Abstract
Hypoxia inducible factor 1 alpha subunit (HIF-1α) is induced in hypoxic conditions and plays a crucial role in the neoangiogenesis and metastasis of cancer. In this study, we aimed to evaluate the expression of HIF-1α in colon adenocarcinoma and to explore its clinicopathological characteristics and prognosis. A tissue microarray involving colon adenocarcinoma tissues and their corresponding paracancerous tissues from 92 patients was utilized to detect HIF-1α. The expression of HIF-1α in colon adenocarcinoma tissues was significantly higher than it was in the corresponding paracancerous tissues (P < 0.001). Furthermore, similar results were observed in HCT116 and RKO human colon adenocarcinoma xenografts in node mice (P < 0.05). Additionally, augmented HIF-1α expression was positively associated with TNM stage III-IV (P = 0.025), the presence of distant metastasis and vascular invasion (P = 0.048), and the presence of positive lymph nodes (P = 0.041). A Kaplan-Meier survival analysis showed that up-regulation of HIF-1α was associated with poor 5-year or 10-year survival (P < 0.05). A multivariable Cox regression analysis also found HIF-1α was an independent risk factor for poor prognosis in colon adenocarcinoma. Thus, targeting HIF-1α might be a viable strategy to treat patients with colon adenocarcinoma.
Keywords: Hypoxia inducible factor 1 alpha subunit, colon adenocarcinoma, survival analysis, prognosis
Introduction
Every year, more than a million patients are diagnosed with colorectal cancer and annual mortality due to colorectal cancer is more than a half million [1]. Though screening of the disease has been suggested to improve the outcome [1,2], there are still many problems to be overcome in order to treat colorectal cancer better. One of the obstacles is the metastasis of colorectal cancer, which still severely impairs the prognosis of these patients [1,3].
Hypoxia and angiogenesis are closely related to cancer metastasis [4-7]. It has been found that many factors regulating pathological angiogenesis, such as phosducin-like 3, EIF5A2, Beclin 1, a vascular endothelial growth factor receptor, and so on, could be altered in hypoxia [8-10], and these factors could then stimulate tumor metastasis. In colorectal cancer, hypoxia could facilitate its aggressiveness, leading to drug resistance and promoting metastasis, which are detrimental to prognosis [11,12].
Hypoxia inducible factor 1 alpha subunit (HIF-1α), a factor commonly induced in hypoxic conditions, plays a crucial role in neoangiogenesis and metastasis in cancer [13]. Moreover, HIF-1α influences experimental angiogenesis and the metastasis of colorectal cancer cells [11,14]. However, clinical data concerning the relationship between HIF-1α and metastasis as well as the prognosis of patients with colorectal cancer are still controversial [15-18].
In this study, we aimed to address whether the expression of HIF-1α was altered in colon adenocarcinoma. Moreover, its effect on the metastasis as well as the prognosis of patients with colon adenocarcinoma was also investigated.
Materials and methods
Tissue microarray (TMA) and frozen tissues
In the current study, the National Engineering Center for Biochip at Shanghai (Shanghai, China) provided colon adenocarcinoma TMA. In total, 92 patients who were diagnosed with colon cancer and had undergone surgery between July 2005 and December 2010 were recruited. From these patients, 52 corresponding paracancerous tissues were also collected. The clinical and pathological information of the participants is shown in Table 1. Follow-up for all patients was carried out from the date of surgery until September 2015. Additionally, colon cancer and the corresponding paracancerous frozen tissues were also collected from 8 patients diagnosed with colon cancer. Written informed consents were received from all the patients. The right of privacy for the patients was well protected. Ethical approval was also obtained from the biobank center related hospitals (the Ethics Committee of Taizhou Hospital of Zhejiang Province). All the experiments were performed in accordance with relevant guidelines and regulations.
Table 1.
Clinicopathological characteristics of 92 colon adenocarcinoma cases
| Characteristics | Number of cases | Percentage (%) |
|---|---|---|
| Total | 92 | 100 |
| Average years | 62.4 ± 11.9 | |
| < 65 | 50 | 54.3 |
| ≥ 65 | 42 | 45.7 |
| Gender | ||
| Male | 55 | 59.8 |
| Female | 37 | 40.2 |
| Histologic grade | ||
| I~II | 70 | 76.1 |
| II~III | 22 | 23.9 |
| TNM stage | ||
| I | 3 | 3.3 |
| II | 51 | 55.4 |
| III | 37 | 40.2 |
| IV | 1 | 1.1 |
| Location (cm) | ||
| Left colon | 39 | 42.4 |
| Right colon | 53 | 57.6 |
| Infiltration degree | ||
| Adventitia | 64 | 69.6 |
| Serosa/muscular/mucosa | 28 | 30.4 |
| Pathological morphology | ||
| Infiltrate/ulcer type | 68 | 73.9 |
| Protrude/basin type | 24 | 26.1 |
| Distant metastasis and Vascular invasion | 18 | 19.6 |
| Positive lymph nodes | 37 | 40.2 |
Cell culture
The 9 human colon cancer cell lines were bought from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Caco2, RKO and RKO-E6 were cultured with minimum essential medium (MEM, HyClone, Logan, UT, USA) containing essential amino acids (Sigma, St. Louis, MO, USA) and 10% fetal bovine serum (FBS, Gibco, Logan, UT, USA). HCT116, Lovo, SW480 and Colo205 cells were performed with PRMI-1640 (HyClone) containing 10% FBS. LS174T and HT29 cells were cultured with Dulbecco’s modified Eagle’s medium (DMEM, HyClone) containing 10% FBS. Cells were incubated in a humidified atmosphere of 37°C, with 5% CO2 in the air.
