Abstract
Objective: To study the relationship between interleukin (IL)-1β and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) expressions in tumor tissue and the recurrence of hepatocellular carcinoma (HCC) after hepatectomy. Methods: The expressions of IL-1β and NFκB in tumor tissues of 92 patients with HCC were measured using immunohistochemical staining. The correlations between IL-1β and NFκB expressions with clinical factors and postoperative recurrence were analyzed. Results: Compared with the low expression group, the disease-free survival (DFS) of HCC with high expression of IL-1β and NFκB was more significantly prolonged (P < 0.05). NFκB is a key molecule in the downstream signaling pathway of IL-1β, and the expression of IL-1β and NFκB is positively associated with serum total bilirubin (TBIL). IL-1β and NFκB levels and platelet (PLT) levels were independent risk factors for postoperative recurrence of HCC (P < 0.05). Conclusions: The low expressions of IL-1β and NFκB in HCC tissues are an independent risk factor for the recurrence of HCC after hepatectomy, but a high expression can significantly prolong DFS.
Keywords: IL-1β, NFκB, hepatocellular carcinoma, recurrence, prognosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. The incidence of liver cancer in China ranks fourth among malignant tumors, and its mortality rate ranks third among malignant tumors [1], which seriously threatens the health and safety of people. There are many therapies for liver cancer, but its high recurrence rate is a key factor limiting the therapies’ efficacy [2]. Therefore, it is an urgent problem to study the prediction of postoperative recurrence indicators of hepatocellular carcinoma and to find a treatment method to prevent recurrence, so as to improve the overall survival rate of patients with liver cancer.
Inflammation and fibrosis of the liver are important factors in promoting the development of HCC. The interleukin (IL)-1 family has 12 factors which are mainly pro-inflammatory cytokines including IL-1α and IL-1β. IL-1β is an important pro-inflammatory factor, which not only promotes the metastasis of renal cell carcinoma [3] and lung cancer [4], but also enhances the invasiveness of the human HCC cell line MHCC97-H in vitro [5]. IL-1β promotes tumor angiogenesis by inducing vascular endothelial growth factor (VGEF) expression [6]. Moreover, IL-1β can induce endothelial cells and tumor cells to express adhesion molecules, promote the adhesion of tumor cells to endothelial cells, and cause distant metastasis of tumor cells [7]. Okamoto et al. studied the relationship between IL-1β and matrix metalloproteinase (MMP)-3 gene polymorphisms and the prognosis of hepatitis C virus (HCV)-associated HCC. It was found that the simultaneous presence of the IL-1β-31T allele and the MMP-3-5A allele was a risk factor for poor prognosis [8]. However, it has been reported that IL-1β can also exert and anti-tumor effect and inhibit the growth and metastasis of tumors by activating a cluster of differentiation (CD)4+ and CD8+ T cells, and producing IL-2, IL-4 and interferon (IFN)-γ [9]. A possible reason is that IL-1β plays a different role in different tumors and different microenvironments. It is this uncertainty that prompted us to do further research.
The nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway is an important and classical downstream signal of IL-1β. In order to study the relationship between the simultaneous expression of IL-1β and NFκB in the HCC microenvironment and the postoperative recurrence of HCC, the expression levels of IL-1β and NFκB in 92 cases of HCC tumor tissues were measured using immunohistochemistry. The relationship of the expressions of the two factors to clinicopathological factors, as well as the effects to postoperative recurrence of HCC, were analyzed.
Materials and methods
Study population
A total of 92 paraffin-embedded specimens of HCC patients who underwent surgical resection in our hospital from January 2000 to December 2012 were collected. There were 75 males and 17 females, with an age range of 31-83 years and a median age of 56 years. Inclusion criteria: (1) pathological diagnosis of hepatocellular carcinoma without distant metastasis; (2) no radical hepatectomy or any anticancer treatment before surgery; (3) exclusion of autoimmune diseases, other malignant tumors or severe heart, lung, kidney or blood disease; (4) complete clinical, pathological and surgical data; (5) exclude patients with non-tumor related deaths.
