Abstract
Esophageal squamous cell carcinoma (ESCC) is generally known to be a highly fatal cancer, and thus novel molecular targets are needed to improve its diagnosis and treatment. AJUBA has been shown to regulate cell cycle, adhesion, proliferation, apoptosis, and migration in many malignant tumors. However, the clinical significance of AJUBA in ESCC tumor metastasis remains unclear. In this study, we explored the role of AJUBA, Yes-associated protein 1 (YAP1), and matrix metalloproteinase 14 (MMP14) in the clinical presentation and survival of ESCC. Immunohistochemical staining showed higher expression of these proteins in cancer tissues than in paired adjacent tissues, and this upregulation was differently related to lymph node metastasis and TNM stage. AJUBA expression was positively correlated with that of YAP1. High expression of MMP14 was associated with reduced survival. In general, our findings reveal that AJUBA, YAP1, and MMP14 might function as oncoproteins and contribute to novel targeted therapy in ESCC.
Keywords: AJUBA, YAP1, MMP14, ESCC, clinical significance
Introduction
Esophageal cancer is one of the most common tumors in the digestive system. Most cases of esophageal cancer derive from esophageal squamous cell carcinoma (ESCC). The advanced stage at initial diagnosis of ESCC results in a poor 5-year overall survival rate of 20.9% [1]. Thus, it is critical to identify the genetic alterations associated with ESCC tumorigenesis or prognosis.
A recent study found that AJUBA, belonging to the LIM domain protein family, could promote tumor invasion and inhibit cisplatin-induced apoptosis in ESCC [2]. The LIM region contains two consecutive zinc finger structures that participate in interactions with multiple proteins and thus contribute to tumor development, with effects on cell mitosis [3], cell adhesion [4], migration [5], transcriptional regulation [6], and immune response [7]. Combined with the serine-threonine kinase aurora A, AJUBA can regulate the cell cycle by promoting mitosis and G2/M phase transition in HeLa cells [8]. AJUBA can also activate the small GTPase Rac1 to regulate cell adhesion in human keratinocytes [9]. AJUBA interacts with Snail to enhance ability epithelial-mesenchymal transition (EMT) in colorectal cancer cells [10].
Yes-associated protein 1 (YAP1) plays an important role in embryonic development and tumor formation as a crucial downstream effector of the Hippo pathway. It usually acts as an oncogene in gastric cancer [11], bladder cancer [12], and cervical cancer [13]. However, there have been a few studies showing a role of YAP1 in the migration of the neoplasm in ESCC. AJUBA can function as a novel regulator of the Hippo pathway by binding to LATS1/2, thus preventing YAP1 activation in Drosophila [14] and malignant mesothelioma [15]. RNA-seq analysis has shown that AJUBA can regulate the MAPK pathway to activate matrix metalloproteinases (MMP) [16-18]. MMP14 is an extracellular matrix enzyme with the ability to degrade collagen and activate MMP2. In ESCC, overexpression of MMP14 is associated with lymphatic invasion and poor prognosis [19,20]. Thus, MMP14 also appears to play a crucial role in ESCC.
In the present study, we detected the expression of AJUBA, YAP1, and MMP14 in ESCC using immunohistochemistry (IHC) and explored the relationship between these expression levels and lymph node metastasis. We also conducted a retrospective study to evaluate the role of AJUBA, YAP1, and MMP14 in overall survival. Our results may provide a new molecular target for ESCC therapy and prevention of metastasis.
Materials and methods
Patients and samples
A total of 142 primary ESCC tumor resection samples were collected from the Department of Pathology at the Second Hospital of Hebei Medical University from 2010 to 2015. In addition, the paired adjacent non-cancer samples at least 5 cm from the lesions were also acquired, but 31 of them were removed for failing to get enough squamous epithelium. All of the samples were formalin fixed paraffin-embedded tissues (FFPE). None of the patients underwent preoperative chemotherapy or radiotherapy. Clinical data such as gender, age, tumor differentiation, lymph metastasis, TNM stage (according to the American Joint Committee on Cancer, 7th edition), and survival were gathered (Table 1). We obtained all patients’ consent and permission according to the guidelines of the Second Hospital of Hebei Medical University ethics committee.
Table 1.
Clinicopathological characteristics of 142 ESCC cases
| Characteristics | N (%) | |
|---|---|---|
| Gender | Male | 111 (78.17%) |
| Female | 31 (21.83%) | |
| Age | ≥60 | 93 (65.49%) |
| <60 | 49 (34.51%) | |
| Pathological grading | Well & Moderate | 101 (71.13%) |
| Poor | 41 (28.87%) | |
| LN metastasis | Present | 45 (31.69%) |
| Absent | 97 (68.31%) | |
| TNM stage | I + II | 70 (49.30%) |
| III + IV | 72 (50.70%) | |
| AJUBA | Positive | 124 (87.32%) |
| Negative | 18 (12.68%) | |
| YAP1 | Positive | 124 (87.32%) |
| Negative | 18 (12.68%) | |
| MMP14 | Positive | 121 (85.21%) |
| Negative | 21 (14.79%) |
Note: LN metastasis is short for lymph node metastasis.
