Abstract
Objective: The present paper aimed to investigate the therapeutic effect of quercetin in a rat model of bone cancer pain, and to further explore the molecular mechanism of quercetin in the treatment of bone cancer pain. Methods: The activation status of the osteoblasts in each group of rats was detected by anti-tartaric acid phosphatase (TRAP) immunohistochemistry, while the activation status of the osteoclasts was detected by alkaline phosphatase (BAP) immunohistochemistry. An ELISA assay was used to detect the levels of serum type I collagen carboxy-terminal pro-peptide (CDC), tartrate-resistant acid phosphatase (TRAP5b), and osteocalcin in each group, as well as the expressions of the main inflammatory mediator membrane protease (trypsin), TNF-α, and IL-1β in the PAR2/TRPV1 pathway in serum. Taking CD68 as a marker, immunohistochemistry was used to detect the positive proportion of macrophages in the left tibia of each group. Western blot was applied to analyze the expressions of the RANKL, OPG, RANK, PTHrP and IGF-1 proteins in rat bone tissue, the inflammatory mediators IL-8, M-CSF and TNF-α, and the PAR2/TRPV1 pathway-related molecules PAR2, TRPV1, PKC-Y and PKA in rat DRG neurons, as well as the neurotransmitters c-Fos, GFAP, PKR, and CGRP in the spinal cord. Results: Quercetin significantly reduces serum CTX, TRAP and osteocalcin expressions in a rat model of bone cancer pain and also significantly reduces the proportion of TRAP-positive cells. The drug can significantly reduce the positive proportion of local bone tissue macrophages in rats with bone cancer pain. It can significantly decrease the expressions of RANKL, RANK, PTHrP and IGF-1 proteins and the inflammatory mediators such as IL-8, M-CSF and TNF-α, significantly increase the expressions of OPG and further significantly inhibit the expressions of the PAR2/TRPV1 pathway-related molecules PAR2, TRPV1, PKC-γ and PKA in DRG neurons, as well as significantly reduce the levels of major inflammatory mediators (trypsin), TNF-α, and IL-1β in the PAR2/TRPV1 pathway. Conclusion: Quercetin can inhibit osteoclast activation and reduce bone destruction in the bone cancer pain model by regulating the RANKL/RANK/OPG signaling pathway and the inflammatory response. It can inhibit peripheral sensitization and central sensitization in bone cancer pain by regulating the PAR2/TRPV1 signaling pathway.
Keywords: Bone cancer pain, quercetin, RANKL/RANK/OPG signaling pathway, PAR2/TRPV1 signaling pathway
Introduction
Cancer pain is one of the main symptoms associated with tumor development and treatment [1,2]. It is reported that about 75-90% of cancer patients have suffered pain, and more than 50% of cancer patients cannot effectively control their pain. Cancer pain is more common in patients with tumor metastasis, and more than 80% of bone metastasis patients are distressed by cancer pain [3]. Cancer induced bone pain (CIBP) is the most common type of cancer pain and one of the most important reasons for the degradation of the quality of life and the living conditions of cancer patients [4].
Although there have been many studies on CIBP in recent years, its mechanism remains unclear. CIBP is associated with a decrease in bone density and/or the destruction of bone structure caused by bone resorption of osteoclasts in the medullary cavity and is also associated with small fracture of the periosteal stretch caused by the direct invasion of cancer cells into the nerve cells and by tumor proliferation. In addition, the tumor stimulates the nociceptive neurons and the innervation of the surface of the periosteum, thereby aggravating CIBP. However, the severity of CIBP is sometimes not directly related to the clinicopathological features of tumors and the extent of osteolysis, and not all bone tumors cause CIBP. Therefore, the pathogenesis of CIBP is characterized by heterogeneity, diversity and complexity [5,6].
Quercetin has a good expectorant and antitussive effect, along with a certain anti-asthmatic effect. In addition, it also has the effects of lowering blood pressure, enhancing capillary resistance, reducing capillary fragility, lowering blood fat, expanding coronary arteries, and increasing coronary blood flow, etc. However, whether it has any effect on bone cancer pain has not been reported, so the present research aims to reveal its molecular mechanism, and to find new targets for the treatment of cancer pain.
