine the presence of vasculogenic mimicry (VM) and expression of Sphingosine kinase 1 (SphK1) and Connexin43 (Cx43) in colorectal cancer (CRC) tissues, and to identify their inter-relationships and associations with multiple pathologic parameters. Methods: Ninety-two CRC specimens and normal pericarcinoma tissues were analyzed for expression of SphK1 and Cx43 using immunohistochemistry, and for identification of VM using CD34-periodic acid-Schiff dual staining. Results: The positive rate of SphK1 expression was greater in CRC cells than pericarcinoma cells (85.87% vs. 33.70%, P < 0.05). In contrast, the positive rate of Cx43 expression was greater in pericarcinoma cells than in CRC cells (58.70% vs. 92.39%, P < 0.05). Analysis of CRC tissues indicated that expression of SphK1 was associated with poor differentiation, advanced tumor stage, lymph node metastasis, and the presence of VM (P < 0.05 for each comparison). Expression of Cx43 was associated with high differentiation and the presence of VM (P < 0.05 for each comparison). Patient sex, age, tumor size, depth of invasion, and distant metastasis were unrelated to the expression of either protein. There was a significant correlation between the expression of SphK1 and Cx43 (P < 0.05). Analysis of overall patient survival indicated that SphK1 positivity and the presence of VM were significantly associated with poor survival, but Cx43 positivity had no relationship with survival. Conclusion: SphK1 protein expression was significantly greater in CRC tissues than pericarcinoma tissues, suggesting this protein may be associated with the pathogenesis of CRC. In addition, the significant correlation between expression of SphK1 and Cx43 in CRC tissues suggests their interaction may impact the pathogenesis of CRC.
Keywords: SphK1, Cx43, colorectal cancer, vasculogenic mimicry, prognosis
Introduction
Colorectal cancer (CRC) is the third-leading cause of cancer-related deaths, and will cause an estimated 50,630 deaths in the United States during 2018 [1]. Thus, despite the implementation of aggressive screening procedures to improve early detection and our extensive knowledge of critical events in the pathogenesis of CRC, this cancer continues to be a major health concern in developed countries.
Sphingolipids, especially ceramide, sphingosine, ceramide-1-phosphate, and sphingosine-1-phosphate (S1P), play key roles in the pathogenesis of cancer [2]. In particular, S1P promotes cell proliferation and survival and regulates angiogenesis, whereas sphingosine and ceramide inhibit cell proliferation and stimulate apoptosis. Sphingosine kinase (SphK) phosphorylates sphingosine to form S1P, and is a critical regulator of sphingolipid-mediated functions [3]. Growing evidence indicates that SphK1 plays a pivotal role in the pathogenesis of CRC [4-8], although the mechanisms are still unclear.
Previous studies suggested that connexins (gap junction proteins) are frequently downregulated or not assembled into identifiable gap junctions during the early stages of many cancers, although more recent research indicated that connexins can be up-regulated during the later phases of metastasis [9]. In addition, accumulating evidence suggests that connexins may facilitate invasion, intravasation, extravasation, and metastasis in a variety of cancers [10-13]. Cx43 is the best known and studied of the 21 connexins. This connexin is highly expressed in epithelial cells, hematopoietic cells, neurons and astrocytes, cardiac neuronal crest cells, and fibroblasts [14]. Recent reports suggest that Cx43 plays multiple roles during the different stages of tumor progression [15,16]. In particular, Cx43 downregulation is associated with cancer phenotype [17,18]. However, the presence of aberrant Cx43 upregulation in advanced carcinomas suggests that cancer cells may communicate with normal host cells across hemichannels, thus suggesting that Cx43 has a role in the migration and growth of metastatic cells. However, it is unknown whether Cx43 level is associated with patient outcome or can be used as a marker of cancer prognosis.
Vasculogenic mimicry (VM) is a newly described process in which highly aggressive tumor cells form fluid-conducting channels that supply nutrition to tumor cells [19]. It is now believed that VM plays an essential role in the pathology of a wide variety of human cancers, including carcinomas, sarcomas, glioblastomas, astrocytomas, and melanomas [8,19-21]. The development of VM strongly correlates with poor prognosis in a number of cancers, including osteosarcoma [7], gastric cancer, [21] and ovarian cancer [22]. However, little is known about the role of VM in CRC.
Protein internalization, lysosomal or proteasomal degradation, and other molecular mechanisms that cause post-translation modifications can regulate the expression of Cx43 [23]. In addition, various kinases can initiate several subsequent events, such as rapid gap-junctional intercellular communication (GJIC), down-regulation or internalization of Cx43, and Cx hexamer stabilization [24,25]. A recent study reported that S1P, a bioactive sphingolipid mainly produced by SphK1 metabolism, can regulate Cx43 protein expression and GJIC in skeletal muscle cells [26]. Thus, we hypothesized that SphK1 may also regulate Cx43 during tumor progression. Therefore, in the present study, we assessed the expression of SphK1 and Cx43 in CRC and pericarcinoma tissues, their associations with clinicopathological characteristics (especially VM formation), and their inter-relationships.