Human colon cancer xenografts
Twelve healthy male BAL b/c nude mice, weighing 18-22 g, were obtained from the Experimental Animal Center of Sichuan University (Chengdu, China). The mice were housed under a 12 hour light/dark cycle, receiving food and water ad libitum with a constant temperature and humidity. Briefly, 1 × 107 of human colon cancer cell HCT116 was subcutaneously injected into the left flank of 6 nude mice, and 1 × 107 of RKO was also subcutaneously injected into the left flank of another 6 nude mice. After 4 weeks, the tumors were aseptically removed, and the corresponding colon was also obtained, after the mice were sacrificed. A portion of the tissues were fixed in 4% neutral buffered paraformaldehyde and embedded with paraffin before histopathologic and immunohistochemical examinations. Other tissues were immediately frozen in liquid nitrogen and stored at -80°C for further protein analysis. The animal procedures were approved by the Animal Use and Care Committee of Sichuan University and were conducted according to the regulations set by Sichuan University.
Hematoxylin and eosin stain (HE)
TMA sections and sections from the mice were routinely deparaffinized in xylene and rehydrated with a series of ethanol dilutions. Then, HE staining was performed according to manufacturer’s instructions (Solarbio, Beijing, China).
Immunohistochemistry (IHC)
TMA sections and paraffin-embedded tissues from the mice were routinely deparaffinized in xylene and rehydrated with a series of ethanol dilutions. Heat induced-antigen retrieval was performed in a 10 mM sodium citrate buffer for 30 minutes. Endogenous peroxidase activity was blocked by H2O2, and antigen blocking was performed with goat serum. The sections were incubated with rabbit anti-HIF-1α (1:500, Proteintech, Wuhan, China) overnight at 4°C. Subsequently, 30 min of incubation with horseradish peroxidase-conjugated secondary antibody kits (ZSGB Bio, Beijing, China) at room temperature was done. Then, the sections were stained with a 3, 3’-diaminobenzidine tetrahydrochloride solution. Finally, the sections were counter-stained with hematoxylin. Negative control slides with the primary antibodies omitted were included in all tests.
IHC scoring
Two independent pathologists blinded to the clinical and pathologic information assessed the abovementioned TMA slides. The expression of HIF-1α in the tissues was scored semi-quantitatively, combining the positive percentage and the intensity of the stained preparation (staining index = positive × intensity score), according to previous studies [36]. The positive percentage of the stained tumor cells was scored as: 0, no staining; 1, < 20%; 2, 20-75%; 3, > 75%. The intensity of the stained tumor cells was graded on the following scale: 0, negative; 1, weak; 2, moderate; 3, strong staining. Based on the staining index, a final total score of 0-4 was considered to be low expression of HIF-1α, and a total score of 5-9 was defined as high expression.
The expressions of the proteins in the paraffin-embedded tissues from the mice were evaluated according to the integrated optical density (IOD) calculated by Image-Pro plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA).
Western blot analysis for HIF-1α protein expression
The extraction of the whole proteins was performed for the cultured cells, frozen human and mice colon cancer, and the corresponding paracancerous tissues using a protein extraction kit (Nanjing Kaiji, Nanjing, China). The same amount of protein (50 μg) from each sample was analyzed by gel electrophoresis and transferred respectively to a PVDF membrane (Millipore, Billerica, MA, USA). The PVDF membranes were treated with 5% non-fat dry milk and then incubated with primary antibodies directed against GAPDH (1:5000, Abcam, Cambridge, UK), HIF-1α (1:2000, Proteintech) at 4°C overnight. After washing, the membranes were incubated with appropriate horseradish-peroxidase-conjugated secondary antibodies (1:10000, Santa Cruz) for 2 h at 37°C. The bands were then visualized with an ECL detection kit (Engreen, Beijing, China), determined by Quantity One software 4.5.0 (Bio-Rad, Hercules, CA, USA), and normalized to GAPDH.
Statistical analysis
Quantitative data were expressed as the mean ± standard deviation (SD). Student’s t test was used for the data analysis. The Kaplan-Meier method was utilized to depict survival curves, which were compared by the log-rank test. The χ2 test was applied to analyze the categorical variables. The hazard ratios of overall survival were determined by the multivariable Cox proportional hazards regression model. All the data were analyzed using SPSS 19.0 software (SPSS, Chicago, IL, USA). A p-value of < 0.05 was considered significant.
