Abstract
Objective: Prostate cancer is one of the most diagnosed malignancies in men worldwide. Novel (nua) kinase family 1 (NUAK1) is a member of adenosine monophosphate (AMP)-related kinase which participates in varying cancers progression. However, the role of NUAK1 in prostate tumorigenesis has not been fully characterized. The aim of this study was to elucidate the potential biological role of NUAK1 in prostate cancer. Methods: Quantitative real-time PCR (qRT-PCR) was performed to determine the expression levels of NUAK1 and microRNA-30b-5p (miRNA-30b-5p) in prostate cancer cell lines and samples. Western blot was conducted to explore the related protein levels of epithelial-mesenchymal transition (EMT) and NUAK1 expression in prostate cancer cells. Trans-well test was used to assay prostate cancer cell migration and invasion. Luciferase assays were employed to probe the interaction between NUAK1 and miR-30b-5p. Results: NUAK1 abundance was enhanced in prostate cancer tissues and cell lines. The knockdown of NUAK1 may inhibit prostate cancer cells EMT, migration and invasion. Luciferase assays suggested NUAK1 was a target gene of miR-30b-5p. Furthermore, miR-30b-5p suppressed EMT, migration, and invasion in prostate cancer cells and introduction of NUAK1 abated the inhibitory effect. Conclusions: Both of NUAK1 and miR-30b-5p were required for prostate cancer progression. NUAK1 interference limited prostate cancer cell EMT, migration and invasion by miRNA-30b-5p modulating, providing a promising therapeutic approach for prostate cancer.
Keywords: Prostate cancer, NUAK1, miR-30b-5p, epithelial-mesenchymal transition, migration, invasion
Introduction
Prostate cancer is one of the most common types of malignancies in men, with an incidence of one in six new cases of male cancer [1]. Patients with prostate cancer have a high risk of metastasis and mortality [2]. There is still a need to determine additional drivers of prostate cancer although a few genes that initiate prostate cancer have been identified [3,4]. Recently, cell migration and invasion is regarded as a major cause of cancer-induced death [5]. Epithelial-mesenchymal transition (EMT) has been identified as a critical player in cancer metastasis [6]. Moreover, cancer cellular adhesion is reduced and detached from each other through EMT in early metastasis [7]. Uncovering the mechanism of the process may provide a novel avenue for prostate cancer treatment.
Novel (nua) kinase family 1 (NUAK1), also termed as the fifth member of adenosine monophosphate-activated protein kinase (AMPK)-related kinase family (ARK5), plays various roles in regulating cellular adhesion, metabolism and response [8]. Overexpression of NUAK1 has been found to support tumor cell viability and reduce the overall survival in gastric cancer [9]. Likewise, NUAK1 is suggested to be involved in cell migration and invasion via EMT in gastric cancer [10]. In addition, addition of NUAK1 promotes EMT, migration, and invasion in ovarian cancer [11]. NUAK1 is strongly associated with tumor migration and metastatic potential in many other cancers, including pancreatic cancer [12], lung cancer [13] and ovarian cancer [14], suggesting that NUAK1 was a potential therapeutic target in many malignancies. However, the regulatory effect of NUAK1 on prostate cancer progression remains poorly understood.
The expression of mRNA is regulated by microRNAs (miRNAs), which function in cancer cell proliferation, invasion and angiogenesis [15]. Inhibition of NUAK1 inhibited cell migration and invasion by miR-204 overexpression in non-small-cell lung carcinoma (NSCLC) cell [16]. Moreover, NUAK1 plays essential role in melanoma invasion by miR-211 targeting [17]. Accordingly, it also reveals a potential role of NUAK1 in malignancies as a common target of series of miRNAs. The available efforts indicate that miR-30b-5p as a tumor suppressor, and plays an essential role in renal cell carcinoma and gastric cancer [18,19]. There is little evidence in support of the interaction between NUAK1 and miR-30b-5p.
In this study, to identify the role of NUAK1 in prostate cancer, mRNA and protein expression levels were detected in prostate cancer tissues and cells. Here we focused on the role of NUAK1 in epithelial-mesenchymal transition (EMT), migration, and invasion in DU145 and PC-3 cells and probed the interaction between NUAK1 and miR-30b-5p.