Methods
The establishment of a rat bone cancer pain model
One hundred Wistar female rats were anesthetized with chloral hydrate, and the skin on the left knee joint was disinfected. A 0.5 cm incision was made with scissors at about 0.5 cm below the knee joint, and the humeral surface was exposed after the separation of muscle tissue. The knee joint was fixed with the left hand, and at about 0.5 cm below the knee, the joint was drilled along the longitudinal direction toward the distal end of the humerus with a 7-gauge needle in the right hand. The depth was about 1.5 cm, and the needle was quickly pulled out. A total of 10 μl Walker-256 cancer cells at a concentration of 1×107/ml were injected into the bone marrow cavity of the rat tibia by a microinjection needle. After the needle was pulled out, the hole was quickly closed with bone wax. The incision was sutured after being washed with saline. The control group rats received 10 μl PBS containing no tumor cells.
Experimental grouping and administration
The rats with successful modeling were randomly divided into the sham operation group and the bone cancer pain group. The bone cancer pain group was randomly divided into the high dose group (20 mg/ml), the medium dose group (10 mg/ml), the low dose group (5 mg/ml), and the model group (same volume of PBS), with 20 rats per group. All model groups were given the drug on the first day after the models were established, and the rats was intragastrically administered the drug once a day for 21 days.
Immunohistochemistry to determine the activation of osteoblasts and osteoclasts and the positive proportion of macrophages
After we obtained the tibia tissues, the paraffin sections were deparaffinized. The dewaxed tibia tissue sections were placed in 3% H2O2 and incubated at room temperature for 5 minutes to eliminate the activities of endogenous peroxidase. The tissue sections were removed from the 3% H2O2, rinsed once with distilled water, and then immersed in PBS for 5 minutes. Goat serum diluted with 5-10% PBS was used to block the tissue sections. The tissue was incubated for 10 minutes at room temperature. Then, the blocking solution was removed, and the corresponding concentration of the primary antibody solution was added dropwise. The tissue was refrigerated overnight at 4°C. Next, the tissue was rinsed three times in PBS for 5 minutes each time. An appropriate amount of biotin-labeled secondary antibody solution was added dropwise. The tissue was incubated for 30 minutes at room temperature, then rinsed the times in PBS for 5 minutes each time. An appropriate amount of horseradish-labeled streptavidin working solution was added dropwise. The tissue was then incubated with PBS for 30 minutes at room temperature. The tissue was DAB developed for 15 minutes, and finally washed three times with pure water, counterstained, dehydrated, clarified, sealed, and observed.
ELISA to determine the contents of Trap5b, CTX, Osteocalcin, Trypsin, TNF-α and IL-1β in rat serum
The serum of the rats from each group administrated for 0, 7, 14, 21 days was obtained, and added with the diluted sample or the standard sample in ELISA plates at 100 μL/well. The control well was set. The biotinylated antibody was added at 50 μL/well and mixed. The plate was covered with the membrane, and then incubated at 37°C for 90 min. The plate was washed 4 times, and then an avidin-horseradish peroxidase Streptavidin-HRP marker at 100 μL/well was added. The plate was incubated at 37°C for 30 min, then washed 4 times. The coloring agent TMB was added at 100 μL/well. The color was developed at 37°C for 10 min. The stop solution was added at 50 μL/well to stop the reaction. The absorbance was read at a wavelength of 450 nm, and the concentrations of Trasp5b, CTX, Osteocalcin, Trypsin, TNF-α and IL-1β were calculated.
Western-blot assay detection of the expressions of the RANKL/RANK/OPG signaling pathway and inflammatory response-related proteins
The tibias, fresh DRG, and the spinal cords from each of the 21 d group rats were obtained. 400 μl of cell lysate and 40 μl of PMSF were added to each tissue. The cell culture flask was gently shaken, then placed on ice for 10 min to make the cells lyse evenly. The cells were repeatedly aspirated with a sterile syringe, and the lysed product was added to the EP tube. The EP tube was ice-bathed for 30 min, and centrifuged at 12000 g for 15 min. The supernatant was transferred to a new EP tube. Each tube was filled with 20 μl of protein sample buffer, boiled for 5 min, mixed, and then stored at -80°C. The above samples were obtained, and the protein was separated by 12% SDS-PAGE electrophoresis. The separated protein bands were transferred to the PVDF membrane using the wet method, then they were closed at room temperature for 1 h. The first anti-body was added (concentration 1:1000). The cells were incubated overnight at 4°C. The primary antibody was eluted. The secondary antibody (BA1026) (concentration 1:1000) was added and incubated for 1 h. The secondary antibody was washed off. The color was developed and fixed by chemiluminescence. The expressions of the RANKL/RANK/OPG signaling pathway and the inflammatory response-related proteins were determined.