Results
Patient characteristics
The clinicopathological characteristics of the 92 patients with colon adenocarcinoma recruited in our study are shown in Table 1. Among the patients, 55 were males (59.8%) and 37 were females (40.2%). The average age was 62.4 ± 11.9 years old. As for the tumor histological grades, 70 cases (76.1%) were in grades I~II, and the other 22 cases (23.9%) were in grades II~III. In terms of the TNM staging system, 3 cases (3.3%) were in stage I, 51 (55.4%) in stage II, 37 (40.2%) in stage III, and only 1 (1.1%) in stage IV. Furthermore, 39 cases (42.4%) had tumors located at the left colon and 53 (57.6%) at the right. An infiltrate/ulcer type of tumor was found in 68 cases (73.9%), and the remaining were of the protruded/basin type (24 cases, 26.1%). Distant metastasis and vascular invasion were identified in 18 patients (19.6%). Moreover, positive lymph nodes were observed in 37 cases (40.2%).
Up-regulation of HIF-1α in colon adenocarcinoma
In the TMA slides from the patients with colon adenocarcinoma, H&E staining clearly identified typical colon adenocarcinoma tissues, while normal colon structures in the corresponding paracancerous tissues could also be observed, highlighting the circular arrangement pattern of crypts and epithelial columnar cells interspersed by goblet cells within (Figure 1A). IHC for HIF-1α was performed, and the positive staining was mainly discerned in the nucleus of the tumor cells in the colon adenocarcinoma tissues (Figure 1B). However, in the corresponding paracancerous tissues, the pattern of HIF-1α staining was notably different, with weak staining in epithelia and strong staining in the immunocytes (Figure 1B). We then evaluated the staining index in both the colon adenocarcinoma tissues and the corresponding paracancerous tissues. A significant difference in the expressions of HIF-1α between the human colon adenocarcinoma tissues and the paracancerous tissues was discovered (6.2 ± 1.7 vs. 4.1 ± 1.7; P < 0.001, Figure 1C). Similarly, the protein levels of HIF-1α quantified by western blot were also remarkably increased in the colon adenocarcinoma tissues compared to the corresponding paracancerous tissues (2.02 ± 0.38 vs. 1.00 ± 0.24; P = 0.007, Figure 1D, 1E).
Figure 1.
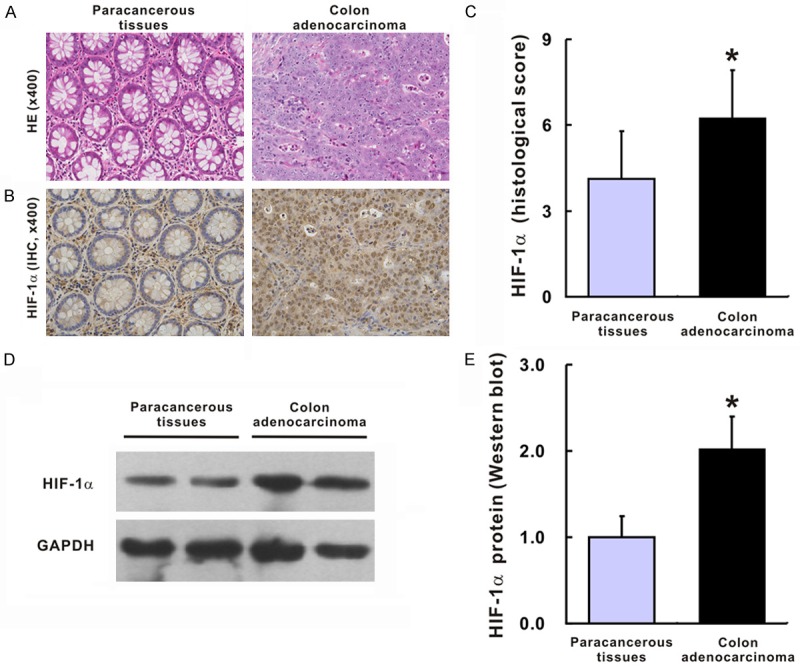
HIF-1α expression in colon adenocarcinoma tissues and their corresponding paracancerous tissues. Representative images for HE and IHC of HIF-1α for colon adenocarcinoma tissues and their corresponding paracancerous tissues (A, B); Histological score of HIF-1α in colon adenocarcinoma tissues and their corresponding paracancerous tissues (C), N = 52 in each group. Western blot (D) and statistic analysis (E) of HIF-1α in colon adenocarcinoma tissues and their corresponding paracancerous tissues, N = 8 in each group. The group of blots was cropped from different gels. *P < 0.05 vs. paracancerous tissues.
Coincidently, this result resembled that of human colon adenocarcinoma xenografts in the node mice (Figure 2). Compared with the normal colon tissues of the nude mice, the H&E staining of the xenografts from HCT116 and RKO was very similar to the colon adenocarcinoma tissues in humans (Figure 2A and 2F). Furthermore, the expression of HIF-1α in the xenografts from HCT116 and RKO was stronger compared with the expressions in the normal colon tissues of the nude mice as assessed by IOD (xenografts from HCT116 and normal colon, 2.5 ± 0.4 vs. 1.0 ± 0.2, P < 0.001, Figure 2B, 2C; xenografts from RKO and normal colon, 1.9 ± 0.5 vs. 1.0 ± 0.3, P = 0.018, Figure 2G, 2H). Consistently, the protein levels detected by Western blot showed a similar pattern, indicating that HIF-1α was substantially increased in the colon adenocarcinoma tissues compared to the normal colon tissues in both HCT116 xenografts (Figure 2D, 2E, 1.00 ± 0.15 vs. 1.98 ± 0.34, P = 0.012) and RKO xenografts (Figure 2I, 2J, 1.00 ± 0.19 vs. 1.65 ± 0.29, P = 0.023).