Materials and methods
Specimens
In this study, prostate cancer samples and adjacent normal tissues from 37 patients at Xiangyang Central Hospital were snap-frozen in liquid nitrogen immediately and stored at -80°C until required. The study was approved by the Institutional Research Ethics Committee of Xiangyang Central Hospital and written informed consent was obtained from all recruited patients. The patients were classified into high and low NUAK1 expression groups for further survival assay according to the statistical analysis of NUAK1 expression level in cancer tissues. So the low NUAK1 expression group (n=18) was defined as those below the mean value of NUAK1 expression, whereas the high NUAK1 expression group (n=19) consisted of those above the mean value in prostate cancer tissues.
Cell culture
Normal Prostate Epithelial Cells (PrEC) and two human prostate cancer cell lines (DU145 and PC-3) were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). Cells were maintained in RPMI-1640 cell culture medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco), 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified incubator with 5% CO2 during the study.
RNA extraction and qRT-PCR
Quantitative real-time PCR (qRT-PCR) was used to detect the relative levels of NUAK1 and miR-30b-5p in prostate cancer tissues and cell lines. Total RNA was extracted from tissues and cells using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Subsequently, first strand cDNA was synthesized with mRNA or miRNA Reverse Transcription Kit (Invitrogen), respectively and then mixed with SYBR green (Applied Biosystems, Foster City, CA, USA) for qRT-PCR detection with the following amplification protocol: 95°C for 1 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min. The gene expression was analyzed with 2-ΔΔCt method. β-actin and U6 small RNA were used as internal control for normalization of NUAK1 and miR-30b-5p, respectively. Primers were listed as follows: NUAK1 (Forward, 5’-CCGCTCACTGATGTAATCGT-3’; Reverse, 5’-GTCATCTCTCAACCATCCTCAT-3’), β-actin (Forward, 5’-AGCAGC ATCGCCCCAAAGTT-3’; Reverse, 5’-GGGCACGAAGGCTCATCATT-3’), miR-30b-5p (Forward, 5’-ATCGCTGTAAACATCCTACAC-3’; Reverse, 5’-GTCG TATC CAGTGCAAGGGTCCGAGGTATTCGCACTGGATACGACAGCTGA-3’), U6 (Forward, 5’-GCTTCGGCAGCACATATACTAAAAT-3’; Reverse, 5’-CGCTTC ACGAATTTGCGTGTCAT-3’).
Cell transfection
NUAK1 small interfering RNA (siNUAK1), NUAK1 overexpression plasmids, pcDNA, miR-30b-5p mimics, inhibitors and negative control (NC) were obtained from GenePharma (Shanghai, China). The transfections were performed into 5×104 cells cultured in a humidified incubator with 5% CO2 at approximately 80% confluence using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. At 24 h post-transfection, transfection efficiencies were analyzed by qRT-PCR.
Transwell assay
Transwell chambers (Costar, Corning, NY, USA) were used to analyze cell migration and invasion ability. For cell invasion assay, chambers were coated with Matrigel (BD, San Jose, CA, USA). DU145 and PC-3 cells (2×104 cells/well) suspended in serum-free medium were placed in the upper chambers. Then the cells were carefully removed with a cotton swab after incubation at 37°C in 5% CO2 for 8 h. Invading cells on the basal side of the membrane were fixed with 100% methanol for 10 min, stained with hematoxylin (Sigma, St. Louis, MO, USA) and counted under a microscope (Olympus, Tokyo, Japan). Three visual fields were randomly selected. The trans-well chambers without Matrigel were selected for cell migration assay following the similar approach.
Luciferase assays
Luciferase assays were used to validate whether NUAK1 is a direct target gene of miR-30b-5p. Plasmids containing wild type (WT) or mutant (MUT) NUAK1 3’ untranslated regions (3’-UTR) as a tracking gene were specifically synthesized (GeneCopoeia, Rockville, MD, USA). Luciferase assays were performed according to the manufacturer’s protocols. The plasmids containing NUAK1 WT or MUT target gene 3’-UTR were co-transfected with miR-30b-5p mimics or inhibitors with Lipofectamine 2000 in DC145 and PC-3 cells. Transfected cells were collected and contributed to luciferase activities analysis using Luciferase Assay Kit (GeneCopoeia) after 48 h.
