Abstract
Apoptosis of microglia is one of the most important pathophysiologic changes after spinal cord injury (SCI). Recently, microRNAs (miRNAs) have been reported to play a crucial role in the regulation of neuronal apoptosis. However, the exact role and underlying mechanisms of miRNAs in the regulation of microglial apoptosis remain unclear. We first performed miRNA microarray to analyze the miRNA expression patterns in a rat SCI model. The expression of microRNA-23b (miR-23b) in spinal cord after contusion SCI was determined by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). BV-2 cells were exposed to H2O2 conditions to establish an in vitro model of SCI. Then, the effects of miR-23b on the apoptosis were investigated through both gain- and loss-of-function studies in this cellular model of SCI. Also, the expression of the main proteins in NF-κB signaling was assessed by Western Blot. Furthermore, bioinformatics analysis was used to predict the target of miR-23b in BV-2 cells, which was validated with a dual-luciferase reporter assay, qRT-PCR, and Western blot analysis. The expression of TGF-β-activated kinase 1 binding protein 3 (TAB3) in cells was overexpressed by transfection with pcDNA-TAB3, and the effects of TAB3 overexpression on miR-23b-mediated apoptosis were detected. Here, we demonstrated that miR-23b was significantly down-regulated in SCI rat model. We also found that the expression level of phosphorylated p65 (p-p65) protein was increased in the SCI rat model. Subsequently, treatment of BV-2 cells with H2O2 decreased the levels of miR-23b and activated NF-κB pathway in a dose dependent manner. Furthermore, overexpression of miR-23b inhibited the BV-2 apoptosis and NF-κB activation, while miR-23b inhibition enhanced the apoptosis and NF-κB activation induced by H2O2. Moreover, our data showed TAB3, an upstream positive regulator of the NF-κB pathway, was proven to be a target of miR-23b in BV-2 cells. Most importantly, we demonstrated that overexpression of miR-23b attenuated the apoptosis by inhibiting the expression of TAB3. These findings suggested that miR-23b protected BV-2 cells from apoptosis by modulating the NF-κB pathway and could serve as a new strategy for the treatment of SCI.
Keywords: Spinal cord injury, microglia, apoptosis, MicroRNA-23b, NF-κB pathway, TAB3
Introduction
Spinal cord injury (SCI) is a serious and disabling condition resulting in severe movement dysfunction, muscle weakness and changes in sensation. Despite the great efforts that have been made, therapeutic advances for patients with SCI have been limited thus far [1]. Therefore, novel therapeutics for SCI are highly needed.
Microglia are one main type of cells present in the spinal cord, which constitute 5-20% of total glial cells in rodents, depending on the specific neuroanatomical region of the central nervous system (CNS). Emerging evidence has confirmed the critical role of microglia in the pathogenesis of SCI through the release of pro-inflammatory factors [2]. Recently, it has been reported that the apoptosis of microglia near the lesion is one of the most important pathophysiologic changes after SCI [3]. An interesting finding was that the increased number of apoptotic microglia in the injured spinal cord by approximately 40%, led to a reduced histological and functional outcome after SCI [4]. The protection of microglia from apoptosis following SCI could, therefore, potentially lead to the amelioration of SCI [5]. However, the precise mechanisms of such apoptosis are not currently fully understood.
MicroRNAs (miRNAs) are a novel class of small non-coding RNAs (approximately 22-25 nt long) that negatively regulate gene expression at the posttranscriptional level by combining with target mRNA in the 3’-untranslated region (3’-UTR) leading to either inhibition of translation or degradation of mRNA [6]. Several miRNAs were found to contribute to the development of SCI. For example, miR-199b was downregulated in microglial cells separated from rat models of SCI and downregulation of miR-199 promoted an acute SCI [7]. In contrast, miR-21 was upregulated in spinal cords in rat with SCI, and treatment with antagomir-miR-21 led to attenuated recovery in hindlimb motor function, increased lesion size, and decreased tissue sparing in rats, as well as increased apoptosis following SCI [8]. These studies provided novel insight to understand further the molecular mechanism of SCI-induced apoptosis following SCI.