Materials and methods
Patients and tissue specimens
Patients with CRC who received surgical resection at the First Affiliated Hospital of Guangxi Medical University (Nanning) from March 2013 to November 2013 were prospectively recruited for this study. Each patient had a diagnosis of CRC based on clinicopathological characteristics, and none of them received radiotherapy or adjuvant chemotherapy before surgery. All patients were followed up until death or when last seen alive at a clinical visit during March 2018 (median follow-up time: 54 months). A total of 92 paraffin-embedded CRC tissues and 92 adjacent normal epithelial tissues were collected for immunohistochemistry (IHC) and CD34/periodic acid Schiff (PAS) double staining. The study protocols were approved by Ethics Committee of First Affiliated Hospital of Guangxi Medical University. Each patient signed a written informed consent agreement before surgery.
IHC procedures
The formalin-fixed paraffin-embedded tissue samples were cut into 4 μm sections, and then baked at 60°C for 30 min. The slides were then dewaxed in xylene, rehydrated in ethanol, and then incubated in 3% hydrogen peroxide to inhibit endogenous peroxidase activity. Next, the slides were boiled in antigen-retrieval EDTA buffer for 10 min. After blocking with goat serum, the slides were incubated with rabbit polyclonal antibody against Cx43 (1:500, Santa Cruz) and rabbit monoclonal antibody against SphK1 (1:700, Sigma), and then incubated at 4°C overnight. After washing with phosphate-buffered saline (PBS), the sections were incubated with a second antibody for 30 min, then washed again with PBS. Finally, the sections were visualized by addition of a diaminobenzidine solution, and counterstained with hematoxylin. Negative controls were performed by incubation with rabbit IgG instead of the primary antibody.
Evaluation of IHC staining
Brown-yellow staining was considered as positive. For each section, 10 visual fields were randomly examined under a microscope (Olympus, Japan) at 400×, and 200 CRC cells were counted in each visual field. The percentage of positive cells in each field was used for scoring. Thus, the cut-off was 10% malignant positive cells for separation between positive and negative results. A four-grade scale was applied: “-” indicated less than 10% staining; “+” indicated 10 to 50% staining; “++” indicated 50 to 75% staining; and “+++” indicated 75% or more staining.
CD34/periodic acid Schiff (PAS) double staining
CD34/PAS double staining was also performed to detect VM formation in the paraffin-embedded sections. First, IHC staining for CD34 using a mouse monoclonal antibody (1:50; cat. no. ZM-0046; Zhongshan Goldenbridge, Beijing, China), was performed to detect endothelial cells. Then, sections were washed in running water for 1 min and incubated with PAS for 30 min to detect the basement membrane of tubular structures. VM is indicated by the presence of tubular structures containing red blood cells, with PAS staining of the basement membrane, that are surrounded by tumor cells with no CD34 staining. The presence of 1 or more red blood cells in the tubular structure was needed for identification of VM.
Statistical analysis
The significance of the relationships between SphK1 expression, Cx43 expression, and clinicopathological characteristics were determined using the Pearson chi-square test or Fisher’s exact test. Survival curves were evaluated by the Kaplan-Meier method, and compared using the log-rank test. Spearman rank correlation analysis was used to assess the association between the expressions of different proteins. Statistical analysis was performed using SPSS software 17.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered significant.
Results
Clinicopathological features of patients
The median age of the 92 CRC patients was 56 years (range: 25 to 83 years), and there were 56 males and 36 females. A total of 78 patients had tubular or papillary adenocarcinoma, 13 had mucinous adenocarcinoma, and 1 had signet-ring cell carcinoma. Cancer cells were highly differentiated in 3 patients, moderately differentiated in 68 patients, and poorly differentiated in 21 patients. Thirty-nine patients had lymph node metastases, 17 patients had distant metastases, and 29 patients had evidence of VM. The median follow-up time was 54 months (range: 3 to 60 months).
SphK1 and Cx43 expression in normal and malignant colorectal tissue
We performed IHC to determine SphK1 and Cx43 expression in colorectal tissue samples and matched pericarcinoma (normal) tissue samples. The expression of SphK1 was predominantly cytoplasmic in tumor cells and pericarcinoma epithelial cells (Table 1; Figure 1). In particular, 31 of 92 normal epithelial cells (34%) expressed SphK1, but 79 of 92 cancer cells (86%) expressed SphK1. Cx43 expression in normal epithelia was in the membranes and cytoplasmic regions (Table 1; Figure 2). The opposite trend occurred for Cx43, in that 85 of 92 normal epithelial cells (92%) expressed Cx43, but 54 of 92 cancer cells (58.7%) expressed Cx43. Most examined cells only had cytoplasmic expression of this protein (Figure 2A-D), although mixed staining (cytoplasmic and membranous) occurred in a few cases (Figure 2B, 2C). Interestingly, Spearman rank correlation analysis indicated a significant positive correlation between expression of SphK1 and Cx43 in CRC cells (r = 0.595, P < 0.01) (Table 2).
Table 1.
Expression of SphK1 and Cx43 in CRC tissues and pericarcinoma (normal) tissues
| Protein | Result | CRC tissues | Pericarcinoma tissues | χ2 | P a |
|---|---|---|---|---|---|
| SphK1 | Positive | 79 | 31 | 52.081 | 0.000 |
| Negative | 13 | 61 | |||
| Cx43 | Positive | 54 | 85 | 28.269 | 0.000 |
| Negative | 38 | 7 |
Chi square tests indicated that SphK1 positivity was significantly higher in CRC tissues, and Cx43 positivity was significantly lower in CRC tissues.