Western blot (WB)
Cell proteins were extracted in RIPA lysis buffer and quantified by BCA assay (Thermo Fisher). Denatured proteins from each sample were separated by SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were blocked in 5% non-fat milk for 1 h and incubated with primary antibodies overnight at 4°C. Fibronectin, Vimentin, E-cadherin, NUAK 1 and β-actin as a control were measured using monoclonal rabbit antibodies (Cell Signaling Technology, Danvers, MA, USA). Then membranes were hatched with anti-rabbit IgG (CST) marked by horseradish peroxidase (HRP) for 2 h after washing with Tris-buffer saline containing 0.1% Tween 20 (TBST). The visualization of immunoreactivity was realized by enhanced chemiluminescence (ECL) chromogenic substrate (GE Healthcare, Amersham, UK) conducted to densitometry analysis using Image Lab software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All experiments were repeated more than three times. Data were presented as the mean ± standard deviation from three independent experiments. All data were analyzed using Student’s t test by SPSS 22.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
NUAK1 is up-regulated in prostate cancer tissues or cells
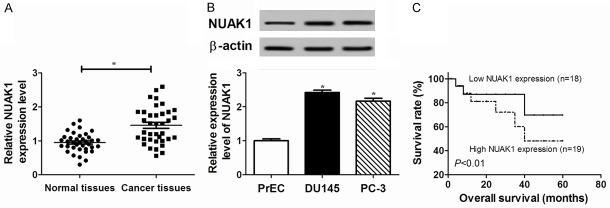
The expression of NUAK1 was evaluated in prostate cancer tissues and cell lines. An increased abundance of NUAK1 was observed at mRNA level in prostate cancer samples compared with adjacent normal tissues (Figure 1A). Moreover, NUAK1 protein levels in DU145 and PC-3 cells were obviously greater than that in PrEC cells (Figure 1B). Furthermore, a survival curve showed that high NUAK1 expression level induced a lower survival rate than the low NUAK1 expression group (Figure 1C) (P<0.01). These results suggested that increased NUAK1 expression resulted in a worse outcome in prostate cancer.
Figure 1.
The expression of NUAK1 was enhanced in prostate cancer tissues and cells. A. The expression of NUAK1 was investigated in prostate cancer tissues compared with adjacent normal tissues by qRT-PCR as well as WB. The number of patients was 37 (n=37). B. The abundance of NUAK1 was investigated in prostate cancer cell lines (DU145 and PC-3) compared with normal Prostate Epithelial Cells (PrEC) by WB. C. The survival rate was detected in the low and high NUAK1 expression group. The low NUAK1 expression group (n=18) was below the mean value of NUAK1 expression, and the high NUAK1 expression group (n=19) was above the mean value in prostate cancer tissues. Data are the mean ± standard deviation from three independent experiments. *P<0.05.
NUAK1 promotes EMT, migration and invasion in prostate cancer cells
Altered mRNA expression occurred in DU145 and PC-3 cells transfected with siNUAK1 compared with the NC group (Figure 2A). To investigate the function of NUAK1 in EMT, EMT-related markers were detected in DU145 and PC-3 cells by WB. Depletion of NUAK1 induced an obvious increase of E-cadherin protein level and great reduction of vimentin and fibronectin compared with the siNC group in DU145 and PC-3 cells, respectively (Figure 2B-D). In particular, abrogation of NUAK1 drastically impaired the ability of cell migration and invasion in DU145 and PC-3 cells (Figure 2E, 2F). Taken together, these findings indicated that NUAK1 knockdown inhibited DU145 and PC-3 cell EMT, migration, and invasion.
Figure 2.
NUAK1 knockdown inhibited EMT, migration, and invasion in prostate cancer cells. (A) The expression of NUAK1 was investigated in DU145 and PC-3 cells transfected by siNUAK1 compared with siNC by qRT-PCR. (B-E) The expression of EMT-related E-cadherin, vimentin and fibronectin was analyzed in DU145 and PC-3 cells compared with controls by WB. (F) Cell migration or (G) invasion ability was detected in DU145 and PC-3 cells by transwell assay. Data are the mean ± standard deviation from three independent experiments. *P<0.05.