In this study, we explored the miRNA expression profile after SCI in rats. Furthermore, the correlation among miR-23b, nuclear factor (NF)-κB pathway and TAB3 was explored, thereby elucidating the underlying mechanisms of miR-23b overexpression in the protection of microglia from apoptosis following SCI. It is anticipated that our study provides a future direction of treatments for SCI.
Materials and methods
Animal preparation
Sprague-Dawley male adult rats, weighing 220-250 g each, were purchased from the Animal Center (Chongqing Medical University, Chongqing, China). All animals were housed in standard conditions of temperature and 12-h light/dark cycle and fed with food and water. The Animal Care and Use Committee of Yongchuan Hospital Affiliated to Chongqing Medical University approved all experiments in accordance with the Declaration of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Spinal cord injury
Spinal cord injury was induced using a weight drop device as previously reported [9]. All animals were anaesthetized through intraperitoneal injection of 4% sodium pentobarbital (40 mg/kg, i.p.). An incision was made along the middle of the back, exposing the paravertebral muscles. A laminectomy was performed at the T9-T11 level, exposing the cord without damaging the dura. The exposed dorsal surface of the cord was subjected to weight drop impact using a 10 g metal rod at a height of 25 mm. All mice were allowed to recover in a room maintained at 24±1°C during the experimental period.
MicroRNA expression profiling
miRNA profiling of spinal cord was performed with the miRCURYTM LNA Array v.16.0 (Agilent Technologies). Briefly, a 10-mm-long spinal cord around the lesion epicenter was harvested and fresh-frozen in liquid nitrogen. Total RNA was isolated with the miRNeasy mini kit (Qiagen, West Sussex, UK). The RNA quality and quantity were measured with a NanoDrop® spectrophotometer (ND-1000, Nanodrop Technologies). The procedure and image processing method as described previously [10].
Cell culture and treatment
The immortalized murine BV-2 cell line was purchased from the Chinese Academy of Medical Science and cultured in DMEM/F12 (Hyclone, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Hyclone), 100 U ml-1 penicillin and streptomycin in 25-cm2 culture flasks at 37°C in a humidified atmosphere with 5% CO2.
Cells were treated with drugs (see below). H2O2 (30% w/w solution; Sigma, St. Louis, MO, USA) was administered to the cells as a 100 mM solution in phosphate-buffered saline (PBS).
Quantitative reverse transcription-PCR (qRT-PCR)
Total RNA from 10 mm spinal cord tissues and cells was isolated using TRIzol (Invitrogen, CA) according to manufacturer’s instructions. After reverse transcription, cDNA was amplified by using SYBR-Green Premix (Takara, Otsu, Japan). The expressions of miR-23b and TAB3 in tissue and cells were, respectively, normalized to the expression of U6 and GAPDH. qRT-PCR was performed using the Applied Biosystems 7900 Fast Real-Time PCR system (Applied Biosystems). The data were analyzed by delta Ct method. The sequences of primers were purchased from Guangzhou RiboBio Co. Ltd: TAB3 forward 5’-CAGCCCACAGCTTGATATTC-3’ and reverse 5’-CATGACTTTGCCCGAGTTAG-3’; GAPDH forward, 5’-GAAGATGGTGATGGGATTTC-3’, and reverse, 5’-GAAGGTGAAGGTCGGAGT-3’; miR-23b forward, 5’-TGACCTGAAACATACACGGGA-3’ and reverse, 5’-GTGCAGGGTCCGAGGT-3’; U6 forward, 5’-AAAGACCTGTACGCCAACAC-3’ and reverse, 5’-GTCATACTCCTGCTTGCTGAT-3’.