Figure 2.
HIF-1α expression in HCT 116 xenograft or RKO xenograft and their corresponding normal colon tissues. Representative images for HE and IHC of HIF-1α for HCT 116 xenograft (A, B) or RKO xenograft and their corresponding normal colon tissues (F, G). IOD of HIF-1α in HCT 116 xenograft or RKO xenograft and their corresponding normal colon tissues (C, H). Western blot and statistical analysis of HIF-1α in HCT 116 xenograft (D, E) or RKO xenograft (I, J) and their corresponding normal colon tissues. N = 6 in each group. The group of blots was cropped from different gels. *P < 0.05 vs. corresponding normal colon.
Correlation of HIF-1α expression with progression in human colon adenocarcinoma
To assess whether HIF-1α expression was associated with the clinicopathological features in colon adenocarcinoma, we then divided the patients into 2 subgroups according to the expression levels of HIF-1α (Table 2). 29 patients were included in the low HIF-1α expression group, while the remaining 63 were included in the high HIF-1α expression group. This indicated that HIF-1α expression was related to the TNM stage of colon adenocarcinoma. Among the 29 patients in the low HIF-1α expression group, 22 (75.9%) were in TNM stages I~II, while 7 (24.1%) were in TNM stages III~IV. And among the 63 patients within the high HIF-1α expression group, 32 (50.8%) were in TNM stages I~II, while 31 (49.2%) were in TNM stages III~IV, indicating that high HIF-1α expression might be related to advanced TNM stages (P = 0.025). Moreover, distant metastasis and vascular invasion were identified in 16 patients (25.4%) within the high HIF-1α expression group but in only 2 patients (6.9%) in the low HIF-1α expression group, suggesting that HIF-1α expression is significantly associated with the possibility of distant metastasis and vascular invasion (P = 0.048). Moreover, 7 patients (24.1%) in the low HIF-1α expression group had positive lymph nodes. In contrast, 30 cases (47.6%) in the high HIF-1α expression group had positive lymph nodes. Thus, HIF-1α expression might also be related to the possibility of lymphatic metastasis (P = 0.041). Consistently, the protein levels of HIF-1α were significantly higher in the metastatic CRC cell lines (Colo205 and Lovo) than they were in the non-metastatic cell lines (Caco2, LS174T, RKO, RKO-E6, SW480, HCT116, HT29; P = 0.031, Figure 3A, 3B). However, no significant correlation between the expression of HIF-1α and other clinicopathological factors, such as age, gender, histologic grade, tumor location, infiltration degree, and pathological morphology, was found (P > 0.05, Table 2).
Table 2.
Relationships between HIF-1α expression and clinicopathological characteristics in 92 colon adenocarcinoma cases
| Characteristics | HIF-1α expression (%) | p value | |
|---|---|---|---|
|
| |||
| Low | High | ||
| Total | 29 | 63 | |
| Average years | 61.3 ± 11.2 | 62.9 ± 12.3 | |
| < 65 | 17 (58.6) | 33 (52.4) | 0.655 |
| ≥ 65 | 12 (41.4) | 30 (47.6) | |
| Gender | |||
| Male | 21 (72.4) | 34 (54.0) | 0.113 |
| Female | 8 (27.6) | 29 (46.0) | |
| Histologic grade | |||
| I~II | 23 (79.3) | 47 (74.6) | 0.794 |
| II~III | 6 (20.7) | 16 (25.4) | |
| TNM stage | |||
| I-II | 22 (75.9) | 32 (50.8) | 0.025 |
| III-IV | 7 (24.1) | 31 (49.2) | |
| Location (cm) | |||
| Left colon | 11 (37.9) | 28 (44.4) | 0.652 |
| Right colon | 18 (62.1) | 35 (55.6) | |
| Infiltration degree | |||
| Adventitia | 19 (65.5) | 45 (71.4) | 0.629 |
| Serosa/muscular/mucosa | 10 (34.5) | 18 (28.6) | |
| Pathological morphology | |||
| Infiltrate/ulcer type | 20 (69.0) | 48 (76.2) | 0.458 |
| Protrude/basin type | 9 (31.0) | 15 (23.8) | |
| Distant metastasis and Vascular invasion | 2 (6.9) | 16 (25.4) | 0.048 |
| Positive lymph nodes | 7 (24.1) | 30 (47.6) | 0.041 |
Figure 3.