NUAK1 is a target gene of miR-30b-5p in prostate cancer cells
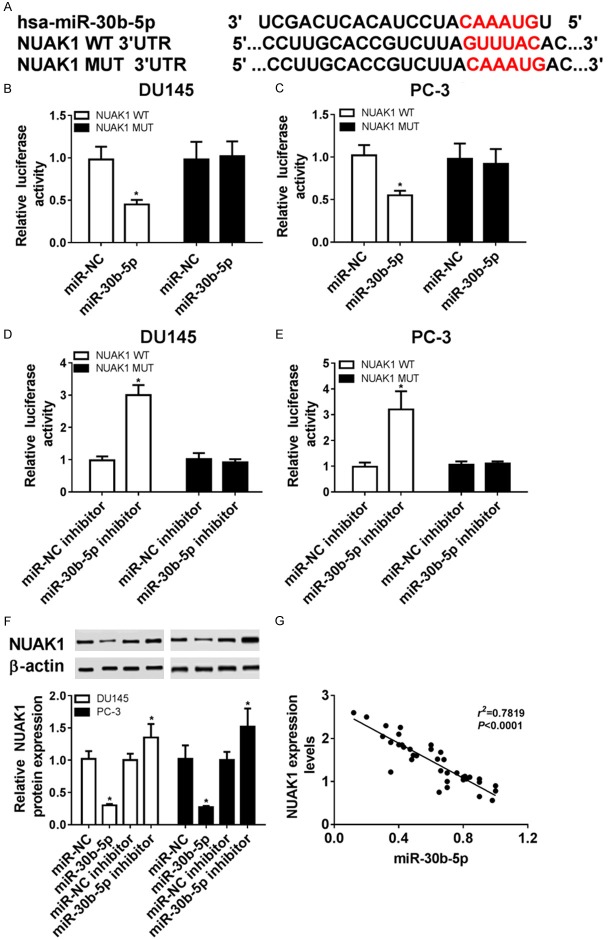
In order to confirm whether NUAK1 level was regulated by miR-30b-5p, we probed a putative miR-30b-5p binding sites of 3’-UTR of NUAK1 by TargetScan (Figure 3A). DU145 and PC-3 cells with the plasmids of WT 3’-UTR of NUAK1 transfection showed a marked decrease of luciferase activity in the presence of miR-30b-5p mimics and elevated luciferase activity in the cells co-transfected with miR-30b-5p inhibitor, but this was not true for MUT 3’-UTR of NUAK1 (Figure 3B-E). Moreover, addition of miR-30b-5p induced a strong reduction of NUAK1 protein level in DU145 and PC-3 cells compared with NC. However, depletion of miR-30b-5p caused an opposite effect (Figure 3F). More particularly, there was a negative correlation (R2=0.7819, P<0.0001) with the abundances of NUAK1 mRNA and miR-30b-5p in prostate cancer tissues from 37 patients (Figure 3G). There results indicated that NUAK1 was directly regulated by miR-30b-5p.
Figure 3.
NUAK1 was identified as a target of miR-30b-5p. A. Wild-type (WT) and mutant (MUT) of the putative miR-30b-5p targeting sequences in NUAK1 3’UTR. B-E. Analysis of luciferase activity was performed in DU145 and PC-3 cells transfected by miR-30b-5p mimic or inhibitor compared with negative control (NC). F. The expression of NUAK1 protein was analyzed in DU145 and PC-3 cells transfected by miR-30b-5p mimic or inhibitor compared with their NC respectively by WB. G. Correlation plot for NUAK1 expression versus miR-30b-5p levels in prostate cancer specimens, n=37. Data are the mean ± standard deviation from three independent experiments. *P<0.05.
MiR-30b-5p inhibits EMT, migration, and invasion in prostate cancer cells
Since NUAK1 was targeted by miR-30b-5p, the role of miR-30b-5p was also analyzed in prostate cancer cells. The expression level of miR-30b-5p obviously decreased in DU145 and PC-3 cells compared with that in PrEC cells (Figure 4A). For further study, DU145 and PC-3 cells were transfected with miR-30b-5p mimics. The qRT-PCR results confirmed that the miR-30b-5p level was specially increased in cells transfected with miR-30b-5p mimics (Figure 4B). In addition, accumulation of miR-30b-5p obviously enhanced E-cadherin protein level and inhibited the levels of Vimentin and Fibronectin compared with NC group in DU145 and PC-3 cells (Figure 4C, 4D). Similarly, addition of miR-30b-5p significantly decreased migrated and invasive abilities in DU145 and PC-3 cells (Figure 4E, 4F). These findings suggested that miR-30b-5p inhibited DU145 and PC-3 cell EMT, migration, and invasion.
Figure 4.
miR-30b-5p expression was decreased and addition of miR-30b-5p inhibited EMT, migration, and invasion in prostate cancer cells. A. The expression of miR-30b-5p was investigated in DU145 and PC-3 cells compared with PrEC by qRT-PCR. B. The alteration of miR-30b-5p was investigated in DU145 and PC-3 cells with miR-30b-5p mimic transfection compared with NC by qRT-PCR. C, D. The expression of EMT-related E-cadherin, vimentin, and fibronectin was analyzed in DU145 and PC-3 cells with miR-30b-5p mimic transfection compared with NC by WB. E, F. Cell migration and invasion abilities were detected in DU145 and PC-3 cells with miR-30b-5p mimic transfection compared with NC. Data re the mean ± standard deviation from three independent experiments. *P<0.05.