Immunohistochemistry and scoring criteria
All 92 wax specimens were serially sectioned to a thickness of 4 μm. IL-1β and NFκB were measured using immunohistochemistry, and the anti-IL-1β and anti-NFκB were purchased from Abcam, USA. Two pathologists read the film without knowing the patients’ clinical data. If there was any disagreement, a third person read the film to discuss the judgment. Under an optical microscope, a comprehensive judgment was made based on the staining intensity and the number of positive cells. The result was judged by randomly selecting 10 high power lens for each slice, and the score was assigned according to the intensity of dyeing (no coloring-0 points, light yellow-1 point, brown yellow-2 points, brown-3 points) and the number of positive cells was counted as the percentage of similar cells (≤ 5% of 0 points, 6%-25% of 1 point, 26%-50% of 2 points, 51%-75% for 3 points, ≥ 76% for 4 points). The result was judged based on the sum of the two kinds of scores. The scores ≤ 4 points were judged as low expression, and the score > 4 points were judged as high expression. Cases with high expression were classified as simultaneous high expression of IL-1β and NFκB, and the remaining cases were classified as being in the low expression group, including a single low expression or simultaneous low expression.
Follow-up of cases
All the cases were followed-up in the form of outpatient visiting, telephone, or letter. The follow-up plan was: review every 3 months within 2 years after surgery, every 6 months between 2 years and 5 years; review every 1 year after 5 years. The review included liver function, α-fetoprotein (AFP), abdominal ultrasound, chest radiograph, and enhanced CT or MRI. Needle biopsies were done when necessary. Recurrence was defined as: imaging studies or biopsy confirmed new lesions in the liver or outside the liver. Disease Free Survival (DFS) was defined as the time from the date of surgery to the time of recurrence or follow-up. Overall Survival (OS) was defined as the time from the date of surgery to the time of death or follow-up. Both DFS and OS were calculated on a monthly basis and the follow-up deadline was March 2017.
Statistical analysis
The statistical analysis was performed using SPSS 24.0 software. The correlation between the clinicopathological factors and the expressions of IL-1β and NFκB were analyzed with a t-test for the measurement of the data and an χ2 test for counting the data. The correlation between IL-1β and NFκB expression was tested using Spearman’s test. The survival analysis was performed using a Kaplan-Meier analysis, and the two-sided log-rank test was used to compare the relationship between the expressions of IL-1β and NFκB and the postoperative recurrence. The univariate analysis was performed using the Cox regression model. The factors that were statistically significant in the univariate analysis were brought into the multivariate Cox proportional-hazards analysis. Bilateral P values < 0.05 were considered to be statistically significant.
Results
Expressions of IL-1β and NFκB in HCC tissue
IL-1β and NFκB are mainly located in the cytoplasms of tumor cells, which are brown-yellow granules. A low expression of the cytoplasm has weak staining, but a high expression has diffuse or scattered brown-yellow granules (Figure 1).
Figure 1.
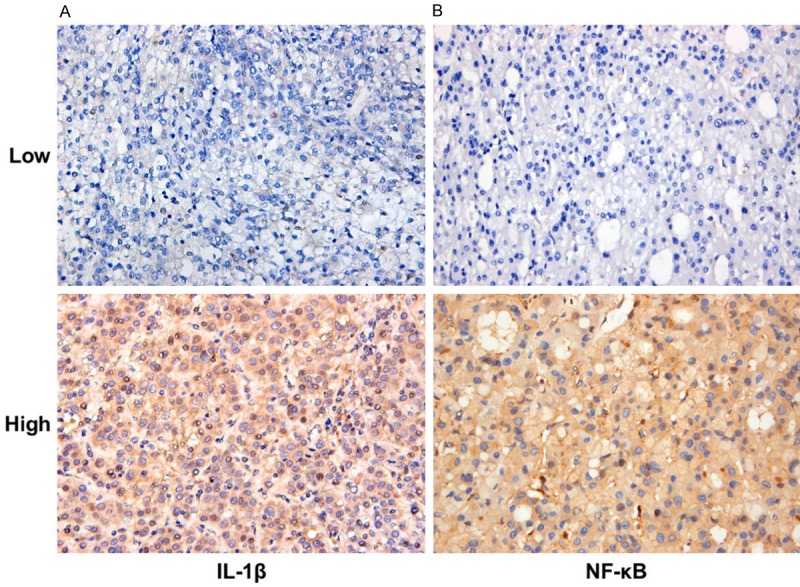
Expression of IL-1β and NFκB in HCC tissue (× 400).