Figure 1.
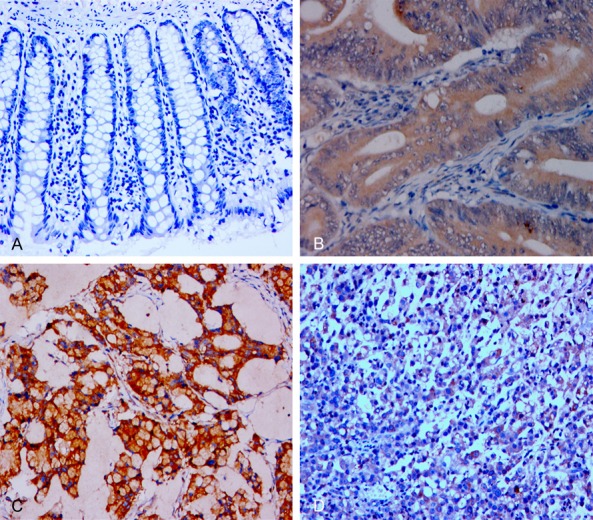
IHC analysis of SphK1 expression in representative pericarcinoma (normal) and CRC tissues (×400). A. No expression in pericarcinoma colorectal cells; B. Positive expression in the cytoplasm of colorectal tubular adenocarcinoma cells; C. Positive expression in colorectal mucinous adenocarcinoma cells; D. Positive expression in the cytoplasm of undifferentiated colorectal carcinoma cells.
Figure 2.
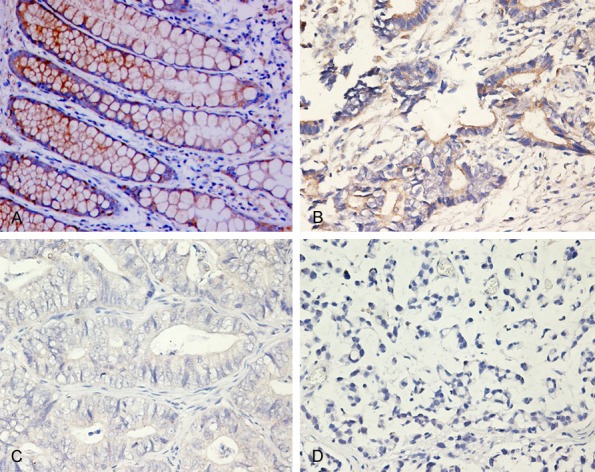
IHC analysis of Cx43 expression in representative pericarcinoma (normal) and CRC tissues (×400). A. Positive expression in the membrane and cytoplasm of pericarcinoma colorectal cells; B. Low expression in the membrane and cytoplasm of well-differentiated colorectal adenocarcinoma cells; C. Low expression in the membrane and cytoplasm of moderately differentiated colorectal adenocarcinoma cells; D. No expression in poorly differentiated colorectal adenocarcinoma cells.
Table 2.
Correlation between the expression of SphK1 and Cx43 in CRC tissues
| Expression | SphK1 expression | rs | P value | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| +++ | ++ | + | - | Total | ||||
| Cx43 | +++ | 10 | 0 | 0 | 1 | 92 | 0.595 | 0.000 |
| ++ | 11 | 1 | 0 | 0 | ||||
| + | 14 | 8 | 6 | 3 | ||||
| - | 4 | 11 | 14 | 9 | ||||
rs: Spearman’s rank correlation coefficient.
Clinical and pathological implications of SphK1 and Cx43 expression in CRC
We also examined the correlations of multiple clinicopathologic characteristics with the expression of SphK1 and Cx43 in CRC cells (Table 3; Figure 3). SphK1 expression was significantly and positively associated with poorly differentiated cells (P = 0.041), more advanced T stage (P = 0.009), lymph node metastases (P = 0.033), and the presence of VM (P = 0.020). Notably, there was no significant association of SphK1 expression with distant metastasis (P = 0.142), but all 17 colon cancer tissues with distant metastasis tested positive for expression of SphK1. Cx43 expression was greater in more highly differentiated cells (P < 0.001) and was associated with the presence of VM (P = 0.023). There were no significant relationships of sex, age, tumor size, depth of invasion, and distant metastases with the expression of Cx43 or SphK1 (Table 3; Figure 3).
Table 3.