Transfection
The miR-23b mimics, miR-23b inhibitor and negative control [11] were synthesized by GenePharma (Shanghai, China). The open reading frame (ORF) of human TAB3 without the 3’-UTR was generated by PCR amplification and then cloned into the mammalian expression vector pcDNA 3.1 (Invitrogen; Life Technologies). Cell transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Detection of cell apoptosis
Flow cytometry was used to detect the effect of miR-23b on cell apoptosis. 24 h after transfection, cells were harvested, washed with ice-cold PBS and re-suspended in 500 μl of binding buffer. After the density of the cells was adjusted to 5 × 105/ml, cells were incubated with 5 μl Annexin V and 5 μl propidium iodide [12] (BD Biosciences) at room temperature in the dark for 15 min. Apoptotic BV-2 cells were quantified by flow cytometry (BD Biosciences).
Western blot
Western blot was performed as previously described [11]. The membranes were blotted with rabbit primary antibody against TAB3 and p-p65 (1:1000, Santa Cruz, CA, USA) or β-actin (1:2000, Santa Cruz, CA, USA) for overnight at 4°C, followed by incubation with goat anti-rabbit IgG-HRP secondary antibody (1:2000, Santa Cruz, CA, USA) at room temperature for 1 h, and then visualized by using an enhanced chemiluminescence detection method (Thermo Scientific). Relative intensities were determined with a PhosphorImager and ImageQuant (Amersham Biosciences, Piscataway, NJ, USA) software analysis.
Luciferase assays
A cDNA fragment of the TAB3 3’-UTR mRNA containing the seed sequence of the miR-23b-binding site or a mutated binding site was cloned into the pmirGLO dual-luciferase vector (Promega, Madison, WI, USA). The dual-luciferase vector was co-transfected with miR-23b mimics, miR-23b inhibitor or NC into HEK293T cells (2.0 × 104) grown in a 96-well plate. Cells were harvested 48 h after transfection for luciferase assay using a luciferase assay kit (Promega) according to the manufacturer’s protocol. Transfection was repeated in triplicate.
Caspase-3 activity assay
Activity of caspase-3 was performed as previously described [13]. Briefly, keratinocytes were lysed and re-suspended in 50 μl of ice-cold cell lysis buffer for 30 min. Caspase-3 activity was determined using a Caspase-3 activity kit (Beyotime). Caspase-3 activity was quantified in the samples with a microplate spectrophotometer (Biotek) at an absorbance of 405 nm.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Differences were analyzed with the Student’s t-test between two groups or with one-way ANOVA among four groups. A p-value of less than 0.05 was considered statistically significant.
Results
miR-23b was downregulated in spinal cord in rats after SCI
To profile the expression of miRNAs after SCI, we first established the animal model of SCI using a weight drop device on the dorsal spinal cord via a two-level T9-T11 laminectomy in accordance with previous descriptions [14] and then a miRNA microarray was performed in spinal cord in rats after SCI. Our data revealed that compared with the normal group, 25 miRNAs were upregulated and 25 miRNAs were downregulated (Figure 1A). Among these aberrantly expressed miRNAs, miR-23b was one of miRNAs being most significantly downregulated. Recently, a study performed by Im et al. showed that miR-23b was decreased in injured spinal cord of SCI rats and played a crucial role in the amelioration of SCI by repressing inflammation [15]. Thus, we chose miR-23b for further study. MiR-23b expression levels were also confirmed by qRT-PCR in injured spinal cord of the rats with SCI (Figure 1B). Recently, several studies have reported that inhibition of the activation of NF-κB pathway could lead to spinal cord tissue repair and improved motor function after SCI [16,17]. Thus, we test the protein expression level of phosphorylated p65 (p-p65), an important mediator of NF-κB activation using Western blot. Consistent with a previous study [7], we observed a remarkably elevated p-p65 protein level in injured spinal cord of the rats with SCI (Figure 1C). These results indicated that miR-23b might be involved in the development of SCI through NF-κB pathway.
Figure 1.