Addition of NUAK1 reversed miR-30b-5p-mediated inhibitory effect on EMT, migration, and invasion in prostate cancer cells
We hypothesized the inhibitory effect of miR-30b-5p might be attenuated by NUAK1 in prostate cancer cells. The results showed that abundant presence of NUAK1 decreased E-cadherin protein level and increased vimentin and fibronectin levels in PC-3 cells in comparisons between NC+NUAK1 and NC+pcDNA3.1 group (Figure 5A-D). Additionally, addition of NUAK1 increased prostate cancer cell migration and invasion abilities compared with NC+pcDNA3.1 group, whereas addition of miR-30b played an opposing role (Figure 5E, 5F). Furthermore, introduction of NUAK1 attenuated the miR-30b-5p-mediated inhibitory effect on EMT, migration, and invasion in prostate cancer cells (Figure 5). Taken together, these findings revealed NUAK1 was a key target of miR-30b, and NUAK1 ablated miR-30b-5p-mediated inhibitory effect in prostate cancer cells.
Figure 5.

Overexpression of NUAK1 attenuated the effect of miR-30b-5p on cell EMT, migration, and invasion. A-D. The expression of EMT-related E-cadherin, vimentin and fibronectin was analyzed in DU145 and PC-3 cells co-transfected with miR-30b-5p mimic and NUAK1 compared with NC-pcDNA3.1 group by WB. E, F. Cell migration and invasion abilities were detected in DU145 and PC-3 cells co-transfected with miR-30b-5p mimic and NUAK1 compared with the NC-pcDNA3.1 group. Data are the mean ± standard deviation from three independent experiments. *P<0.05.
Discussion
In the present study, we first showed that deficiency of NUAK1 inhibited EMT, migration, and invasion by regulation of miR-30b-5p in prostate cancer cells. It was previously suggested that NUAK1 was up-regulated with poor prognosis in NSCLC and pancreatic cancer, suggesting NUAK1 as a tumorigenic gene [12,13]. Similarly, NUAK1 expression was abnormally elevated in prostate cancer tissues and cell lines, suggesting that NUAK1 played a key role in prostate cancer progression.
Consistently, this study first investigated the potential effect of NUAK1 on EMT, migration, and invasion in prostate cancer cells. We found that NUAK1 knockdown blocked the migration and invasion in prostate cancer cells in accordance with its role in squamous cell carcinoma and intrahepatic cholangiocarcinoma [20,21]. Significant insight has been gained that invasion and metastasis of prostate cancer are the biggest obstacles in the clinic [22]. Such work showed that NUAK1 is involved in cell migration and invasion in NSCLC cells and NUAK1 knockdown decreased lung metastasis in a murine xenograft model [13]. In gastric cancer, ARK5 (NUAK1) was increased and promoted cell migration and invasion by EMT [10]. Besides, epithelial-mesenchymal transition (EMT) also played a pivotal role in cancer metastasis [6]. The main hallmark of EMT is down-regulation of epithelial markers like E-cadherin, cytokeratin and up-regulation of mesenchymal markers including N-cadherin, fibronectin, and vimentin [23-25]. Mounting evidence indicates EMT is involved in prostate cancer formation and metastasis [26,27]. Recently, NUAK1 was reported as an inducer of EMT in nasopharyngeal carcinoma [28]. However, there are few studies on the function of NUAK1 in prostate cancer. In this study, absence of NUAK1 inhibited EMT in prostate cancer cells. Therefore, the view was reported that NUAK1 might play a key role in prostate cancer by affecting EMT, migration and invasion.