Statistical methods
All data are expressed as the mean ± standard deviation. The t-test was used for comparison between the two groups, and the comparisons among multiple groups of data (> 2) were calculated using the one-way ANOVA technique. There was a statistical significance at P < 0.05. The data was analyzed using Graphpad Prism 5.0 (Graphpad Software. San Diego, CA).
Results
Effects of quercetin on osteoblasts and osteoclasts in rat bone cancer pain model
Bone cancer pain is usually accompanied by osteoclast dysfunction, which leads to bone absorption and destruction, and also the activation of osteoblasts. In order to elucidate the mechanism of quercetin in relieving bone cancer pain, the activation status of the osteoblasts was detected by TRAP immunohistochemistry, and the activation status of the osteoclasts was detected by BAP immunohistochemistry. Significant osteoblast and osteoclast staining were observed in the model group. The proportion of TRAP-positive cells in all the drug-administered groups was significantly lower than it was in the model group. See Figures 1, 2.
Figure 1.
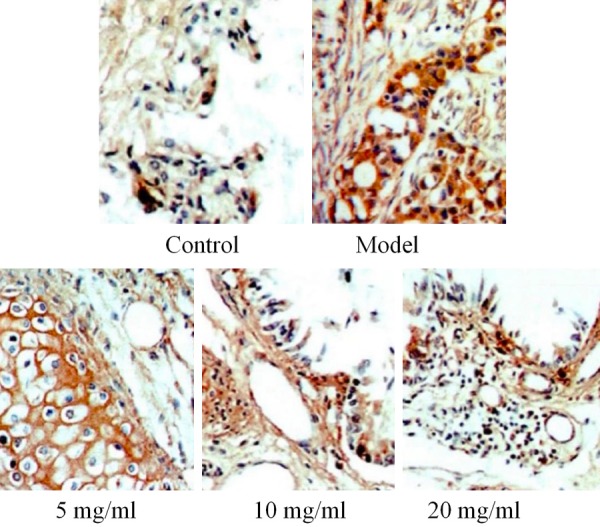
Effects of drugs on the number of osteoblasts in a rat bone cancer pain model.
Figure 2.

Effects of drugs on numbers of osteoclasts in a rat bone cancer pain model. **P < 0.01 vs Model *P < 0.05 vs Model ##P < 0.01 vs 5 mg/kg, #P < 0.05 vs 5 mg/kg, &&P < 0.01 vs 10 mg/kg, &P < 0.05 vs 10 mg/k, !!P < 0.01 vs 20 mg/k, !P < 0.05 vs 20 mg/kg.
Effects of drugs on the expressions of Trasp5b, CTX, Osteocalcin, Trypsin, TNF-α, and IL-1β in rat serum
The results showed that different concentrations of drugs can significantly reduce the expressions of Trasp5b, Trypsin, TNF-α, and IL-1β. High-dose drugs can reduce the expressions of CTX and osteocalcin, and the effects are more obvious with an increase in the drug administration period, as shown in Figures 3, 4, 5, 6, 7 and 8.
Figure 3.
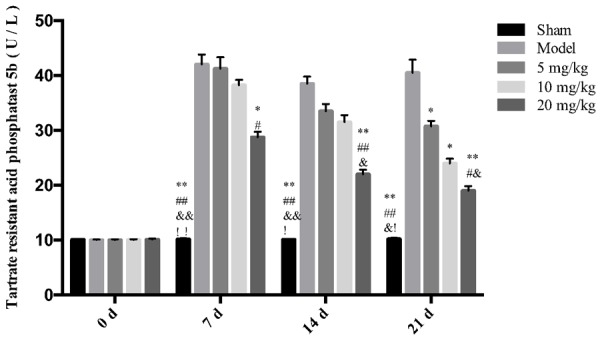
The effect of drugs on the expression of Trasp5b in rat serum. **P < 0.01 vs Model *P < 0.05 vs Model ##P < 0.01 vs 5 mg/kg, #P < 0.05 vs 5 mg/kg, &&P < 0.01 vs 10 mg/kg, &P < 0.05 vs 10 mg/kg.
Figure 4.