Relationship of SphK1 and Cx43 expression with clinical and pathological characteristics of patients with CRC
| Groups | Total | SphK1 | Cx43 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Positive (n) | Positive (%) | χ2 | P | Positive (n) | Positive (%) | χ2 | P | ||
| Sex | |||||||||
| Male | 56 | 46 | 82.1 | 1.638 | 0.201 | 32 | 57.1 | 0.142 | 0.706 |
| Female | 36 | 33 | 91.7 | 22 | 61.1 | ||||
| Age | |||||||||
| ≤ 50 | 32 | 30 | 93.8 | 1.614 | 0.204 | 18 | 56.2 | 0.121 | 0.728 |
| > 50 | 60 | 49 | 81.7 | 36 | 60.0 | ||||
| Tumor diameter | |||||||||
| < 5 cm | 41 | 36 | 87.8 | 0.228 | 0.633 | 28 | 68.3 | 2.810 | 0.094 |
| ≥ 5 cm | 51 | 43 | 84.3 | 26 | 51.0 | ||||
| Tumor Differentiation | |||||||||
| High | 3 | 1 | 33.3 | 6.308 | 0.041 | 2 | 66.7 | 13.525 | 0.000 |
| Moderate | 68 | 58 | 85.3 | 47 | 69.1 | ||||
| Low | 21 | 20 | 95.2 | 5 | 23.8 | ||||
| Tumor stage | |||||||||
| T1/T2 | 47 | 36 | 76.6 | 6.811 | 0.009 | 28 | 59.6 | 0.031 | 0.861 |
| T3/T4 | 45 | 43 | 95.6 | 26 | 57.8 | ||||
| Lymph node metastasis | |||||||||
| Yes | 39 | 37 | 94.9 | 4.521 | 0.033 | 23 | 59.0 | 0.002 | 0.963 |
| No | 53 | 42 | 79.2 | 31 | 54.5 | ||||
| Invasion depth | |||||||||
| Mucosal/superficial muscularis | 5 | 4 | 80.0 | 0.542 | 3 | 60.0 | 1.000 | ||
| Deep muscle or full layer | 87 | 75 | 86.2 | 51 | 58.6 | ||||
| Distant metastasis | |||||||||
| Yes | 17 | 17 | 100.0 | 2.152 | 0.142 | 12 | 70.6 | 1.217 | 0.270 |
| No | 75 | 62 | 82.7 | 42 | 56.0 | ||||
| Vasculogenic mimicry | |||||||||
| Yes | 29 | 29 | 100.0 | 5.372 | 0.020 | 22 | 75.9 | 5.148 | 0.023 |
| No | 63 | 50 | 79.4 | 32 | 50.8 | ||||
Figure 3.
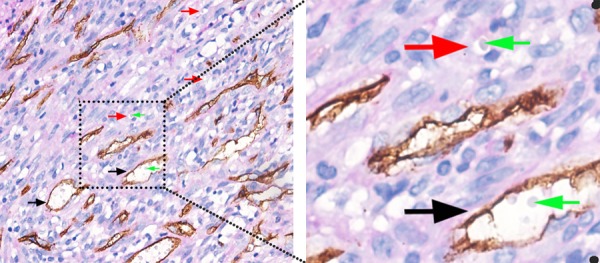
CD34/PAS double staining of colorectal cancer tissue samples for detection of VM. Black arrows indicate typical blood vessels, with brown CD34+ staining; green arrows indicate red blood cells; and red arrows indicate VM channels formed by tumor cells.
Association of SphK1 expression, Cx43 expression, and the presence of VM with clinical outcome
The Kaplan-Meier survival analysis indicated that overall survival was worse for patients with SphK1 positivity (P = 0.0443) and with the presence of VM in colorectal tumor infiltrates (P = 0.0388) (Figure 4A, 4C). However, Cx43 positivity had no significant relationship with overall survival (P = 0.7732, Figure 4B).
Figure 4.
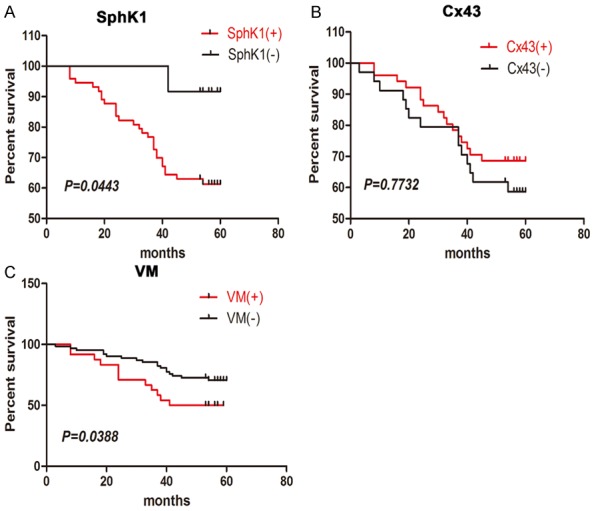
Kaplan-Meier analysis of the overall survival of CRC patients with positive and negative expression of SphK1 (A), positive and negative expression of Cx43 (B), and the presence of VM (C). Log rank tests showed that SphK1 positivity (P = 0.0443) and the presence of VM (P = 0.0388) were significantly associated with poor survival, but Cx43 positivity (P = 0.7732) was not associated with survival.
Discussion
Previous studies demonstrated elevated expression of SphK1 in the colons of patients with CRC, suggesting its potential use as a biomarker for CRC, and that increased expression of SphK1 was associated with poor outcomes (mainly due to metastasis to the lymph nodes, liver, and other organs) [27]. In this study, we found that SphK1 expression was significantly correlated with tumor differentiation, lymph node metastasis, and T stage, and that the expression of SphK1 was higher in CRC tissues than adjacent non-cancer tissues. Although SphK1 expression was not significantly different in patients with distant metastasis, all 17 CRC tissues with distant metastasis had expression of SphK1. Furthermore, Kaplan-Meier survival analysis showed that SphK1-positive CRC patients had significantly shorter OS than SphK1-negative patients. These findings are consistent with previous studies that examined the role of this protein in CRC [28].