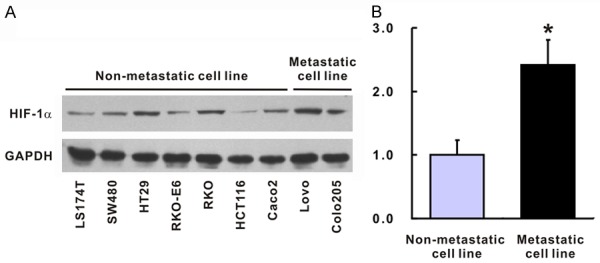
HIF-1α expression in metastatic CRC cell lines and low-metastatic cell lines. Western blot (A) and statistic analysis (B) of HIF-1α in metastatic CRC cell lines and low-metastatic cell lines, N = 3 in each cell. The group of blots was cropped from different gels. *P < 0.05 vs. low-metastatic cell lines.
High expression of HIF-1α was associated with poor survival in colon adenocarcinoma
Following the above-mentioned expression levels of HIF-1α, the Kaplan-Meier method was administered to estimate the 5-year and 10-year survival rates (Figure 4).
Figure 4.
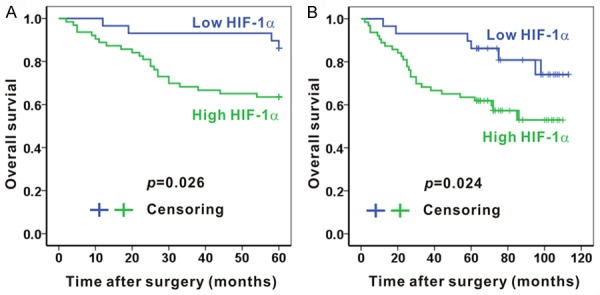
Associations of the HIF-1α expression and 5-year or 10-year survival in colon adenocarcinoma tissues. The 5-year survival (A) and 10-year survival (B) were better in patients with low HIF-1α expression comparing with those with high HIF-1α expression. N = 29 and N = 63 in low HIF-1α expression group and high HIF-1α expression group, respectively.
The overall 5-year survival of 92 patients with colon cancer was 70.7%, and the median 5-year survival of these patients was about 49 months. A significant difference was observed between HIF-1α expression and the 5-year survival rate (P = 0.026; Figure 4A). The survival rate of patients with high HIF-1α expression at 5 years was 63.5%, and their median 5-year survival was 46 months. In contrast, the overall 5-year survival rate of those with low HIF-1α expression was 86.2%, with a median 5-year survival of about 57 months.
Then, with regard to the 10-year survival rate, 59 out of 92 patients (64.1%) with colon cancer were still alive, and the median 10-year survival of the patients was about 83 months. We also detected a significant difference between HIF-1α expression and the 10-year survival rate (P = 0.024; Figure 4B). For patients with high HIF-1α expression, the survival rate at 10 years was 57.1%, and their median 10-year survival was 74 months. However, for those with low HIF-1α expression, the overall 10-year survival rate was 79.3%, with a median 10-year survival of about 99 months.
HIF-1α is an independent risk factor for the prognosis of colon adenocarcinoma
To assess whether HIF-1α is an independent risk factor for the prognosis of colon adenocarcinoma, we first evaluated whether other factors were potentially related with survival. Indeed, age (< 65 vs. ≥ 65), infiltration degree (adventitia vs. Serosa/muscular/mucosa), metastasis (present vs. absent), positive lymph nodes (present vs. absent), and TNM stage (I-II vs. III-IV) were significantly associated with the survival of these patients (P < 0.05; Figure 5). However, no other factors were related with survival.
Figure 5.

Factors significantly related with poor survival. Age ≥ 65 (A), adventitia infiltration (B), the presence of metastasis (C), positive lymph nodes (D), and TNM stage III-IV (E) were significantly associated with the poor survival of patients with colon adenocarcinoma. N = 92 in each analysis.
In this case, we further utilized the multivariable Cox proportional hazards regression model to identify the independent factors affecting overall survival. Among the factors, we found age > 65 years, the presence of metastasis, and high HIF-1α expression were independent risk factor for the prognosis of colon adenocarcinoma and were significantly related with poor overall survival (P = 0.013, P = 0.003, P = 0.035, respectively; Table 3; Figure 6).
Table 3.
Multivariable Cox regression analysis of overall survival in 92 colon adenocarcinoma cases
| Features | HR | (95% CI) | p value |
|---|---|---|---|
| Average years (< 65 vs. ≥ 65) | 3.832 | 1.287-8.370 | 0.013 |
| TNM stage (I-II vs. III-IV) | 1.793 | 0.624-5.156 | 0.278 |
| Infiltration degree (Adventitia vs. Serosa/muscular/mucosa) | 0.290 | 0.082-1.032 | 0.056 |
| Metastasis (No vs. Yes) | 3.331 | 1.498-7.403 | 0.003 |
| Positive lymph nodes (No vs. Yes) | 1.221 | 0.988-1.509 | 0.065 |
| HIF-1α expression (High vs. Low) | 0.318 | 0.110-0.921 | 0.035 |
HR: hazard ratio; CI: confidence interval.
Figure 6.