There is a positive correlation between IL-1β and NFκB expression in the high-low expression group of single factor (Table 1). Both are key molecules in the same signaling pathway, so we classify the cases with simultaneous high expression of IL-1β and NFκB as the high expression groups, indicating that the pathway is activated. The rest of the cases are classified as the low expression groups. By comparing the baseline data, it was found that the expressions of IL-1β and NFκB were positively correlated with serum total bilirubin (TBIL) levels (Table 2).
Table 1.
Correlation between IL-1β and NFκB expression
| Characteristics | NFκB | Correlation coefficient | P | ||
|---|---|---|---|---|---|
|
| |||||
| High | Low | ||||
| IL-1β | High | 50 | 19 | 0.301 | 0.004 * |
| Low | 9 | 14 | |||
Spearman’s χ2 test.
P < 0.05, statistically significant.
IL-1β, interleukin-1β. NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells.
Table 2.
The clinicopathological characteristics in the Low and High groups of IL-1β and NFκB
| Characteristics | Low | High | T/χ2 Test | P |
|---|---|---|---|---|
| Gender, male/female | 35/7 | 40/10 | 0.168 | 0.682 |
| Age (y) | 55.0 ± 9.9 | 55.5 ± 9.6 | -0.223 | 0.824 |
| AFP (ng/L) | 305.8 ± 471.9 | 279.0 ± 454.8 | -0.091 | 0.928 |
| ALB (g/L) | 38.2 ± 3.3 | 38.9 ± 4.4 | -0.816 | 0.416 |
| ALT (U/L) | 51.8 ± 32.6 | 50.1 ± 40.0 | 0.222 | 0.824 |
| AST (U/L) | 41.0 ± 28.9 | 40.6 ± 34.3 | 0.059 | 0.953 |
| PLT (109/L) | 143.4 ± 56.8 | 139.2 ± 62.5 | 0.330 | 0.742 |
| TBIL (μmol/L) | 14.7 ± 5.2 | 17.5 ± 6.7 | -2.209 | 0.030* |
| Tumor number, single/multiple | 39/3 | 49/1 | 1.452 | 0.228 |
| Tumor size (cm) | 3.9 ± 1.4 | 3.8 ± 1.6 | 0.168 | 0.867 |
| Tumor margin (mm) | 9.4 ± 13.6 | 11.8 ± 14.3 | -0.811 | 0.420 |
| Differentiation, high and middle/low | 36/6 | 48/2 | 3.042 | 0.081 |
| Microvascular invasion, yes/no | 6/36 | 10/40 | 0.519 | 0.471 |
| Capsule invasion, yes/no | 24/18 | 35/15 | 1.640 | 0.200 |
| HBV infection, yes/no | 38/4 | 44/6 | 0.144 | 0.704 |
| Liver cirrhosis, yes/no | 3/39 | 8/42 | 1.701 | 0.192 |
| Child-Pugh grade, A/B | 40/2 | 49/1 | 0.552 | 0.458 |
P < 0.05, statistically significant.
IL-1β, interleukin-1β; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; AFP, α-fetoprotein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PLT, platelet; TBIL, total bilirubin; HBV, hepatitis B virus.
Prognosis of HCC patients in the high and low groups
The 1-, 3-, and 5-year DFS rates were 48.9%, 32.6%, and 27.2%, respectively, and the median DFS was 11.7 months (95% CI: 4.3-19.1). The median DFS of the IL-1β and NFκB high and low expression groups was 22.2 months (95% CI: 0.0-48.1) and 9.3 months (95% CI: 6.1-12.5), and the 1-, 3- and 5-year DFS rates were 58.0%, 44.0%, 38.0% and 38.1%, 19.0%, 14.3% respectively. The DFS of the high expression group was significantly longer than that of the low expression group, and the difference between the two groups was statistically significant (χ2 = 5.137, P = 0.023) (Figure 2A).