Generally, the expression of mRNA was controlled by miRNAs and in turn led to an impact on the outcome of cancer progression [15]. Concordantly, NUAK1, as an oncogene, promoted cell proliferation, migration and invasion by miR-96 targeting in pancreatic cancer [12]. Moreover, NUAK1 was reported as a target of miR-145 and inhibition of NUAK1 decreased proliferation, growth and invasion in intrahepatic cholangiocarcinoma [21]. Overexpression of miR-204 inhibited NSCLC cell migration and invasion via suppressing NUAK1 expression [16]. Recently, various studies have described hundreds of miRNAs that were differentially expressed in prostate cancer samples [29,30]. MiR-30b-5p, as a member of miR-30 family, played a crucial regulator role in the progression of several cancers [31,32]. Accumulating studies have suggested that miR-30b-5p repressed cancer cell migration and invasion as a tumor suppressor [33,34]. In view of above, it is urgent to investigate whether NUAK1 is a target gene of miR-30-5p. Interestingly, luciferase assays suggested that NUAK1 might be a direct target gene of miR-30b-5p.
Since functional NUAK1 that seemed to be regulated by miR-30b-5p, the related mechanism of miR-30b-5p was investigated in prostate cancer cells. miR-30b-5p was suggested to be required for hepatoma cells, revealed by inhibitory effect on proliferation, EMT, migration as well as invasion [35]. Moreover, addition of miR-30b-5p might participate in cell proliferation and colorectal cancer growth in vivo [36]. Similarly, this study showed that miR-30b-5p overexpression suppressed EMT, migration, and invasion in prostate cancer cells. Furthermore, we described that introduction of NUAK1 counteracted the miR-30b-5p-mediated role, which also uncovered that the presence of NUAK1 exacerbated a worse outcome in prostate cancer cells. This is also consistent with other targets of miR-30b-5p as described previously. For instance, all of KRAs, PIK3CD and BCL2 were suggested as potential targets of miR-30b-5p, and addition of those mRNAs attenuated the inhibitory effect of miR-30b-5p on colorectal cancer cell proliferation [36]. In addition, investigators have reported that Cthrc1, regulated by miR-30b-5p, was required for cell invasion and migration in NSCLC cells [33]. Likewise in NSCLC, Rab18 was also controlled by miR-30b-5p and contributed to cell proliferation [37].
In conclusion, NUAK1 is up-regulated in prostate cancer tissues or cells. The interaction between miR-30b-5p and NUAK1 was validated in prostate cancer cells. Moreover, NUAK1 facilitated EMT, migration, and invasion in prostate cancer cells, and this effect might be abrogated by introduction of miR-30b-5p. It was expected that NUAK1 could be exploited as a novel therapeutic involved in prostate cancer.
Disclosure of conflict of interest
None.
References
- 1.Liu C, Liu T, Zhang N, Liu Y, Li N, Du P, Yang Y, Liu M, Gong K, Yang X, Zhu H, Yan K, Yang Z. (68) Ga-PSMA-617 PET/CT: a promising new technique for predicting risk stratification and metastatic risk of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2018;45:1852–1861. doi: 10.1007/s00259-018-4037-9. [DOI] [PubMed] [Google Scholar]
- 2.Berg KD, Thomsen FB, Mikkelsen MK, Ingimarsdottir IJ, Hansen RB, Kejs AM, Brasso K. Improved survival for patients with de novo metastatic prostate cancer in the last 20 years. Eur J Cancer. 2017;72:20–27. doi: 10.1016/j.ejca.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Liu W. DNA alterations in the tumor genome and their associations with clinical outcome in prostate cancer. Asian J Androl. 2016;18:533–542. doi: 10.4103/1008-682X.177120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shtivelman E, Beer TM, Evans CP. Molecular pathways and targets in prostate cancer. Oncotarget. 2014;5:7217–7259. doi: 10.18632/oncotarget.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han T, Kang D, Ji D, Wang X, Zhan W, Fu M, Xin H, Wang J. How does cancer cell metabolism affect tumor migration and invasion? Cell Adh Migr. 2013;7:395–403. doi: 10.4161/cam.26345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye X, Brabletz T, Kang Y, Longmore G, Nieto M, Stanger B, Yang J, Weinberg R. Upholding a role for EMT in breast cancer metastasis. Nature. 2017;547:E1–E3. doi: 10.1038/nature22816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteverde T, Muthalagu N, Port J, Murphy DJ. Evidence of cancer-promoting roles for AMPK and related kinases. FEBS J. 2015;282:4658–4671. doi: 10.1111/febs.13534. [DOI] [PubMed] [Google Scholar]