Previously, Cx43 was considered as a tumor suppressor [9,29], but there is increasing recognition that it has multiple effects in the pathogenesis of cancer. Thus, recent research suggests that Cx43 can promote cancer progression [30], angiogenesis [31], invasion [32], intravasation, extravasation, metastasis [33], and drug resistance of cancer cells [34]. Kanczuga-Koda et al. [35] used IHC analysis to demonstrate that either upregulation of Cx43 occurred as tumors progressed to an invasive phenotype, or that a dominant cell type within the tumor which expressed cytoplasmic Cx43 made up the bulk of the invasive tumors. Teleki et al. [36] used mRNA platforms to evaluate connexin gene expression and found that high expression of Cx43 was associated with poorer relapse-free survival (RFS) and overall survival (OS) in patients with ER-negative breast cancer. Other research reported that enhanced Cx43 expression was also associated with increased cell migration, tumor chemoresistance, and metastasis of different types of tumors [37,38]. The role of Cx43 in cancer is tissue- and stage-specific [9,39]. In particular, Cx43 has a role in the later stages of breast cancer progression, in that it mediates the interaction between the tumor and endothelial cells, and facilitates adhesion and extravasation at secondary sites [40,41]. Cx43 is also expressed at higher levels in lymph node metastases than in primary breast tumors [42]. Other studies reported aberrant Cx43 expression in advanced stages of prostate and colon adenocarcinomas [43,44].
In this study, we found that Cx43 expression was significantly lower in CRC tissues than adjacent non-cancerous tissues, and that expression was inversely associated with tumor grade. Furthermore, our Kaplan-Meier analysis showed that Cx43-positive and Cx43-negative CRC patients had similar overall survival times. Our evidence thus suggests that the role of Cx43 depends on the cancer subtype, consistent with previous studies [45].
VM is the formation of a microcirculation pattern that differs from the traditional vascular system, and plays an important role in transferring blood and other nutrients to tumors. Researchers have identified VM in many solid tumors, such as melanoma, hepatocellular carcinoma, bladder cancer, and prostate cancer [46-49]. Furthermore, there is a strong correlation between VM and the presence of more malignant features of cancer, such as advanced stage or grade, poor cell differentiation, and short overall survival. In our study, we identified VM in 29 of 92 specimens of CRC tissues (31.5%), similar to a previous study of CRC [50], and confirming the presence of VM in CRC. Moreover, our Kaplan-Meier analysis indicated that VM-positive CRC patients had significantly shorter OS than VM-negative patients (P = 0.0388). These findings suggest that VM could be useful as a biomarker for predicting progression and metastasis in CRC. Our results thus agree with a previous immunohistochemical study which concluded that VM in CRC patients is associated with metastasis and poor prognosis [50].
Cx43 interacts with various proteins via its C-terminal region, including kinases (such as SphK1), cell-adhesion proteins, and Bax (a pro-apoptotic protein). These molecular interactions may affect the expression and function of these proteins, and thereby modulate cellular physiology. Recent studies reported that epithelial-mesenchymal transition (EMT) contributed to VM, and identified the upregulation of EMT-associated transcription factors in tumor cells that had VM [48,51]. Mounting evidence also indicates that cancer stem cell (CSCs) can differentiate into tumor or endothelial lineages [52], as well as into vascular smooth muscle-like cells [53]. VM-engaging tumor cells have the phenotypes of endothelial and tumor tissues [54,55], so VM may represent the incomplete progression of CSC differentiation into different endothelial lineages. Another study found that epithelial cancer cells may have the capacity of self-renewal of the stem cell phenotype via EMT [56]. VM allows tumor cells to express the endothelial phenotype and function as endothelial cells in forming blood vessel-like structures. In fact, both epithelial and mesenchymal markers are present in tumor cells undergoing VM [54,57]. It is plausible that CSCs function in VM by induction of the EMT, because EMT plays a key role in tumorigenesis and in VM [48,58,59].
Furthermore, our previous studies showed that SphK1 modulates the expression of EMT-related markers and cell migration in CRC cells [60]. Thus, SphK1 may have a role in mediating EMT in CRC. Inoculation with CSCs overexpressing SphK1 increases the development of tumors in nude mice, relative to parent cells or CSCs that do not overexpress SphK1 [61]. Shipitsin et al. [62] studied breast tumor heterogeneity and found that Cx43 was significantly expressed in the subset of CD44+ stem-like cells, and considered it as a CD44+ specific gene. Another study found that Cx43, in addition functioning as a channel, also functioned as an adhesion site that enhanced cell aggregation, and that the adhesive effects did not require the formation of functional channels [63]. Thus, Cx43 and other connexins have multiple functions during the pathogenesis of cancer.