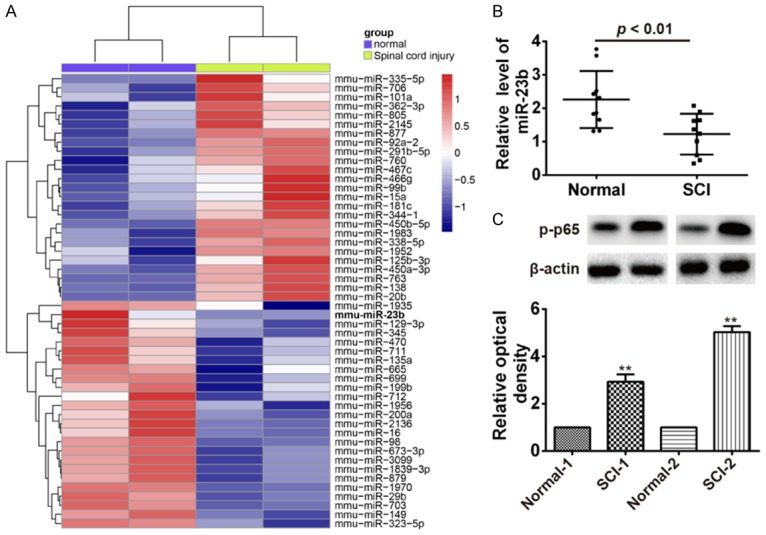
Downregulation of miR-23b and activation of NF-κB were observed in rats with SCI. A. Heatmap of normalized expression levels of miRNAs in spinal cord in rats (n=2) with SCI and normal spinal cord in rats (n=2). Green indicates low expression levels; red indicates high expression levels. B. qRT-PCR was performed to determine the expression levels of miR-23b in spinal cord in rats (n=10) with SCI and normal spinal cord in rats (n=10). P < 0.01 vs. Normal group. C. Western Blot was used to detect the protein level of p-p65 in spinal cord in rats (n=3 with SCI and normal spinal cord in rats (n=3). Data represent the mean ± SD of three independent experiments. **P < 0.01 compared with Normal-1 or Normal-2.
Downregulation of miR-23b and activation of NF-κB in H2O2 treated BV-2 cells
It is well known that the apoptosis of microglia is an important feature of SCI [3,18]. Because reactive oxygen species (ROS), such as H2O2, superoxide anion, and hydroxyl radicals can activate various pathways of apoptosis, we treated murine BV-2 cells with H2O2 to generate a cellular model of SCI for detection of the role of miR-23b in the apoptosis. First, we employed different concentrations of H2O2 (50, 100, 200 and 400 μM) to activate BV-2 cells, which have been widely used to explore the pathologic factors after SCI as it is reported to share many characteristics with primary microglia [19]. Consistent with the results in injured spinal cord of the rats with SCI, the level of miR-23b was significantly reduced in BV-2 cells after H2O2 treatment in a dose-dependent manner (Figure 2A). Similarly, the protein level of p-p65 was also higher in BV-2 cells after H2O2 treatment in a dose dependent manner (Figure 2B). These data suggest that miR-23b may affect the apoptosis through regulation of NF-κB pathway in H2O2 induced cell model of SCI.
Figure 2.

Downregulation of miR-23b and activation of NF-κB in H2O2 treated BV-2 cells. BV-2 cells were treated with different concentrations of H2O2 (50, 100, 200 and 400 μM) for 48 h. A. qRT-PCR was performed to determine the expression levels of miR-23b in H2O2 treated BV-2 cells. B. Western blot was used to detect the protein level of p-p65 in H2O2 treated BV-2 cells. Data represent the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with Control group.
Overexpression of miR-23b attenuated the microglia apoptosis through suppressing NF-κB activation
Given the important role of microglial apoptosis in the pathogenesis of SCI, we assess the role of miR-23b on the microglia apoptosis. The BV-2 cells were transfected with miR-23b mimics, miR-23b inhibitor and the corresponding scramble control following H2O2 treatment, then, the apoptosis and the activity of caspase-3 were measured. As shown in Figure 3A, 3B, the apoptosis and the activity of caspase-3 were significantly reduced in the miR-23b mimics + H2O2 group compared with H2O2 + mimics NC group. In contrast, the apoptosis and the activity of caspase-3 were significantly increased in the miR-23b inhibitor + H2O2 group compared with H2O2 + inhibitor NC group. (Figure 3D, 3E). A recent study revealed miR-23b could activate the NF-κB pathway by targeting TAB2, TAB3 and IKK-α [20]. Therefore, to further illustrate whether miR-23b modulates the NF-κB pathway in the BV-2 cells treated with H2O2, we examined the expression of p-p65 using Western blot. As shown in Figure 3C, 3F, overexpression of miR-23b significantly attenuated the expression of p-p65, whereas inhibition of miR-23b increased the expression of p-p65. These data suggest that miR-23b could attenuate the microglia apoptosis through suppressing NF-κB activation.