Figure 2.
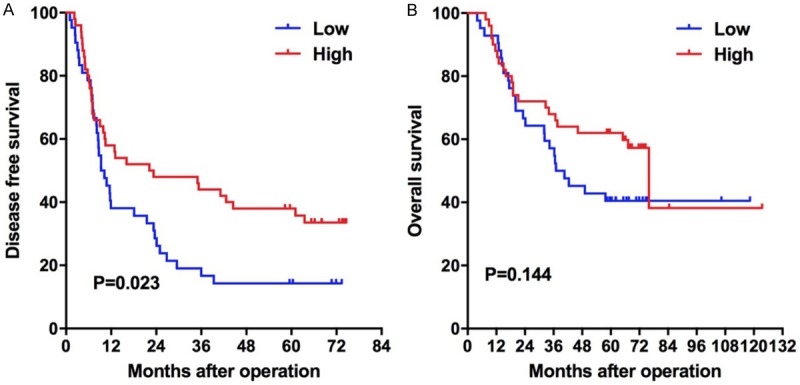
Kaplan-Meier survival plots comparing OS and DFS for patients stratified as IL-1β and NFκB high and low groups. A. DFS of patients in the high group was longer than that in the low group (P = 0.023, log-rank test); B. There is no difference in the OS of patients in the high and low groups (P = 0.144, log-rank test).
The 1-, 3-, 5-year OS rates were 90.2%, 63.0%, and 52.1%, respectively, with a median OS of 67.3 months (95% CI: 45.5-89.1). The median OS of the IL-1β and NFκB high and low expression groups was 76.0 months (95% CI: 61.4-90.6) and 37.0 months (95% CI: 21.3-52.7) respectively, and the 1, 3, and 5-year OS rates were 88.0%, 68.0%, 62.0% and 82.9%, 57.1%, 40.2% respectively. The OS of the high group was longer, but the difference between the two groups was not statistically significant (χ2 = 2.135, P = 0.144) (Figure 2B).
The univariate and multivariate COX analysis of DFS
A univariate analysis showed that the IL-1β and NFκB levels, the alanine aminotransferase (ALT) levels, the aspartate aminotransferase (AST) levels, and the platelet (PLT) levels were risk factors for DFS, and the difference was statistically significant (P < 0.05; some univariate analysis data was cited from the previous article published by our team [10], Table 3). A multivariate analysis of the above factors showed that the IL-1β and NFκB levels and the PLT levels were independent risk factors for postoperative DFS. A further analysis of the hazard ratio (HR) values revealed that the effect of the IL-1β and NFκB levels for DFS is greater than the effect of the PLT level (Table 3).
Table 3.
Univariate and multivariate analysis of variables with disease-free survival
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender, male/female | 0.686 (0.359-1.309) | 0.253 | ||
| Age (y, < 50 vs. ≥ 50) | 1.193 (0.709-2.009) | 0.506 | ||
| AFP (ng/L, ≤ 400 vs. > 400) | 1.495 (0.888-2.517) | 0.131 | ||
| ALB (g/L, < 35 vs. ≥ 35) | 0.714 (0.326-1.562) | 0.399 | ||
| ALT (U/L, ≤ 60 vs. > 60) | 1.855 (1.055-3.261) | 0.032 * | 1.477 (0.673-3.244) | 0.331 |
| AST (U/L, ≤ 42 vs. > 42) | 2.145 (1.289-3.568) | 0.003 * | 1.637 (0.807-3.317) | 0.172 |
| PLT (109/L, < 100 vs. ≥ 100) | 1.875 (1.110-3.166) | 0.019 * | 0.529 (0.302-0.926) | 0.026 * |
| TBIL (μmol/L, ≤ 22 vs. > 22) | 1.713 (0.897-3.270) | 0.103 | ||
| Tumor number, single/multiple | 2.666 (0.962-7.383) | 0.059 | ||
| Tumor size (cm, ≤ 5 vs. > 5) | 1.924 (0.914-4.051) | 0.085 | ||
| Tumor margin (cm, ≤ 2 vs. > 2) | 0.445 (0.178-1.110) | 0.082 | ||
| Differentiation, High vs. Middle and Low | 1.216 (0.526-2.812) | 0.648 | ||
| Microvascular invasion, yes/no | 1.720 (0.955-3.100) | 0.071 | ||
| Capsule invasion, yes/no | 1.246 (0.500-3.104) | 0.637 | ||
| HBV infection, yes/no | 1.196 (0.546-2.619) | 0.655 | ||
| Liver cirrhosis, yes/no | 1.228 (0.609-2.476) | 0.567 | ||
| Child-Pugh grade, A/B | 1.292 (0.316-5.284) | 0.722 | ||
| IL-1β and NFκB (Low vs. High) | 0.578 (0.358-0.935) | 0.025 * | 0.555 (0.342-0.901) | 0.017 * |
P < 0.05, statistically significant.