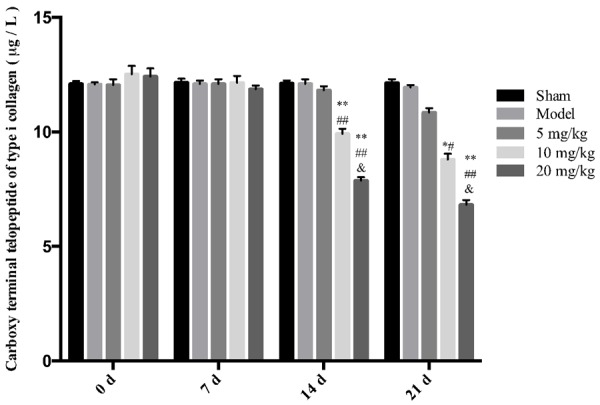
The effect of drugs on serum CTX levels in rats. **P < 0.01 vs Model *P < 0.05 vs Model ##P < 0.01 vs 5 mg/kg, &&P < 0.01 vs 10 mg/k, !!P < 0.01 vs 20 mg/kg.
Figure 5.
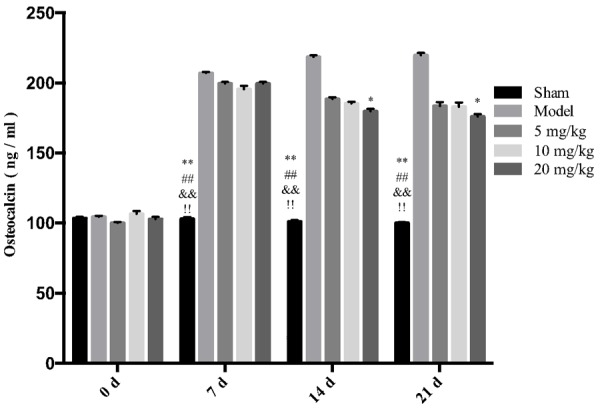
The effect of drugs on the expression of osteocalcin in rat serum. **P < 0.01 vs Model *P < 0.05 vs Model ##P < 0.01 vs 5 mg/kg, &&P < 0.01 vs 10 mg/kg, &P < 0.05 vs 10 mg/kg, !!P < 0.01 vs 20 mg/kg.
Figure 6.
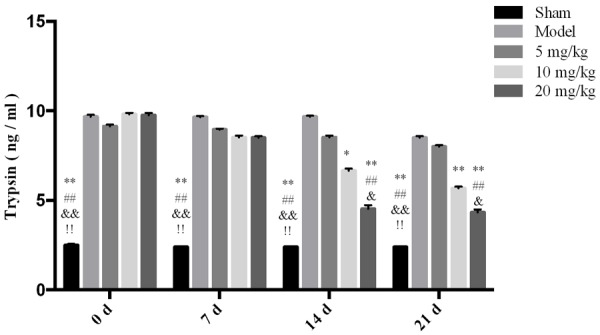
The effect of drugs on the trypsin level in rat serum. **P < 0.01 vs Model *P < 0.05 vs Model ##P < 0.01 vs 5 mg/kg, &&P < 0.01 vs 10 mg/kg, &P < 0.05 vs 10 mg/kg, !!P < 0.01 vs 20 mg/kg.
Figure 7.

The effect of drugs on the IL-1β level in rat serum. **P < 0.01 vs Model *P < 0.05 vs Model ##P < 0.01 vs 5 mg/kg, #P < 0.05 vs 5 mg/kg, &&P < 0.01 vs 10 mg/kg, &P < 0.05 vs 10 mg/kg, !!P < 0.01 vs 20 mg/kg, !P < 0.05 vs 20 mg/kg.
Figure 8.
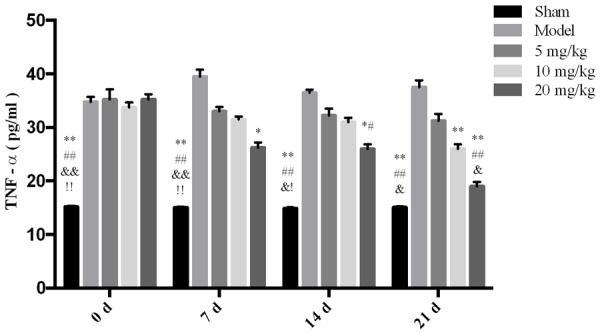
The effect of drugs on TNF-α expression in rat serum.