Figure 3.

Overexpression of miR-23b attenuated microglial apoptosis by suppressing NF-κB activation. The BV-2 cells were transfected with miR-23b mimics and mimics NC following H2O2 treatment for 48 h. (A) The apoptosis portion of BV-2 cells was measured by flow cytometry assay. (B) The activity of caspase-3 was measured by a caspase-3 activity kit. (C) The protein level of p-p65 was determined by Western Blot. The BV-2 cells were transfected with miR-23 inhibitor or inhibitor NC following H2O2 treatment for 48 h. Then, the apoptosis portion of BV-2 cells was measured by flow cytometry assay (D), the activity of caspase-3 was measured by a caspase-3 activity kit (E) and the protein level of p-p65 was determined by Western Blot (F) respectively. Western Blot. Data represent the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with control group. ##P < 0.01 compared with H2O2 + mimics NC or H2O2 + inhibitor NC.
TAB3 was a direct target of miR-23b
Recently, a study from Zhu et al. showed that miR-23b suppressed NF-κB activation by targeting TAB3, and, consequently, represses autoimmune inflammation [20]. Thus, we sought to determine whether miR-23b also inhibit NF-κB pathway by targeting TAB3 in our cell model. First, two publicly available databases TargetScan and miRanda were used to predict the potential downstream targets of miR-23b. As suggested in Figure 4A, the complementary sequence of miR-23b was found in the 3’-UTR of TAB3 mRNA. To test whether that miR-23b could directly target 3’-UTR of TAB3, a luciferase reporter assay was performed. The results showed that overexpression of miR-23b significantly decreased the luciferase activity of TAB3 with wt 3’-UTR, whereas knockdown of miR-23b increased the luciferase activity of TAB3 with wt 3’-UTR (Figure 4B). Likewise, cells co-transfected with miR-23b mimics, miR-23b inhibitor, and TAB3-mut-3’UTR showed no obvious change in their luciferase activity (Figure 4B). We further examined whether miR-23b could modulate the expression of TAB3 in BV-2 cells. The results of qRT-PCR and Western blot showed that overexpression of miR-23b significantly reduced the expressions of TAB3 at protein and mRNA levels, whereas inhibition of miR-23b promoted the expressions of TAB3 at mRNA and protein levels (Figure 4C, 4D). Taken together, these data indicate that miR-23b modulates the NF-κB pathway by targeting TAB3 in BV-2 cells.
Figure 4.
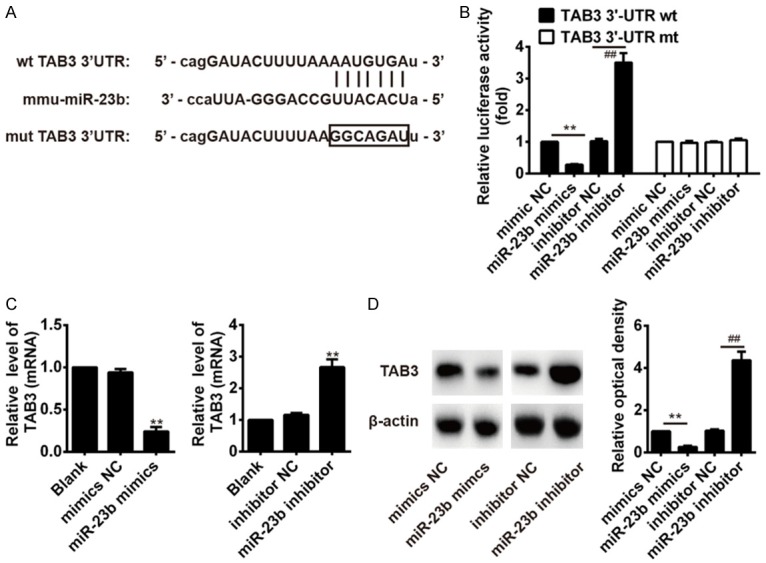
TAB3 was a direct target of miR-23b. A. The putative binding site of miR-23b and TAB3 is shown. B. Luciferase assay of HEK293 cells co-transfected with firefly luciferase constructs containing the TAB3 wild-type or mutated 3’-UTRs and miR-23b mimics, mimics NC, miR-23b inhibitor or inhibitor NC. **P < 0.01 vs. mimics NC, ##P < 0.01 vs. inhibitor NC. C. The mRNA expression level of TAB3 after transfection with miR-23b mimic or miR-23b inhibitor was measured by qRT-PCR. **P < 0.01 vs. mimics NC, inhibitor or Blank. D. The protein expression level of TAB3 after transfection with miR-23b mimic or miR-23b inhibitor was measured by western blot. Data represent the mean ± SD of three independent experiments. **P < 0.01 vs. mimics NC, ##P < 0.01 vs. inhibitor NC.