- 9.Ye X, Guo A, Yin P, Cao X, Chang J. Overexpression of NUAK1 is associated with disease-free survival and overall survival in patients with gastric cancer. Med Oncol. 2014;31:61. doi: 10.1007/s12032-014-0061-1. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Liu G, Xu N, You X, Zhou H, Zhao X, Liu Q. Knockdown of ARK5 expression suppresses invasion and metastasis of gastric cancer. Cell Physiol Biochem. 2017;42:1025–1036. doi: 10.1159/000478685. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Li J, Li G, Wang S. Activation of ARK5/miR-1181/HOXA10 axis promotes epithelial-mesenchymal transition in ovarian cancer. Oncol Rep. 2015;34:1193–1202. doi: 10.3892/or.2015.4113. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Lv W, Zhang J, Lu D. miR-96 functions as a tumor suppressor gene by targeting NUAK1 in pancreatic cancer. Int J Mol Med. 2014;34:1599–1605. doi: 10.3892/ijmm.2014.1940. [DOI] [PubMed] [Google Scholar]
- 13.Chen P, Li K, Liang Y, Li L, Zhu X. High NUAK1 expression correlates with poor prognosis and involved in NSCLC cells migration and invasion. Exp Lung Res. 2013;39:9–17. doi: 10.3109/01902148.2012.744115. [DOI] [PubMed] [Google Scholar]
- 14.Phippen NT, Bateman NW, Wang G, Conrads KA, Ao W, Teng PN, Litzi TA, Oliver J, Maxwell GL, Hamilton CA, Darcy KM, Conrads TP. NUAK1 (ARK5) is associated with poor prognosis in ovarian cancer. Front Oncol. 2016;6:213. doi: 10.3389/fonc.2016.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigalotti L, Fratta E, Parisi G, Coral S, Maio M. Epigenetic markers of prognosis in melanoma. Methods Mol Biol. 2014;1102:481–499. doi: 10.1007/978-1-62703-727-3_25. [DOI] [PubMed] [Google Scholar]
- 16.Shi L, Zhang B, Sun X, Lu S, Liu Z, Liu Y, Li H, Wang L, Wang X, Zhao C. MiR-204 inhibits human NSCLC metastasis through suppression of NUAK1. Br J Cancer. 2014;111:2316–2327. doi: 10.1038/bjc.2014.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell RE, Khaled M, Netanely D, Schubert S, Golan T, Buxbaum A, Janas MM, Postolsky B, Goldberg MS, Shamir R, Levy C. Transcription factor/microRNA axis blocks melanoma invasion program by miR-211 targeting NUAK1. J Invest Dermatol. 2014;134:441–451. doi: 10.1038/jid.2013.340. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Li H, Wang Y, Zhao X, Guo Y, Jin J, Chi R. MiR-30b-5p functions as a tumor suppressor in cell proliferation, metastasis and epithelial-to-mesenchymal transition by targeting G-protein subunit α-13 in renal cell carcinoma. Gene. 2017;626:275–281. doi: 10.1016/j.gene.2017.05.040. [DOI] [PubMed] [Google Scholar]
- 19.Qiao F, Zhang K, Gong P, Wang L, Hu J, Lu S, Fan H. Decreased miR-30b-5p expression by DNMT1 methylation regulation involved in gastric cancer metastasis. Mol Biol Rep. 2014;41:5693–5700. doi: 10.1007/s11033-014-3439-4. [DOI] [PubMed] [Google Scholar]
- 20.Benaich N, Woodhouse S, Goldie SJ, Mishra A, Quist SR, Watt FM. Rewiring of an epithelial differentiation factor, miR-203, to inhibit human squamous cell carcinoma metastasis. Cell Rep. 2014;9:104–117. doi: 10.1016/j.celrep.2014.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong X, Sun D, Chai H, Shan W, Yu Y, Pu L, Cheng F. MiR-145 functions as a tumor suppressor targeting NUAK1 in human intrahepatic cholangiocarcinoma. Biochem Biophys Res Commun. 2015;465:262–269. doi: 10.1016/j.bbrc.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Helgstrand JT, Roder MA, Klemann N, Toft BG, Lichtensztajn DY, Brooks JD, Brasso K, Vainer B, Iversen P. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer-A population-based analysis of 2 national cohorts. Cancer. 2018;124:2931–2938. doi: 10.1002/cncr.31384. [DOI] [PubMed] [Google Scholar]