The effect of drugs on macrophages
In the present study, CD68 was used as a marker to detect the positive proportion of macrophages in the left tibia of each group by immunohistochemistry. There was almost no macrophage infiltration in the bone tissue of the sham group, and a large number of macrophages were detected in the model groups, and the infiltration was significantly improved after drug intervention. See Figure 9.
Figure 9.
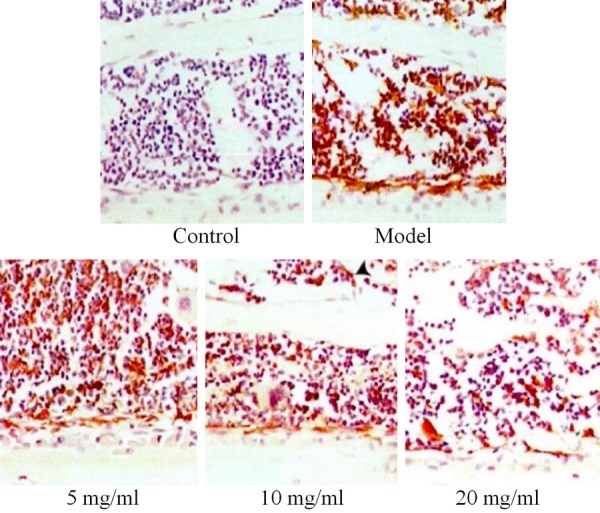
The effect of drugs on macrophages.
The effects of drugs on the expressions of the RANKL/RANK/OPG signaling pathway and inflammatory factor-related proteins
The results showed that the drug significantly decreased the expressions of the RANKL, RANK, PTHrP, and IGF-1 proteins and the inflammatory mediators such as IL-8, M-CSF, and TNF-α, significantly increased the expression of OPG and inhibited expressions of the PAR2/TRPV1 pathway-related molecules PAR2, TRPV1, PKC-γ, and PKA in the DRG neurons in rats with bone cancer pain. The drug also significantly reduced the expressions of the main inflammatory mediators (trypsin), TNF-α, and IL-1β in the PAR2/TRPV1 pathway, as shown in Figures 10, 11, 12 and 13.
Figure 10.
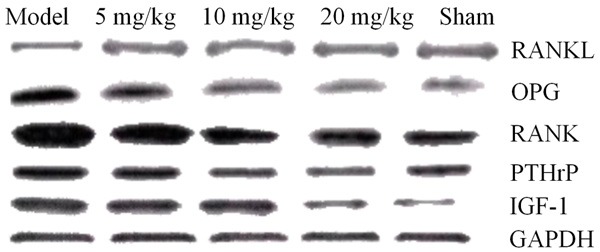
The effect of drugs on RANK pathway-associated proteins.
Figure 11.

The effect of drugs on inflammatory factor-related proteins.
Figure 12.
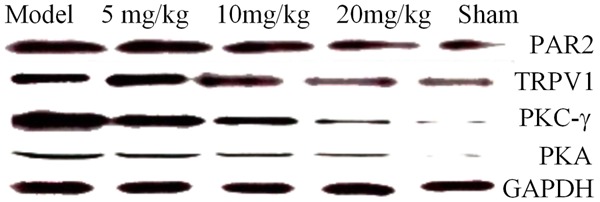
The effect of drugs on the PAR2/TRPV1 signaling pathway-associated proteins.
Figure 13.
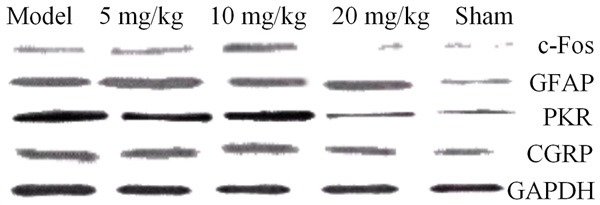
The effect of drugs on the neurotransmitter-related proteins in the spinal cord.