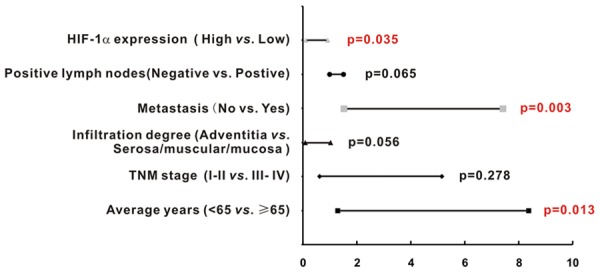
High HIF-1α expression was an independent risk factor for poor prognosis of colon adenocarcinoma. High HIF-1α expression was an independent risk factor for the poor prognosis of colon adenocarcinoma (P = 0.035). Other independent risk factors for poor prognosis included the presence of metastasis (P = 0.003) and age ≥ 65 (P = 0.013).
Discussion
The burden brought about by colorectal cancer is still heavy worldwide [1,19]. To cope with it, it is critical to identify markers related to metastasis and poor prognosis. HIF-1α was reported to promote metastasis in cancer, including colorectal cancer [11,13,14]. However, clinically speaking, the results are conflicting concerning the association between HIF-1α expression and metastasis as well as prognosis. Some found HIF-1α expression was related with tumor stage and metastases [16], but others failed to find such results [15,17]. Thus, in this study TMA was applied to further discover whether there is an association between HIF-1α and metastasis as well as prognosis in patients with colon adenocarcinoma. We found that the expression of HIF-1α in colon adenocarcinoma tissues was higher than it was in the corresponding paracancerous tissues, and the result was also confirmed in human colon adenocarcinoma xenografts in node mice. Further, it was also found that high HIF-1α expression could predict a higher TNM stage, distant metastasis and vascular invasion, and positive lymph nodes. Moreover, high HIF-1α expression was detrimental to 5-year and 10-year survival and was an independent risk factor for poor prognosis. These findings provided new insights into the role of HIF-1α in colon adenocarcinoma and it could potentially work as a biomarker in the prediction of metastasis and poor prognosis.
HIF-1α is a transcriptional factor which could promote tumor progression by regulating the expressions of various hypoxically induced genes, whose function is enhanced when cells become hypoxic [20]. In our study, we found that high HIF-1α expression was predictive of higher TNM stages, distant metastasis and vascular invasion, and positive lymph nodes. These phenomena could be understood since increased HIF-1α can promote tumor growth and enhance the migration capacity of tumor cells by the activation of small GTPases and the induction of fibroblast phenotypes [21,22].
Higher TNM stages, distant metastasis and vascular invasion, and positive lymph nodes observed in patients with a high HIF-1α expression was indicative of a more aggressive phenotype of colon adenocarcinoma in these patients. Thus, it was not surprising that our results suggested that augmented HIF-1α could lead to poor prognosis independently. This might be attributed to the effect of HIF-1α on tumor angiogenesis, which plays a crucial role in the progression of colon cancer [23]. Multiple proangiogenic factors, VEGF for example, could be upregulated by HIF-1α [24]. Moreover, it is known that in xenografts, HIF-1α expression was correlated with tumor growth and angiogenesis [25]. In addition, another study found that aberrant HIF-1α expression could also downregulate E-cadherin [26], which might further facilitate the invasion of cancer.
In general, the detrimental role of HIF-1α has been established in colorectal cancer. Our above-mentioned results are similar to those of Cao and Wu [16,18]. Another study from Baba demonstrated that HIF-1α expression is associated with poor prognosis in colorectal cancer, but they did not find any relationship between HIF-1α expression and TNM stage [24]. This discrepancy might be due to their method of scoring HIF-1α expression level. They only judged the presence or absence of HIF-1α in cancer tissues but in our study, we used a staining index as described in our methods section, which might be more accurate. Moreover, though they also utilized TMA, but they did not compare the strength of HIF-1α staining to the paracancerous tissues, which might also influence the accuracy. By comparing the expression of HIF-1α in TMA (IHC), frozen human colon tissues (Western blot), as well as HCT116 and RKO xenografts (IHC and Western blot), an increased expression of HIF-1α was found in colon adenocarcinoma when compared with the expression in the corresponding paracancerous tissues. However, some studies failed to identify the relationship between HIF-1α expression and the prognosis of patients with colorectal cancer. For example, Saka et al. did not find any associations between HIF-1α and clinicopathological characteristics or prognosis in patients with colorectal adenocarcinoma [15]. We suspect that this might be attributed to the racial disparity because patients from that study were Turkish. In support of this, a meta-analysis demonstrated that overexpressed HIF-1α was associated with poor survival particularly in Asian countries but not in European or other countries [27].
Currently, many experiments concerning cancer therapy are targeting HIF-1α, which is closely associated with tumor angiogenesis, metabolism, immunoregulation, and proliferation [28-30]. In colon cancer, a number of drugs are found to downregulate HIF-1α in vivo, which suppress tumor vascularity and growth [31-33]. A novel HIF-1α inhibitor, IDF-11774, is reported to decrease the energy production of colorectal cancer [34]. Combination treatment with a specific HIF-1α inhibitor was also recommended for drug resistant colorectal cancer, as an in vitro study found that PX-478, a HIF-1α inhibitor, could enhance the therapeutic effects of EGFR inhibition [35]. However, clinical experiments using an HIF-1α inhibitor to treat colorectal cancer are still lacking. Since promising results in basic studies have been reported, and high HIF-1α expression is associated with advanced colon cancer and poor survival, clinical studies applying the HIF-1α inhibitor should be carried out to treat colorectal cancer.