Overexpression of TAB3 reversed the inhibitory effects of miR-23b on the apoptosis of BV-2 cells
To further investigate whether miR-23b reduces the apoptosis of microglia via TAB3, BV-2 cells were first co-transfected with miR-23b mimic and pcDNA-TAB3 plasmids, following H2O2 treatment. The apoptosis and the activity of caspase-3 were measured by flow cytometry assay and a caspase activity kit. As shown in Figure 5A, 5B, overexpression of TAB3 reversed the inhibitory effects of miR-23b mimics on the apoptosis and the activity of caspase-3 in H2O2 treated BV-2 cells. These data indicated that miR-23b attenuated the apoptosis of BV-2 cells induced by H2O2 via targeting TAB3.
Figure 5.
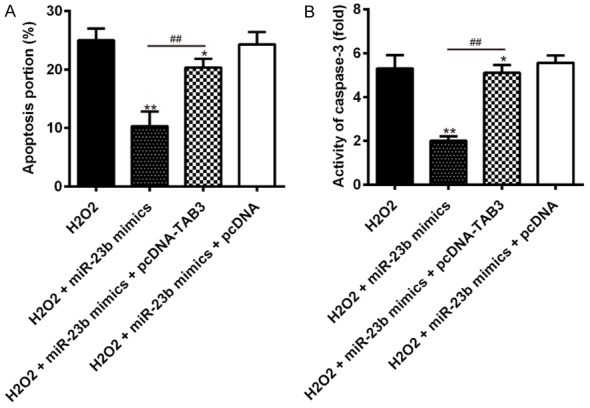
Overexpression of TAB3 reversed the inhibitory effects of miR-23b on microglial apoptosis in H2O2 treated BV-2 cells. The BV-2 cells were co-transfected with miR-23b mimics, pcDNA-TAB3, or the corresponding scrambled control following H2O2 treatment for 48 h. A. The apoptosis portion of BV-2 cells was measured by flow cytometry assay. B. The activity of caspase-3 was measured by a caspase-3 activity kit. Data represent the mean ± SD of three independent experiments. **P < 0.01 vs. H2O2 alone group, ##P < 0.01 vs. H2O2 + miR-23b mimics.
Discussion
In the present study, we found that low expression of miR-23b and activation of NF-κB were observed in rat after SCI. Moreover, miR-23b showed a protective role in H2O2-induced apoptosis in BV2 cells by targeting TAB3/NF-κB pathway. Our findings implicate that miR-23b/TAB3/NF-κB axis may reveal ideal therapeutic targets for SCI treatment.