Overall, our findings suggest that complex associations among SphK1, VM, and Cx43 affect tumor progression and metastasis. Thus, we believe that examination of the interactions of these biomarkers could help to predict the biological behavior of CRC cells, and may allow identification of targets for novel therapeutic strategies.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81760516), Hainan Medical College Research and Development Fund (No. HY2015-20), 2018 Innovation Project of Guangxi Graduate Education (No. YCBZ2018046) and Guangxi Zhuang Autonomous Region Health and Family Planning Commission Self-financing Research Projects (No. Z20170086).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol. 2002;71:493–511. doi: 10.1016/s0079-6603(02)71049-0. [DOI] [PubMed] [Google Scholar]
- 4.Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, Hannun YA, Obeid LM. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuya H, Tamashiro PM, Shimizu Y, Iino K, Peres R, Chen R, Sun Y, Hannun YA, Obeid LM, Kawamori T. Sphingosine kinase 1 expression in peritoneal macrophages is required for colon carcinogenesis. Carcinogenesis. 2017;38:1218–1227. doi: 10.1093/carcin/bgx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, Wargovich MJ, Reddy BS, Hannun YA, Obeid LM, Zhou D. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- 7.Ren K, Zhang J, Gu X, Wu S, Shi X, Ni Y, Chen Y, Lu J, Gao Z, Wang C, Yao N. Migration-inducing gene-7 independently predicts poor prognosis of human osteosarcoma and is associated with vasculogenic mimicry. Exp Cell Res. 2018;369:80–89. doi: 10.1016/j.yexcr.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Ding J, Jia X, Zuo B, He J, Yang J, He Y. A novel monoclonal antibody targeting a novel epitope of VEcadherin inhibits vasculogenic mimicry of lung cancer cells. Oncol Rep. 2018;39:2837–2844. doi: 10.3892/or.2018.6374. [DOI] [PubMed] [Google Scholar]
- 9.Naus CC, Laird DW. Implications and challenges of connexin connections to cancer. Nat Rev Cancer. 2010;10:435–441. doi: 10.1038/nrc2841. [DOI] [PubMed] [Google Scholar]
- 10.Tang B, Peng ZH, Yu PW, Yu G, Qian F, Zeng DZ, Zhao YL, Shi Y, Hao YX, Luo HX. Aberrant expression of Cx43 is associated with the peritoneal metastasis of gastric cancer and Cx43-mediated gap junction enhances gastric cancer cell diapedesis from peritoneal mesothelium. PLoS One. 2013;8:e74527. doi: 10.1371/journal.pone.0074527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh S, Kumar A, Tripathi RP, Chandna S. Connexin-43 regulates p38-mediated cell migration and invasion induced selectively in tumour cells by low doses of gamma-radiation in an ERK-1/2-independent manner. Carcinogenesis. 2014;35:383–395. doi: 10.1093/carcin/bgt303. [DOI] [PubMed] [Google Scholar]
- 12.Zhang A, Hitomi M, Bar-Shain N, Dalimov Z, Ellis L, Velpula KK, Fraizer GC, Gourdie RG, Lathia JD. Connexin 43 expression is associated with increased malignancy in prostate cancer cell lines and functions to promote migration. Oncotarget. 2015;6:11640–11651. doi: 10.18632/oncotarget.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa K, Pitchakarn P, Suzuki S, Chewonarin T, Tang M, Takahashi S, Naiki-Ito A, Sato S, Takahashi S, Asamoto M, Shirai T. Silencing of connexin 43 suppresses invasion, migration and lung metastasis of rat hepatocellular carcinoma cells. Cancer Sci. 2012;103:860–867. doi: 10.1111/j.1349-7006.2012.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuuchi L, Naus CC. Gap junction proteins on the move: connexins, the cytoskeleton and migration. Biochim Biophys Acta. 2013;1828:94–108. doi: 10.1016/j.bbamem.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Sirnes S, Lind GE, Bruun J, Fykerud TA, Mesnil M, Lothe RA, Rivedal E, Kolberg M, Leithe E. Connexins in colorectal cancer pathogenesis. Int J Cancer. 2015;137:1–11. doi: 10.1002/ijc.28911. [DOI] [PubMed] [Google Scholar]
- 16.Lamiche C, Clarhaut J, Strale PO, Crespin S, Pedretti N, Bernard FX, Naus CC, Chen VC, Foster LJ, Defamie N, Mesnil M, Debiais F, Cronier L. The gap junction protein Cx43 is involved in the bone-targeted metastatic behaviour of human prostate cancer cells. Clin Exp Metastasis. 2012;29:111–122. doi: 10.1007/s10585-011-9434-4. [DOI] [PubMed] [Google Scholar]
- 17.Bates DC, Sin WC, Aftab Q, Naus CC. Connexin43 enhances glioma invasion by a mechanism involving the carboxy terminus. Glia. 2007;55:1554–1564. doi: 10.1002/glia.20569. [DOI] [PubMed] [Google Scholar]
- 18.Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M. Gap junctions and cancer: new functions for an old story. Antioxid Redox Signal. 2009;11:323–338. doi: 10.1089/ars.2008.2153. [DOI] [PubMed] [Google Scholar]