Discussion
The specific pathogenesis of bone cancer pain is still unclear. Recent studies have confirmed that the excessive activation of primary afferent sensory DRG neurons is closely related to the occurrence and development of bone cancer pain [7]. Primary afferent sensory DRG neurons are the first-order neurons of emotional sensory afferents. The unmyelinated C-type fibers emitted by small-diameter DRG neurons can directly transmit pain information and regulate thermal and mechanical hyperalgesia and spontaneous pain [8]. Cytokines secreted by tumors and surrounding inflammatory cells, such as endothelin, nerve growth factor, transforming growth factor TNF-α, IL-1β, and other local factors, such as local compression, ischemia and necrosis, can continuously stimulate nerve endings [9,10], changing the intrinsic membrane properties of DRG neurons, such as the polarization of resting membrane potential, reducing input resistance, reducing action potential voltage, current threshold, and post-hyperpolarization duration and amplitude, inducing DRG neuron excitation, and resulting in peripheral sensitization, which in turn increases the excitability of nociceptive neurons in the dorsal horn of the spinal cord, leading to central sensitization and the formation of bone cancer pain [11]. Therefore, peripheral sensitization and central sensitization by DRG are the foundations of the formation of bone cancer pain.
The amino terminus of PAR2 is cleaved by a protease or a protease analog such as a PAR2 agonist, to form a new amino terminus and coordinate with a specific closed region of PAR2, which form an activation coordination loop with the exposed extracellular domain, resulting in PAR2 activation and neural signal transmission. Protease-activated receptors are distributed in the central and peripheral nervous systems. In the dorsal horn of the spinal cord, PAR2 receptors are located in the microglia, house glial cells, neurons and afferent fibers, participating in a variety of physiological and pathological processes [12]. Recent studies have confirmed that PAR2 regulates the excitability of DRG neurons and plays an important role in the development of pain signaling and pain occurrence. Many recent experiments have demonstrated that PAR2 is expressed on DRG neurons in the thoracolumbar region of rats and mice [13,14]. Intestinal injection of PAR2 agonists can cause visceral nociceptor sensitization, while the injection of a PAR2 agonist in the foot can cause the secretion of substance P and a calcitonin gene-related peptide in the peripheral nerve tissues, causing thermal and mechanical hyperalgesia [15]. The tumor microenvironment is rich in protease and proteolytic peptides that directly activate PAR2 on DRG neurons, leading to spontaneous acute pain which can become persistent chronic cancer pain [16]. The regulation of the PAR2-induced excitability of the DRG neurons and the transmission of pain signals depends on the activation of TRPV1. TCPV1 is a cation channel that is non-selective and expressed on nociceptors, and it can regulate neuronal excitability and the transmission of pain signals, peripheral sensitization, and the centralization of inflammatory pain, neuropathic pain, and bone cancer pain. It plays an important role in the sensitization process. Exogenous factors such as ethanol, capsaicin, and endogenous factors such as protons and cannabinoids can directly activate TRPV1 [17]. In addition, PAR2 agonists and other inflammatory factors such as bradykinin and prostaglandins can indirectly activate TRPV1 and lead to Ca2+ influx, which induces DRG neurons via a calcium-dependent protein kinase II-cAMP reaction element binding protein cascade. The expression and phosphorylation of CGRP, ERK, and CREB produce peripheral sensitization, which in turn causes an increase in nociceptive nerve excitability of the spinal dorsal horn, leading to central sensitization, further leading to bone cancer pain. Recent studies have confirmed that the mechanism by which PAR2 activates TRPV1 is mainly achieved by activating PKC and PKA [18]. After activation, PAR2 can either be coupled with the GaQll gene to activate phospholipase (PLCP) to further activate PKC, or PAR2 can be coupled with GAS gene which directly activates PKA, and the activated PKC and PKA can phosphorylate TRPV1.
In conclusion, the experiment showed that quercetin inhibits the peripheral and central sensitization of bone cancer pain by inhibiting the PAR2/TRPV1 signaling pathway, inhibits bone cancer by inhibiting the expression of inflammatory stromal cells such as macrophages and inflammatory mediators, and inhibits the peripheral sensitization of pain to achieve the goal of relieving and treating bone cancer pain, thus providing a scientific basis for the treatment of bone cancer pain.
Acknowledgements
This work was supported by the National Natural Science Foundation of China, No. 31572217.
Disclosure of conflict of interest
None.