A large body of evidence has indicated that alteration of miRNAs may be involved in the progression of a variety of neurological diseases including SCI [21,22]. Wei et al. showed that miR-146a could suppress the inflammation induced by SCI through downregulating TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated kinase 1 (IRAK1) expression [23]. Zhou et al. found that upregulation of miR-494 improved functional recovery, reduced tissue damage, and inhibited apoptotic cell death induced by SCI in rats [24], indicating its protective role after SCI. These studies suggest that alteration of miRNAs after SCI may be involved in the pathogenesis of SCI. Therefore, we used the miRNA microarray to determine the miRNA level in SCI rat model and found a large set of miRNAs were abnormally expressed in injured spinal cord compared with sham group. Among them, miR-23b was one of the miRNAs that was most significantly downregulated and its expression was further confirmed by qRT-PCR analysis. To explore the miR-23b effect on SCI, we treated murine BV-2 cells with H2O2 to generate a cellular model of SCI. According to our data, miR-23b expression was reduced by the H2O2 treatment in a dose-dependent manner. Moreover, overexpression of miR-23b significantly suppressed the apoptosis induced by SCI, whereas inhibition of miR-23b enhanced the apoptosis induced by SCI. These data indicated that downregulation of miR-23b may contribute to the progression of SCI.
NF-κB is an important factor of inflammatory processes and autoimmune diseases which consists of homo- and heterodimers of different Rel family proteins (p65, RelB, c-Rel, p52, and p50). Recently, NF-κB has been proved to be involved in SCI [18]. For example, Zhou et al. found that the protein level of IKKβ and p-p65 were dramatically elevated in activated microglia [7]. Lu et al. found that targeting IKK/NF-κB pathway improved the recovery of locomotor function by reducing the infiltration of inflammatory cells and apoptosis after SCI in rats [16]. However, the molecular regulation mechanism of NF-κB pathway in SCI remains unclear. Our results are in agreement with those of previous studies, which reported that p-p65 was elevated in the spinal cord of SCI rats and in ROS-induced BV-2 cells. Moreover, we also found that overexpression of miR-23b significantly suppressed the protein expression of p-p65, while inhibition of miR-23b promoted the expression of p-p65, indicating that miR-23b protects microglia from apoptosis after SCI through suppressing NF-κB pathway.
TAB3 is a transforming growth factor-β-activated kinase 1 (TAK1) binding protein, and plays a crucial role in the activation of the NF-κB pathway [25,26]. Kanayama et al. showed that TAB3 could activate the NF-κB pathway through binding to polyubiquitin chains [26]. Recently, it has been reported that miR-23b suppresses IL-17-, tumor necrosis factor α (TNF-α)- or IL-1β-induced NF-κB activation via TAB3 [20]. Based on these results, we supposed that miR-23b also inhibited NF-κB pathway by targeting TAB3 in microglia cell. As expected, our data showed that miR-23b inhibited the protein and mRNA levels for TAB3 by targeting its 3’-UTR in BV-2 cells. These data indicate that miR-23b regulates the NF-κB pathway via inhibiting TAB3 level after SCI. More importantly, we found that overexpression of TAB3 could reverse the inhibitory effects of miR-23b overexpression on the apoptosis in H2O2 treated BV-2 cells. Our findings suggest that miR-23b inhibits the NF-κB activation via targeting TAB3 and, consequently, attenuated the apoptosis after SCI.
In summary, our study confirms that miR-23b inhibited NF-κB activation by targeting TAB3 and subsequently attenuated the apoptosis of microglia induced by SCI, revealing its protective effects. Our results further indicate that targeting miR-23b/TAB3/NF-κB axis may be useful in the treatment of SCI.
Acknowledgements
This work was supported by the Chongqing Yongchuan Science and Technology Research Project Foundation (Ycstc2014bf5003).
Disclosure of conflict of interest
None.