AFP, α-fetoprotein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PLT, platelet; TBIL, total bilirubin; HBV, hepatitis B virus; IL-1β, interleukin-1β; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells.
Discussion
We found that the expressions of IL-1β and NFκB are positively correlated, and a high expression of IL-1β and NFκB can reflect the activation status of this pathway to a certain extent. The expressions of IL-1β and NFκB are positively correlated with the level of TBIL. The average follow-up time of this study was more than 7 years, and it was found that the DFS was significantly prolonged in the IL-1β and NFκB high expression groups. But the OS in the high and low expression groups was not significantly different. The COX regression analysis found that low expressions of IL-1β and NFκB and the PLT levels were independent risk factors for the postoperative recurrence of HCC.
IL-1β was the first interleukin to be discovered, and its initial function was mainly to participate in the inflammatory response as an endogenous heat source. IL-1β is produced and released by many different immune and non-immune cells, all of which respond rapidly to inflammatory signals, and IL-1β plays a role as a signal amplifier [11]. Numerous studies have found that IL-1β has both tumor-promoting and anti-tumor effects. It has been reported that high levels of IL-1β in body fluids are associated with a poor prognosis, tumorigenesis, and invasion in cancer patients [12,13]. The key mechanism by which IL-1β promotes tumor development is to drive non-specific chronic inflammation, activate endothelial cells, and promote tumor angiogenesis [12,14,15]. However, Allen et al. reported that IL-1β has protective effects in chemical colitis and colon cancer mouse models [16]. In myeloma-resistant T cell receptors (TCR), transgenic severe combined immunodeficiency (SCID) mice were injected with myeloma cells, neutralizing IL-1 results in a decrease in IFN production in tumor-specific Th1 cells and attenuating macrophage infiltration and tumor growth [17,18].
IL-1β binds to IL-1R on the cell surface and activates E3 ubiquitin ligase TRAF6, which catalyzes the binding of the non-anchored K63 polyubiquitin chain to the TAB2 subunit in the TAK1 kinase complex, resulting in TAK1 activation, and TAK1 will activate NF-κB ultimately by the phosphorylating of IKKβ [19-21]. We found that the expression of IL-1β and NF-κB in HCC tissues is positively correlated. According to the results of immunohistochemistry, in the case of the high expression of both, it can be considered that the signaling pathways of IL-1β and NF-κB in cancer tissues are activated to some extent. If both are low expressed or one of them is low expressed, the pathway is considered to be inactive. The downstream pathway of IL-1β is not only the NF-κB pathway [22,23]. Meanwhile NF-κB also accepts multiple sources of signaling [24-26]. Perhaps there is no positive result, if only one of the two factors is analyzed for its effects on prognosis.
We analyzed IL-1β and NF-κB together and found that the DFS of the high expression groups was significantly prolonged. It indicated that the activation of this pathway may play a role in inhibiting the recurrence of HCC. However, we also found that IL-1β and NF-κB co-expression had no significant effect on the OS. When comparing the baseline data of the high and low expression groups, the average value of TBIL in the IL-1β and NF-κB high expression group was significantly higher than it was in the low expression group. Regardless of the cause of liver fibrosis or liver cancer, there is a different degree of TBIL elevation. And the inflammatory response induced by the co-expression of IL-1β and NF-κB may cause edema or increase the permeability of the capillary bile duct, leading to a release of bilirubin into the blood.