Immunohistochemistry
Cancer tissues were formalin-fixed and paraffin-embedded into tissue blocks. Then, 4-μm thick paraffin sections were prepared for analysis with a streptavidin-peroxidase (SP) method [21]. After deparaffinization and hydration, antigen retrieval was performed in 0.01 M citrate buffer (pH 6.0) for 10 min. Endogenous peroxidases were quenched with 3% H2O2 for 10 min. After treatment with Block Solution (Zhongshanjinqiao, Beijing, China) for 60 min at temperature of 25°C sections were incubated at 4°C overnight with anti-AJUBA (1:300; Cell Signaling Technology, Danvers, MA, USA), anti-YAP (1:400; Abcam, Cambridge, UK), and anti-MMP14 (1:400; Abcam) antibody. An SP IHC Kit (Zhongshanjinqiao) was used for color development with diaminobenzidine tetrahydrochloride. Counterstaining was performed with hematoxylin. Concurrent IHC staining of tissue sections in the absence of the primary antibody was performed as a negative control.
Evaluation of IHC staining results
Two independent pathologists evaluated the IHC staining semi-quantitatively for each case within five randomly selected high-power fields (20× magnification) under a light microscope using the following scoring system: grade 0, <10% immunoreactive cells; grade 1, 10-30% immunoreactive cells; grade 2, 30-50% immunoreactive cells; grade 3, > 50% immunoreactive cells. Cases with grade 0 were regarded as negative, and those with grades 1 to 3 were regarded as positive.
Statistical analysis
Statistical analyses were carried out using SPSS 17.0. Clinicopathological features associated with AJUBA, YAP1, and MMP14 expression were analyzed for statistical significance using the χ2 or Fisher’s exact test. Spearman analysis was used to calculate correlations among factors.
For survival analysis, we underwent a retrospective research. Kaplan-Meier survival curves were constructed for groups based on univariate predictors, and differences between groups were assessed with the log-rank test. Within limitations of telephone follow-up, some useful information we failed to get, such as patients’ cause of death. So we chose to use overall survival instead of disease-free survival. Multivariate survival analyses were performed using the likelihood ratio test of the Cox proportional hazards model. Differences were assessed with a two-sided test and considered significant at P<0.05.
Results
AJUBA, YAP1, and MMP14 proteins are overexpressed in ESCC
We analyzed the expression levels of AJUBA, YAP1, and MMP14 in 142 tumor tissues and 111 paired adjacent tissues using IHC (Figure 1). The results showed that 87.32% (124/142) of tumor tissues exhibited positive expression of AJUBA, whereas 3.60% (4/111) of adjacent tissues were positive for AJUBA. Similarly, 87.32% (124/142) of tumor tissues showed positive expression of YAP1, whereas only 8.11% (9/111) of adjacent tissues did. Finally, 85.21% (121/142) of tumor tissues were positive for MMP14 expression, whereas 5.41% (6/111) of adjacent tissues were positive. These results demonstrated that AJUBA, YAP1, and MMP14 are more highly expressed in tumor than in adjacent tissue samples (P<0.05).
Figure 1.
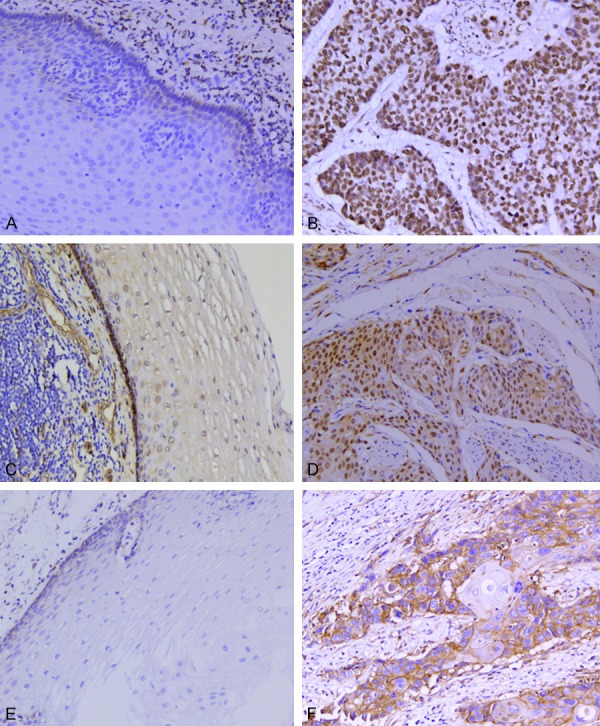
Expression of AJUBA, YAP1, and MMP14 in ESCC and adjacent tissues. IHC staining of AJUBA in the nucleus, YAP1 mainly in the nucleus with some staining in the cytoplasm, and MMP14 in the membrane and cytoplasm. A. No AJUBA expression in adjacent epithelium (×200). B. High AJUBA expression in ESCC (×200). C. No YAP1 expression in adjacent epithelium (×200). D. High YAP1 expression in ESCC (×200). E. No MMP14 expression in adjacent epithelium (×200). F. High MMP14 expression in ESCC (×200).