- 23.Hanrahan K, O’Neill A, Prencipe M, Bugler J, Murphy L, Fabre A, Puhr M, Culig Z, Murphy K, Watson R. The role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in mediating docetaxel-resistant prostate cancer. Mol Oncol. 2017;11:251–265. doi: 10.1002/1878-0261.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Dedhar S, Kalluri R, Thompson E. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph J, Harishankar M, Pillai A, Devi A. Hypoxia induced EMT: a review on the mechanism of tumor progression and metastasis in OSCC. Oral Oncol. 2018;80:23–32. doi: 10.1016/j.oraloncology.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Yue J, Sun H, Liu S, Yu F, Wang S, Wang F, Shen R, Zhu F, Zhang L, Shao C. Downregulation of NDR1 contributes to metastasis of prostate cancer cells via activating epithelial-mesenchymal transition. Cancer Med. 2018 doi: 10.1002/cam4.1532. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, Wang X, Wang Y, Dai Y, Xie Y, Ping Y, Yin B, Yu P, Liu Z, Duan X, Liao Z, Chen Y, Liu C, Li X, Tao Z. SGK1 inhibition-induced autophagy impairs prostate cancer metastasis by reversing EMT. J Exp Clin Cancer Res. 2018;37:73. doi: 10.1186/s13046-018-0743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lan X, Liu X. LncRNA SNHG1 functions as a ceRNA to antagonize the effect of miR-145a-5p on the down-regulation of NUAK1 in nasopharyngeal carcinoma cell. J Cell Mol Med. 2018 doi: 10.1111/jcmm.13497. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar B, Rosenberg A, Choi S, Fox-Talbot K, De Marzo A, Nonn L, Brennen W, Marchionni L, Halushka M, Lupold S. Cell-type specific expression of oncogenic and tumor suppressive microRNAs in the human prostate and prostate cancer. Sci Rep. 2018;8:7189. doi: 10.1038/s41598-018-25320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latonen L, Afyounian E, Jylhä A, Nättinen J, Aapola U, Annala M, Kivinummi K, Tammela T, Beuerman R, Uusitalo H, Nykter M, Visakorpi T. Integrative proteomics in prostate cancer uncovers robustness against genomic and transcriptomic aberrations during disease progression. Nat Commun. 2018;9:1176. doi: 10.1038/s41467-018-03573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Zhang X, Li N, Liu Q, Chen D. miR-30b inhibits cancer cell growth, migration, and invasion by targeting homeobox A1 in esophageal cancer. Biochem Biophys Res Commun. 2017;485:506–512. doi: 10.1016/j.bbrc.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Coebergh van den Braak R, Sieuwerts A, Lalmahomed Z, Smid M, Wilting S, Bril S, Xiang S, van der Vlugt-Daane M, de Weerd V, van Galen A, Biermann K, van Krieken J, Kloosterman W, Foekens J, Martens J, IJzermans J. Confirmation of a metastasis-specific microRNA signature in primary colon cancer. Sci Rep. 2018;8:5242. doi: 10.1038/s41598-018-22532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S, Li P, Yang R, Cheng R, Zhang F, Wang Y, Chen X, Sun Q, Zang W, Du Y, Zhao G, Zhang G. microRNA-30b inhibits cell invasion and migration through targeting collagen triple helix repeat containing 1 in non-small cell lung cancer. Cancer Cell Int. 2015;15:85. doi: 10.1186/s12935-015-0236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian S, Yu J, Liu Y, Kang W, Ma Z, Ye X, Yan C. MiR-30b suppresses tumor migration and invasion by targeting EIF5A2 in gastric cancer. World J Gastroenterol. 2015;21:9337–9347. doi: 10.3748/wjg.v21.i31.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun X, Zhao S, Li H, Chang H, Huang Z, Ding Z, Dong L, Chen J, Zang Y, Zhang J. MicroRNA-30b suppresses epithelial-mesenchymal transition and metastasis of hepatoma cells. J Cell Physiol. 2017;232:625–634. doi: 10.1002/jcp.25466. [DOI] [PubMed] [Google Scholar]
- 36.Liao W, Ye Y, Zhang N, Li T, Wang S, Cui Y, Qi L, Wu P, Jiao H, Xie Y, Zhang C, Wang J, Ding Y. MicroRNA-30b functions as a tumour suppressor in human colorectal cancer by targeting KRAS, PIK3CD and BCL2. J Pathol. 2014;232:415–427. doi: 10.1002/path.4309. [DOI] [PubMed] [Google Scholar]
- 37.Zhong K, Chen K, Han L, Li B. MicroRNA-30b/c inhibits non-small cell lung cancer cell proliferation by targeting Rab18. BMC Cancer. 2014;14:703. doi: 10.1186/1471-2407-14-703. [DOI] [PMC free article] [PubMed] [Google Scholar]