References
- 1.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 2.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 3.Li GL, Brodin G, Farooque M, Funa K, Holtz A, Wang WL, Olsson Y. Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J Neuropathol Exp Neurol. 1996;55:280–289. doi: 10.1097/00005072-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Vidal PM, Lemmens E, Avila A, Vangansewinkel T, Chalaris A, Rose-John S, Hendrix S. ADAM17 is a survival factor for microglial cells in vitro and in vivo after spinal cord injury in mice. Cell Death Dis. 2013;4:e954. doi: 10.1038/cddis.2013.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou HJ, Wang LQ, Xu QS, Fan ZX, Zhu Y, Jiang H, Zheng XJ, Ma YH, Zhan RY. Downregulation of miR-199b promotes the acute spinal cord injury through IKKbeta-NF-kappaB signaling pathway activating microglial cells. Exp Cell Res. 2016;349:60–67. doi: 10.1016/j.yexcr.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Hu JZ, Huang JH, Zeng L, Wang G, Cao M, Lu HB. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J Neurotrauma. 2013;30:1349–1360. doi: 10.1089/neu.2012.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. discussion 126-128. [DOI] [PubMed] [Google Scholar]
- 10.Yu DS, Lv G, Mei XF, Cao Y, Wang YF, Wang YS, Bi YL. MiR-200c regulates ROS-induced apoptosis in murine BV-2 cells by targeting FAP-1. Spinal Cord. 2014 doi: 10.1038/sc.2014.185. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F, Capogrossi MC. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011;18:1628–1639. doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Criollo A, Niso-Santano M, Malik SA, Michaud M, Morselli E, Marino G, Lachkar S, Arkhipenko AV, Harper F, Pierron G, Rain JC, Ninomiya-Tsuji J, Fuentes JM, Lavandero S, Galluzzi L, Maiuri MC, Kroemer G. Inhibition of autophagy by TAB2 and TAB3. EMBO J. 2011;30:4908–4920. doi: 10.1038/emboj.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Jia R, Wang C, Hu T, Wang F. Piceatannol promotes apoptosis via up-regulation of microRNA-129 expression in colorectal cancer cell lines. Biochem Biophys Res Commun. 2014;452:775–781. doi: 10.1016/j.bbrc.2014.08.150. [DOI] [PubMed] [Google Scholar]
- 14.Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- 15.Im YB, Jee MK, Choi JI, Cho HT, Kwon OH, Kang SK. Molecular targeting of NOX4 for neuropathic pain after traumatic injury of the spinal cord. Cell Death Dis. 2012;3:e426. doi: 10.1038/cddis.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimenez-Garza O, Camacho J, Ibarra A, Martinez A, Guizar-Sahagun G. Early effects of modulating nuclear factor-kappaB activation on traumatic spinal cord injury in rats. Ann N Y Acad Sci. 2005;1053:148–150. doi: 10.1196/annals.1344.012. [DOI] [PubMed] [Google Scholar]
- 18.Carson MJ, Thrash JC, Walter B. The cellular response in neuroinflammation: the role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin Neurosci Res. 2006;6:237–245. doi: 10.1016/j.cnr.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 20.Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W, Shi Y, Harley JB, Shen N, Qian Y. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Kwon EJ, Tsai LH. MicroRNAs in learning, memory, and neurological diseases. Learn Mem. 2012;19:359–368. doi: 10.1101/lm.026492.112. [DOI] [PubMed] [Google Scholar]
- 22.Martirosyan NL, Carotenuto A, Patel AA, Kalani MY, Yagmurlu K, Lemole GM Jr, Preul MC, Theodore N. The role of microRNA markers in the diagnosis, treatment, and outcome prediction of spinal cord injury. Front Surg. 2016;3:56. doi: 10.3389/fsurg.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei J, Wang J, Zhou Y, Yan S, Li K, Lin H. MicroRNA-146a contributes to SCI recovery via regulating TRAF6 and IRAK1 expression. Biomed Res Int. 2016;2016:4013487. doi: 10.1155/2016/4013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu H, Xie R, Liu X, Shou J, Gu W, Gu S, Che X. MicroRNA-494 improves functional recovery and inhibits apoptosis by modulating PTEN/AKT/mTOR pathway in rats after spinal cord injury. Biomed Pharmacother. 2017;92:879–887. doi: 10.1016/j.biopha.2017.05.143. [DOI] [PubMed] [Google Scholar]
- 25.Cheung PC, Nebreda AR, Cohen P. TAB3, a new binding partner of the protein kinase TAK1. Biochem J. 2004;378:27–34. doi: 10.1042/BJ20031794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]