Cox analysis of DFS found that IL-1β and NF-κB low expressions (HR = 0.555) and PLT ≥ 100 × 109/L (HR = 0.529) were independent risk factors for the postoperative recurrence of HCC. According to the comparison of HR values, the effect of IL-1β and NF-κB low expressions on recurrence was more significant than the PLT level. Numerous studies have reported a significant increase in postoperative recurrence rates in HCC patients with platelet counts < 100 × 109/L [27-29]. We did not find a correlation between IL-1β and NF-κB expression and serum PLT levels (P > 0.05, Table 2). The possible reason is that it is difficult for the expression levels of IL-1β and NF-κB in local tissues to affect the level of PLT in the whole circulatory system. The effect of IL-1β and NF-κB expressions on prognosis is mainly postoperative recurrence.
In summary, the low expressions of IL-1β and NF-κB in hepatocellular carcinoma are an independent risk factor for DFS, but they have nothing to do with OS. The expressions of IL-1β and NF-κB were positively correlated with serum TBIL levels.
Acknowledgements
The authors thank the patients and their families for their cooperation and participation in the study, as well as the staff involved with the study for data collection. The study was supported by grants from the Key Research and Development Plan of Shandong Province (grant no. 2018GSF118233), the Science and Technology for People’s Livelihood Project of Qingdao (grant no. 18-6-1-89-nsh), and the Science and Technology Plan of Qingdao City Shinan District (grant no. 2018-4-018-YY).
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R, Kishimoto T, Nakatani T. Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer. 2002;86:1396–1400. doi: 10.1038/sj.bjc.6600257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexandrakis MG, Passam FH, Boula A, Chris-tophoridou A, Aloizos G, Roussou P, Kyriakou DS. Relationship between circulating serum soluble interleukin-6 receptor and the angiogenic cytokines basic fibroblast growth factor and vascular endothelial growth factor in multiple myeloma. Ann Hematol. 2003;82:19–23. doi: 10.1007/s00277-002-0558-0. [DOI] [PubMed] [Google Scholar]
- 5.Huang XY, Wang L, Liu BB, Qiu SJ, Fan J, Ye SL, Tang ZY. [An in vitro research on interleukin-1 beta promoted invasiveness of human hepatocellular carcinoma MHCC97-H cells] . Zhonghua Gan Zang Bing Za Zhi. 2008;16:309–310. [PubMed] [Google Scholar]
- 6.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yano S, Nokihara H, Yamamoto A, Goto H, Ogawa H, Kanematsu T, Miki T, Uehara H, Saijo Y, Nukiwa T, Sone S. Multifunctional interleukin-1beta promotes metastasis of human lung cancer cells in SCID mice via enhanced expression of adhesion-, invasion- and angiogenesis-related molecules. Cancer Sci. 2003;94:244–252. doi: 10.1111/j.1349-7006.2003.tb01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamoto K, Ishida C, Ikebuchi Y, Mandai M, Mimura K, Murawaki Y, Yuasa I. The genotypes of IL-1 beta and MMP-3 are associated with the prognosis of HCV-related hepatocellular carcinoma. Intern Med. 2010;49:887–895. doi: 10.2169/internalmedicine.49.3268. [DOI] [PubMed] [Google Scholar]
- 9.Roy D, Sarkar S, Felty Q. Levels of IL-1 beta control stimulatory/inhibitory growth of cancer cells. Front Biosci. 2006;11:889–898. doi: 10.2741/1845. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Zhang S, Yang Z, Hu J, Hu W, Sun P, Wu L, Han B. Association between the expression levels of IL-6 and IL-6R in the hepatocellular carcinoma microenvironment and postoperative recurrence. Oncol Lett. 2018;16:7158–7165. doi: 10.3892/ol.2018.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinarello CA. An expanding role for interleukin-1 blockade from gout to cancer. Mol Med. 2014;20(Suppl 1):S43–58. doi: 10.2119/molmed.2014.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apte RN, Krelin Y, Song X, Dotan S, Recih E, Elkabets M, Carmi Y, Dvorkin T, White RM, Gayvoronsky L, Segal S, Voronov E. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. Eur J Cancer. 2006;42:751–759. doi: 10.1016/j.ejca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev. 2018;281:57–61. doi: 10.1111/imr.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steel JL, Terhorst L, Collins KP, Geller DA, Vodovotz Y, Kim J, Krane A, Antoni M, Marsh JW, Burke LE, Butterfield LH, Penedo FJ, Buysse DJ, Tsung A. Prospective analyses of cytokine mediation of sleep and survival in the context of advanced cancer. Psychosom Med. 2018;80:483–491. doi: 10.1097/PSY.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haabeth OA, Lorvik KB, Hammarstrom C, Donaldson IM, Haraldsen G, Bogen B, Corthay A. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun. 2011;2:240. doi: 10.1038/ncomms1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haabeth OA, Lorvik KB, Yagita H, Bogen B, Corthay A. Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. Oncoimmunology. 2016;5:e1039763. doi: 10.1080/2162402X.2015.1039763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E, Yamamoto T, Inoue J. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem. 1996;271:28745–28748. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 21.Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 22.Saquib Q, Al-Khedhairy AA, Ahmad J, Siddiqui MA, Dwivedi S, Khan ST, Musarrat J. Zinc ferrite nanoparticles activate IL-1b, NFKB1, CCL21 and NOS2 signaling to induce mitochondrial dependent intrinsic apoptotic pathway in WISH cells. Toxicol Appl Pharmacol. 2013;273:289–297. doi: 10.1016/j.taap.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Ganguly K, Upadhyay S, Irmler M, Takenaka S, Pukelsheim K, Beckers J, Hamelmann E, Schulz H, Stoeger T. Pathway focused protein profiling indicates differential function for IL-1B, -18 and VEGF during initiation and resolution of lung inflammation evoked by carbon nanoparticle exposure in mice. Part Fibre Toxicol. 2009;6:31. doi: 10.1186/1743-8977-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biswas R, Bagchi A. Inhibition of TRAF6-Ubc13 interaction in NFkB inflammatory pathway by analyzing the hotspot amino acid residues and protein-protein interactions using molecular docking simulations. Comput Biol Chem. 2017;70:116–124. doi: 10.1016/j.compbiolchem.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Jana A, Krett NL, Guzman G, Khalid A, Ozden O, Staudacher JJ, Bauer J, Baik SH, Carroll T, Yazici C, Jung B. NFkB is essential for activin-induced colorectal cancer migration via upregulation of PI3K-MDM2 pathway. Onco-target. 2017;8:37377–37393. doi: 10.18632/oncotarget.16343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain AR, Ahmed SO, Ahmed M, Khan OS, Al Abdulmohsen S, Platanias LC, Al-Kuraya KS, Uddin S. Cross-talk between NFkB and the PI3-kinase/AKT pathway can be targeted in primary effusion lymphoma (PEL) cell lines for efficient apoptosis. PLoS One. 2012;7:e39945. doi: 10.1371/journal.pone.0039945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei Y, Yee LW, Ping ZW. Target patients for partial hepatectomy and relationship between PLT and prognosis in BCLC B HCC. J Hepatol. 2015;62:750. doi: 10.1016/j.jhep.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Amano H, Tashiro H, Oshita A, Kobayashi T, Tanimoto Y, Kuroda S, Tazawa H, Itamoto T, Asahara T, Ohdan H. Significance of platelet count in the outcomes of hepatectomized patients with hepatocellular carcinoma exceeding the milan criteria. J Gastrointest Surg. 2011;15:1173–1181. doi: 10.1007/s11605-011-1538-2. [DOI] [PubMed] [Google Scholar]
- 29.Carr BI, Guerra V, Giannini EG, Farinati F, Ciccarese F, Rapaccini GL, Di Marco M, Benvegnù L, Zoli M, Borzio F, Caturelli E, Chiaramonte M, Trevisani F Italian Liver Cancer Group. Significance of platelet and AFP levels and liver function parameters for HCC size and survival. Int J Biol Markers. 2014;29:e215–223. doi: 10.5301/jbm.5000064. [DOI] [PubMed] [Google Scholar]