In conclusion, HIF-1α expression is associated with metastasis and poor survival in colon adenocarcinoma. It is an independent risk factor for poor prognosis in patients with colon adenocarcinoma. Targeting HIF-1α might enable the inhibition of metastasis and promote a better prognosis in colon adenocarcinoma.
Acknowledgements
This study was supported by grants from the Natural Science Fund of China (81873584, 81670551, U1702281 and 81400637), Chinesisch-Deutsches Zentrum fǖr Wissenschaftsfǒrderung (GZ 1065), the National Key R&D Program of China (2017YFA0205400) and the Science and Technology Support Program of Sichuan province (2016SZ0041).
Disclosure of conflict of interest
None.
References
- 1.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 3.Zhao S, Sun H, Jiang W, Mi Y, Zhang D, Wen Y, Cheng D, Tang H, Wu S, Yu Y, Liu X, Cui W, Zhang M, Sun X, Zhou Z, Peng Z, Yan D. miR-4775 promotes colorectal cancer invasion and metastasis via the Smad7/TGFbeta-mediated epithelial to mesenchymal transition. Mol Cancer. 2017;16:12. doi: 10.1186/s12943-017-0585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen LD. The circadian clock and hypoxia in tumor cell de-differentiation and metastasis. Biochim Biophys Acta. 2015;1850:1633–1641. doi: 10.1016/j.bbagen.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 5.De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol. 2011;8:393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- 6.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 7.Gao JH, Wang CH, Tong H, Wen SL, Huang ZY, Tang CW. Targeting inhibition of extracellular signal-regulated kinase kinase pathway with AZD6244 (ARRY-142886) suppresses growth and angiogenesis of gastric cancer. Sci Rep. 2015;5:16382. doi: 10.1038/srep16382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasan S, Chitalia V, Meyer RD, Hartsough E, Mehta M, Harrold I, Anderson N, Feng H, Smith LE, Jiang Y, Costello CE, Rahimi N. Hypoxia-induced expression of phosducin-like 3 regulates expression of VEGFR-2 and promotes angiogenesis. Angiogenesis. 2015;18:449–462. doi: 10.1007/s10456-015-9468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SJ, Kim HP, Jin Y, Choi AM, Ryter SW. Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis. Autophagy. 2011;7:829–839. doi: 10.4161/auto.7.8.15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Fu L, Li JB, Qin Y, Zeng TT, Zhou J, Zeng ZL, Chen J, Cao TT, Ban X, Qian C, Cai Z, Xie D, Huang P, Guan XY. Increased expression of EIF5A2, via hypoxia or gene amplification, contributes to metastasis and angiogenesis of esophageal squamous cell carcinoma. Gastroenterology. 2014;146:1701–1713. e1709. doi: 10.1053/j.gastro.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Rokavec M, Jiang L, Horst D, Hermeking H. Antagonistic effects of p53 and HIF1A on microrna-34a regulation of ppp1r11 and stat3 and hypoxia-induced epithelial to mesenchymal transition in colorectal cancer cells. Gastroenterology. 2017;153:505–520. doi: 10.1053/j.gastro.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Ioannou M, Paraskeva E, Baxevanidou K, Simos G, Papamichali R, Papacharalambous C, Samara M, Koukoulis G. HIF-1α in colorectal carcinoma: review of the literature. J BUON. 2015;20:680–689. [PubMed] [Google Scholar]
- 13.Kizaka-Kondoh S, Tanaka S, Harada H, Hiraoka M. The HIF-1-active microenvironment: an environmental target for cancer therapy. Adv Drug Deliv Rev. 2009;61:623–632. doi: 10.1016/j.addr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Xiang J, Sun H, Su L, Liu L, Shan J, Shen J, Yang Z, Chen J, Zhong X, Ávila MA, Yan X, Liu C, Qian C. Myocyte enhancer factor 2D promotes colorectal cancer angiogenesis downstream of hypoxia-inducible factor 1alpha. Cancer Lett. 2017;400:117–126. doi: 10.1016/j.canlet.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 15.Saka B, Ekinci O, Dursun A, Akyurek N. Clinicopathologic and prognostic significance of immunohistochemical expression of HIF-1α, CXCR4 and CA9 in colorectal carcinoma. Pathol Res Pract. 2017;213:783–792. doi: 10.1016/j.prp.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Cao D, Hou M, Guan YS, Jiang M, Yang Y, Gou HF. Expression of HIF-1alpha and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. BMC Cancer. 2009;9:432. doi: 10.1186/1471-2407-9-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SJ, Kim JG, Sohn SK, Chae YS, Moon JH, Kang BW, Park JS, Park JY, Choi GS. No association of the hypoxia-inducible factor-1alpha gene polymorphisms with survival in patients with colorectal cancer. Med Oncol. 2011;28:1032–1037. doi: 10.1007/s12032-010-9618-9. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Jin M, Xu H, Shimin Z, He S, Wang L, Zhang Y. Clinicopathologic significance of HIF-1α, CXCR4, and VEGF expression in colon cancer. Clin Dev Immunol. 