- 19.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’Er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B, Mao X, Wang H, Su G, Mo C, Cao K, Qiu S. Vasculogenic mimicry in bladder cancer and its association with the aberrant expression of ZEB1. Oncol Lett. 2018;15:5193–5200. doi: 10.3892/ol.2018.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Q, Yuan Y, Jin Z, Xu T, Gao Y, Wei H, Li C, Hou W, Hua B. Association between tumor vasculogenic mimicry and the poor prognosis of gastric cancer in China: an updated systematic review and meta-analysis. Biomed Res Int. 2016;2016:2408645. doi: 10.1155/2016/2408645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang J, Yang B, Cao Q, Wu X. Association of vasculogenic mimicry formation and CD133 expression with poor prognosis in ovarian cancer. Gynecol Obstet Invest. 2016;81:529–536. doi: 10.1159/000445747. [DOI] [PubMed] [Google Scholar]
- 23.Klotz LO. Posttranscriptional regulation of connexin-43 expression. Arch Biochem Biophys. 2012;524:23–29. doi: 10.1016/j.abb.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Axelsen LN, Calloe K, Holstein-Rathlou NH, Nielsen MS. Managing the complexity of communication: regulation of gap junctions by post-translational modification. Front Pharmacol. 2013;4:130. doi: 10.3389/fphar.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solan JL, Lampe PD. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 2014;588:1423–1429. doi: 10.1016/j.febslet.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Squecco R, Sassoli C, Nuti F, Martinesi M, Chellini F, Nosi D, Zecchi-Orlandini S, Francini F, Formigli L, Meacci E. Sphingosine 1-phosphate induces myoblast differentiation through Cx43 protein expression: a role for a gap junction-dependent and -independent function. Mol Biol Cell. 2006;17:4896–4910. doi: 10.1091/mbc.E06-03-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long J, Xie Y, Yin J, Lu W, Fang S. SphK1 promotes tumor cell migration and invasion in colorectal cancer. Tumour Biol. 2016;37:6831–6836. doi: 10.1007/s13277-015-4542-4. [DOI] [PubMed] [Google Scholar]
- 28.Bao Y, Guo Y, Zhang C, Fan F, Yang W. Sphingosine kinase 1 and sphingosine-1-phosphate signaling in colorectal cancer. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aasen T, Mesnil M, Naus CC, Lampe PD, Laird DW. Gap junctions and cancer: communicating for 50 years. Nat Rev Cancer. 2016;16:775–788. doi: 10.1038/nrc.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czyz J. The stage-specific function of gap junctions during tumourigenesis. Cell Mol Biol Lett. 2008;13:92–102. doi: 10.2478/s11658-007-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gartner C, Ziegelhoffer B, Kostelka M, Stepan H, Mohr FW, Dhein S. Knock-down of endothelial connexins impairs angiogenesis. Pharmacol Res. 2012;65:347–357. doi: 10.1016/j.phrs.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Czyz J, Piwowarczyk K, Paw M, Luty M, Wrobel T, Catapano J, Madeja Z, Ryszawy D. Connexindependent intercellular stress signaling in tisVasculogenic mimicry, SphK1 and Cx43 in colorectal cancer sue homeostasis and tumor development. Acta Biochim Pol. 2017;64:377–389. doi: 10.18388/abp.2017_1592. [DOI] [PubMed] [Google Scholar]
- 33.Kotini M, Mayor R. Connexins in migration during development and cancer. Dev Biol. 2015;401:143–151. doi: 10.1016/j.ydbio.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 34.Murphy SF, Varghese RT, Lamouille S, Guo S, Pridham KJ, Kanabur P, Osimani AM, Sharma S, Jourdan J, Rodgers CM, Simonds GR, Gourdie RG, Sheng Z. Connexin 43 inhibition sensitizes chemoresistant glioblastoma cells to temozolomide. Cancer Res. 2016;76:139–149. doi: 10.1158/0008-5472.CAN-15-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanczuga-Koda L, Sulkowska M, Koda M, Reszec J, Famulski W, Baltaziak M, Sulkowski S. Expression of connexin 43 in breast cancer in comparison with mammary dysplasia and the normal mammary gland. Folia Morphol (Warsz) 2003;62:439–442. [PubMed] [Google Scholar]
- 36.Teleki I, Szasz AM, Maros ME, Gyorffy B, Kulka J, Meggyeshazi N, Kiszner G, Balla P, Samu A, Krenacs T. Correlations of differentially expressed gap junction connexins Cx26, Cx30, Cx32, Cx43 and Cx46 with breast cancer progression and prognosis. PLoS One. 2014;9:e112541. doi: 10.1371/journal.pone.0112541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein A, Sagi-Assif O, Meshel T, Telerman A, Izraely S, Ben-Menachem S, Bayry J, Marzese DM, Ohe S, Hoon D, Erez N, Witz IP. CCR4 is a determinant of melanoma brain metastasis. Oncotarget. 2017;8:31079–31091. doi: 10.18632/oncotarget.16076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen DR, Lu DY, Lin HY, Yeh WL. Mesenchymal stem cell-induced doxorubicin resistance in triple negative breast cancer. Biomed Res Int. 2014;2014:532161. doi: 10.1155/2014/532161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Saghir JA, El-Habre ET, El-Sabban ME, Talhouk RS. Connexins: a junctional crossroad to breast cancer. Int J Dev Biol. 2011;55:773–780. doi: 10.1387/ijdb.113372je. [DOI] [PubMed] [Google Scholar]
- 40.El-Sabban ME, Pauli BU. Adhesion-mediated gap junctional communication between lungmetastatatic cancer cells and endothelium. Invasion Metastasis. 1994;14:164–176. [PubMed] [Google Scholar]