References
- 1.Marie N, Luckett T, Davidson PM, Lovell M, Lal S. Optimal patient education for cancer pain: a systematic review and theory-based meta-analysis. Support Care Cancer. 2013;21:3529–3537. doi: 10.1007/s00520-013-1995-0. [DOI] [PubMed] [Google Scholar]
- 2.Swarm RA, Abernethy AP, Anghelescu DL, Benedetti C, Buga S, Cleeland C, Deleon-Casasola OA, Eilers JG, Ferrell B, Green M, Janjan NA, Kamdar MM, Levy MH, Lynch M, McDowell RM, Moryl N, Nesbit SA, Paice JA, Rabow MW, Syrjala KL, Urba SG, Weinstein SM, Dwyer M, Kumar R National Comprehensive Cancer Network. Adult cancer pain. J Natl Compr Canc Netw. 2013;11:992–1022. doi: 10.6004/jnccn.2013.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Running A, Seright T. Integrative oncology: managing cancer pain with complementary and alternative alerapies. Curr Pain Headache Rep. 2012;16:325–331. doi: 10.1007/s11916-012-0275-x. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Clinical features of metastatic bope disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–49s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 5.Bao Y, Hua B, Hou W, Shi Z, Li W, Li C, Chen C, Liu R, Qin Y. Involvement of protease activated receptor 2 in nociceptive behavior in a rat model of bone cancer. J Mol Neurosci. 2014;52:566–76. doi: 10.1007/s12031-013-0112-7. [DOI] [PubMed] [Google Scholar]
- 6.Bao Y, Hou W, Hua B. Protease activated receptor 2 signalling pathways: a role in pain processing. Expert Opin Ther Targets. 2014;18:15–27. doi: 10.1517/14728222.2014.844792. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Q, Fang D, Cai J, Wan Y, Han JS, Xing GG. Enhanced excitability of small dorsal root ganglion neurons in mts with bone cancer pain. Mol Pain. 2012;8:24. doi: 10.1186/1744-8069-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ke CB, He WS, Li CJ, Shi D, Gao F, Tian YK. Enhanced SCN7A/Nax expression contributes to bone cancer pain by increasing excitability of neurons in dorsal root ganglion. Neuroscience. 2012;227:80–9. doi: 10.1016/j.neuroscience.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Villiere V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J Neurophysio. 1996;76:1924–1941. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- 10.Middlemiss T, Laird BJ, Fallon MT. Mechanisms of cancer-induced bone pain. Clin Oncol (R Coll Radiol) 2011;23:387–392. doi: 10.1016/j.clon.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, Mantyh PW. Bone cancer pain. Ann N Y Acad Sci. 2010;1198:173–181. doi: 10.1111/j.1749-6632.2009.05429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao Y, Hou W, Hua B. Protease activated receptor 2 signalling pathways: a role in pain processing. Expert Opin Ther Targets. 2014;18:15–27. doi: 10.1517/14728222.2014.844792. [DOI] [PubMed] [Google Scholar]
- 13.Bao Y, Hou W, Liu R, Gao Y, Kong X, Yang L, Shi Z, Li W, Zheng H, Jiang S, Li C, Qin Y, Hua B. PAR2-mediated upregulation of BDNF contributes to central sensitization in bone cancer pain. Mol Pain. 2014;10:28. doi: 10.1186/1744-8069-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanke T, Takizawa T, Kabeya M, Kawabata A. Physiology and pathophysiology of proteinaseactivated receptors (PARs): PAR-2 as a potential therapeutic target. J Pharmaeol Sci. 2005;97:38–42. doi: 10.1254/jphs.fmj04005x7. [DOI] [PubMed] [Google Scholar]
- 15.García PS, Gulati A, Levy JH. The role of thrombin and protease-activated receptors in pain mechanisms. Thromb Haemost. 2010;103:1145–51. doi: 10.1160/TH09-12-0848. [DOI] [PubMed] [Google Scholar]
- 16.Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P, Hollenberg MD, Wallace JL. Protease-activated receptor-2 and hyperalgesia: anovel pain pathway. Nat Med. 2001;7:821–6. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Yang C, Wang ZJ. Proteinase activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxd-induced neuropathic pain. Neuroscience. 2011;193:440–451. doi: 10.1016/j.neuroscience.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 18.Yoneda T, Hata K, Nakanishi M, Nagae M, Nagayama T, Wakabayashi H, Nishisho T, Sakurai T, Hiraga T. Involvement of acidic microenvironment in the pathophysiology of cancer-associated bone pain. Bone. 2011;48:100–105. doi: 10.1016/j.bone.2010.07.009. [DOI] [PubMed] [Google Scholar]