2010:2010. doi: 10.1155/2010/537531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68:7–11. doi: 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 20.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatrai E, Bartal A, Gacs A, Paku S, Kenessey I, Garay T, Hegedus B, Molnár E, Cserepes MT, Hegedűs Z, Kucsma N, Szakács G, Tóvári J. Cell type-dependent HIF1 alpha-mediated effects of hypoxia on proliferation, migration and metastatic potential of human tumor cells. Oncotarget. 2017;8:44498–44510. doi: 10.18632/oncotarget.17806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiavarina B, Whitaker-Menezes D, Migneco G, Martinez-Outschoorn UE, Pavlides S, Howell A, Tanowitz HB, Casimiro MC, Wang C, Pestell RG, Grieshaber P, Caro J, Sotgia F, Lisanti MP. Hif1-alpha functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells: autophagy drives compartment-specific oncogenesis. Cell Cycle. 2010;9:3534–3551. doi: 10.4161/cc.9.17.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi H, Jiang J, Ji J, Shi M, Cai Q, Chen X, Yu Y, Liu B, Zhu Z, Zhang J. Anti-angiogenesis participates in antitumor effects of metronomic capecitabine on colon cancer. Cancer Lett. 2014;349:128–135. doi: 10.1016/j.canlet.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Baba Y, Nosho K, Shima K, Irahara N, Chan AT, Meyerhardt JA, Chung DC, Giovannucci EL, Fuchs CS, Ogino S. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol. 2010;176:2292–2301. doi: 10.2353/ajpath.2010.090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62–67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 26.Mladenova DN, Dahlstrom JE, Tran PN, Benthani F, Bean EG, Ng I, Pangon L, Currey N, Kohonen-Corish MR. HIF1alpha deficiency reduces inflammation in a mouse model of proximal colon cancer. Dis Model Mech. 2015;8:1093–1103. doi: 10.1242/dmm.019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, He X, Xia W, Huang Q, Zhang Z, Ye J, Ni C, Wu P, Wu D, Xu J, Qiu F, Huang J. Prognostic value and clinicopathological differences of HIFs in colorectal cancer: evidence from meta-analysis. PLoS One. 2013;8:e80337. doi: 10.1371/journal.pone.0080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kheshtchin N, Arab S, Ajami M, Mirzaei R, Ashourpour M, Mousavi N, Khosravianfar N, Jadidi-Niaragh F, Namdar A, Noorbakhsh F, Hadjati J. Inhibition of HIF-1α enhances anti-tumor effects of dendritic cell-based vaccination in a mouse model of breast cancer. Cancer Immunol Immunother. 2016;65:1159–1167. doi: 10.1007/s00262-016-1879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubey R, Levin MD, Szabo LZ, Laszlo CF, Kushal S, Singh JB, Oh P, Schnitzer JE, Olenyuk BZ. Suppression of tumor growth by designed dimeric epidithiodiketopiperazine targeting hypoxia-inducible transcription factor complex. J Am Chem Soc. 2013;135:4537–4549. doi: 10.1021/ja400805b. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Li G, Wang Y, Tang S, Sun X, Feng X, Li Y, Bao G, Li P, Mao X, Wang M, Liu P. Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1α/VEGF secretion axis. Oncotarget. 2015;6:44579–44592. doi: 10.18632/oncotarget.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shay JE, Imtiyaz HZ, Sivanand S, Durham AC, Skuli N, Hsu S, Mucaj V, Eisinger-Mathason TS, Krock BL, Giannoukos DN, Simon MC. Inhibition of hypoxia-inducible factors limits tumor progression in a mouse model of colorectal cancer. Carcinogenesis. 2014;35:1067–1077. doi: 10.1093/carcin/bgu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui H, Zhao J, Zhou L, Wen H, Deng W, Li C, Ji Q, Liu X, Feng Y, Chai N, Zhang Q, Cai J, Li Q. Tanshinone IIA inhibits β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments in human colorectal cancer. Cancer Lett. 2017;403:86–97. doi: 10.1016/j.canlet.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68:2043–2050. doi: 10.1158/0008-5472.CAN-07-6247. [DOI] [PubMed] [Google Scholar]
- 34.Ban HS, Kim BK, Lee H, Kim HM, Harmalkar D, Nam M, Park SK, Lee K, Park JT, Kim I, Lee K, Hwang GS, Won M. The novel hypoxia-inducible factor-1alpha inhibitor IDF-11774 regulates cancer metabolism, thereby suppressing tumor growth. Cell Death Dis. 2017;8:e2843. doi: 10.1038/cddis.2017.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Lei F, Rong W, Zeng Q, Sun W. Positive feedback between oncogenic KRAS and HIF-1αlpha confers drug resistance in colorectal cancer. Onco Targets Ther. 2015;8:1229–1237. doi: 10.2147/OTT.S80017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen S, Gao J, Zhang L, Zhou H, Fang D, Feng S. P53 increase mitochondrial copy number via up-regulation of mitochondrial transcription factor a in colorectal cancer. Oncotarget. 2016;7:75981–75995. doi: 10.18632/oncotarget.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]