- 41.Elzarrad MK, Haroon A, Willecke K, Dobrowolski R, Gillespie MN, Al-Mehdi AB. Connexin-43 upregulation in micrometastases and tumor vasculature and its role in tumor cell attachment to pulmonary endothelium. BMC Med. 2008;6:20. doi: 10.1186/1741-7015-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanczuga-Koda L, Sulkowski S, Lenczewski A, Koda M, Wincewicz A, Baltaziak M, Sulkowska M. Increased expression of connexins 26 and 43 in lymph node metastases of breast cancer. J Clin Pathol. 2006;59:429–433. doi: 10.1136/jcp.2005.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamiche C, Clarhaut J, Strale PO, Crespin S, Pedretti N, Bernard FX, Naus CC, Chen VC, Foster LJ, Defamie N, Mesnil M, Debiais F, Cronier L. The gap junction protein Cx43 is involved in the bone-targeted metastatic behaviour of human prostate cancer cells. Clin Exp Metastasis. 2012;29:111–122. doi: 10.1007/s10585-011-9434-4. [DOI] [PubMed] [Google Scholar]
- 44.Han Y, Zhang PJ, Chen T, Yum SW, Pasha T, Furth EE. Connexin43 expression increases in the epithelium and stroma along the colonic neoplastic progression pathway: implications for its oncogenic role. Gastroenterol Res Pract. 2011;2011:561719. doi: 10.1155/2011/561719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sirnes S, Lind GE, Bruun J, Fykerud TA, Mesnil M, Lothe RA, Rivedal E, Kolberg M, Leithe E. Connexins in colorectal cancer pathogenesis. Int J Cancer. 2015;137:1–11. doi: 10.1002/ijc.28911. [DOI] [PubMed] [Google Scholar]
- 46.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’Er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma JL, Han SX, Zhu Q, Zhao J, Zhang D, Wang L, Lv Y. Role of Twist in vasculogenic mimicry formation in hypoxic hepatocellular carcinoma cells in vitro. Biochem Biophys Res Commun. 2011;408:686–691. doi: 10.1016/j.bbrc.2011.04.089. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z, Sun B, Qi L, Li H, Gao J, Leng X. Zinc finger E-box binding homeobox 1 promotes vasculogenic mimicry in colorectal cancer through induction of epithelial-to-mesenchymal transition. Cancer Sci. 2012;103:813–820. doi: 10.1111/j.1349-7006.2011.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Lin H, Pan J, Mo C, Zhang F, Huang B, Wang Z, Chen X, Zhuang J, Wang D, Qiu S. Vasculogenic mimicry in prostate cancer: the roles of EphA2 and PI3K. J Cancer. 2016;7:1114–1124. doi: 10.7150/jca.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu B, Zhou L, Yu L, Wu S, Song W, Gong X, Wang D. Evaluation of the correlation of vasculogenic mimicry, ALDH1, KAI1 and microvessel density in the prediction of metastasis and prognosis in colorectal carcinoma. BMC Surg. 2017;17:47. doi: 10.1186/s12893-017-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW, Che N, Wang XH, Du J, Liu YX, Sun BC. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology. 2010;51:545–556. doi: 10.1002/hep.23311. [DOI] [PubMed] [Google Scholar]
- 52.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 53.El HS, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL, Eichmann A, Delattre JY, Maniotis AJ, Sanson M. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133:973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su M, Feng YJ, Yao LQ, Cheng MJ, Xu CJ, Huang Y, Zhao YQ, Jiang H. Plasticity of ovarian cancer cell SKOV3ip and vasculogenic mimicry in vivo. Int J Gynecol Cancer. 2008;18:476–486. doi: 10.1111/j.1525-1438.2007.01034.x. [DOI] [PubMed] [Google Scholar]
- 55.Dong J, Zhao Y, Huang Q, Fei X, Diao Y, Shen Y, Xiao H, Zhang T, Lan Q, Gu X. Glioma stem/progenitor cells contribute to neovascularization via transdifferentiation. Stem Cell Rev. 2011;7:141–152. doi: 10.1007/s12015-010-9169-7. [DOI] [PubMed] [Google Scholar]
- 56.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 58.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Sun T, Sun BC, Zhao XL, Zhao N, Dong XY, Che N, Yao Z, Ma YM, Gu Q, Zong WK, Liu ZY. Promotion of tumor cell metastasis and vasculogenic mimicry by way of transcription coactivation by Bcl-2 and Twist1: a study of hepatocellular carcinoma. Hepatology. 2011;54:1690–1706. doi: 10.1002/hep.24543. [DOI] [PubMed] [Google Scholar]
- 60.Xu CY, Liu SQ, Qin MB, Zhuge CF, Qin L, Qin N, Lai MY, Huang JA. SphK1 modulates cell migration and EMT-related marker expression by regulating the expression of p-FAK in colorectal cancer cells. Int J Mol Med. 2017;39:1277–1284. doi: 10.3892/ijmm.2017.2921. [DOI] [PubMed] [Google Scholar]
- 61.Hirata N, Yamada S, Shoda T, Kurihara M, Sekino Y, Kanda Y. Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation. Nat Commun. 2014;5:4806. doi: 10.1038/ncomms5806. [DOI] [PubMed] [Google Scholar]
- 62.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, Polyak K. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Lin JH, Takano T, Cotrina ML, Arcuino G, Kang J, Liu S, Gao Q, Jiang L, Li F, Lichtenberg-Frate H, Haubrich S, Willecke K, Goldman SA, Nedergaard M. Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J Neurosci. 2002;22:4302–4311. doi: 10.1523/JNEUROSCI.22-11-04302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