AJUBA, YAP1, and MMP14 are associated with tumor invasion and metastasis
Relationships between AJUBA, YAP1, and MMP14 expression and clinicopathological features were further analyzed (Table 2). YAP1 and MMP14 were expressed at higher levels in cases with lymph node metastasis than in those without (95.56% vs. 83.51%, P = 0.045; 95.56% vs. 80.41%, P = 0.018, respectively). Stage III/IV cancer tissues exhibited higher expression of AJUBA and MMP14 than stage I/II tissues (94.44% vs. 80.00%, P = 0.010; 91.67% vs. 78.57%, P = 0.028, respectively), indicating that AJUBA and MMP14 expression is associated with ESCC tumor malignancy. Taken together, these findings indicate that AJUBA, YAP1, and MMP14 are correlated with tumor invasion and metastasis in ESCC.
Table 2.
Characteristics of AJUBA, YAP1, and MMP14 expression in ESCC tissues
| Characteristics | n | AJUBA | P | YAP1 | P | MMP14 | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| + | - | + | - | + | - | |||||
| Gender | ||||||||||
| Male | 111 | 98 | 13 | 0.545 | 98 | 13 | 0.513 | 95 | 16 | 0.780 |
| Female | 31 | 26 | 5 | 26 | 5 | 26 | 5 | |||
| Age | ||||||||||
| ≥60 | 93 | 79 | 14 | 0.241 | 82 | 11 | 0.676 | 82 | 11 | 0.171 |
| <60 | 49 | 45 | 4 | 42 | 7 | 39 | 10 | |||
| Pathological grading | ||||||||||
| Well-Moderate | 101 | 87 | 14 | 0.505 | 90 | 11 | 0.316 | 88 | 13 | 0.312 |
| Poor | 41 | 37 | 4 | 34 | 7 | 33 | 8 | |||
| LN metastasis | ||||||||||
| Absent | 97 | 82 | 15 | 0.143 | 81 | 16 | 0.045 | 78 | 19 | 0.018 |
| Present | 45 | 42 | 3 | 43 | 2 | 43 | 2 | |||
| TNM stage | ||||||||||
| I + II | 70 | 56 | 14 | 0.010 | 58 | 12 | 0.115 | 55 | 15 | 0.028 |
| III + IV* | 72 | 68 | 4 | 66 | 6 | 66 | 6 | |||
Note: Statistically significant values (P<0.05) are in boldface type. P-values are derived from two-sided tests.
For limitation of early medical work, the stage classification was based on clinical information after surgery.
Some patients of stage III or IV received surgery.
AJUBA expression positively correlated with YAP1 but not with MMP14
We analyzed the relationships among AJUBA, YAP1, and MMP14 expression and found that YAP1 expression was positively correlated with AJUBA expression (r = 0.300, P = 0.001) and MMP14 expression (r = 0.438, P = 0.001), while AJUBA expression was not correlated with MMP14 expression (r = 0.020, P = 0.812). This indicates that AJUBA may directly regulate YAP1, which may then regulate MMP14 expression in ESCC.
The prognostic significance of AJUBA, YAP1, and MMP14 expression
To examine whether expression of AJUBA, YAP1, and MMP14 was related to overall survival, we performed a Kaplan-Meier analysis and found that lymph node metastasis, stage III/IV, and high expression of MMP14 were associated with reduced survival (P<0.05; Table 3; Figure 2). Further, we performed a multivariable Cox regression analysis and found that only MMP14 were independent risk factors of poor overall survival in ESCC (HR = 6.250, 95% CI = 1.668-23.412, P = 0.007; Table 3).
Table 3.
Correlation of ESCC prognosis with AJUBA, YAP1, and MMP14 expression and clinicopathological factors
| Characteristics | Univariate | Multivariate* | |||
|---|---|---|---|---|---|
|
| |||||
| χ2 | P | HR | 95% CI | P | |
| Gender | |||||
| Male vs. Female | 0.031 | 0.860 | - | - | - |
| Age | |||||
| ≥60 vs. <60 | 0.152 | 0.697 | - | - | - |
| Pathological grading | |||||
| Poor vs. Well-Moderate | 0.583 | 0.445 | - | - | - |
| LN metastasis | |||||
| Present vs. Absent | 31.400 | 0.001 | 1.201 | 0.665-2.167 | 0.544 |
| TNM stage | |||||
| III + IV vs. I + II | 48.266 | 0.001 | 8.406 | 3.678-19.209 | 0.001 |
| AJUBA expression | |||||
| Positive vs. Negative | 2.573 | 0.109 | 1.548 | 0.540-4.437 | 0.416 |
| YAP1 expression | |||||
| Positive vs. Negative | 1.104 | 0.293 | 0.340 | 0.117-0.989 | 0.048 |
| MMP14 expression | |||||
| Positive vs. Negative | 6.598 | 0.010 | 6.250 | 1.668-23.412 | 0.007 |
Note: Statistically significant values (P<0.05) are in boldface type. Log-rank tests were used for univariate analysis, and the enter method was used for multivariate analysis. P-values are derived from two-sided tests.
Multivariate test was performed only if the univariate survival test was significant.
Figure 2.
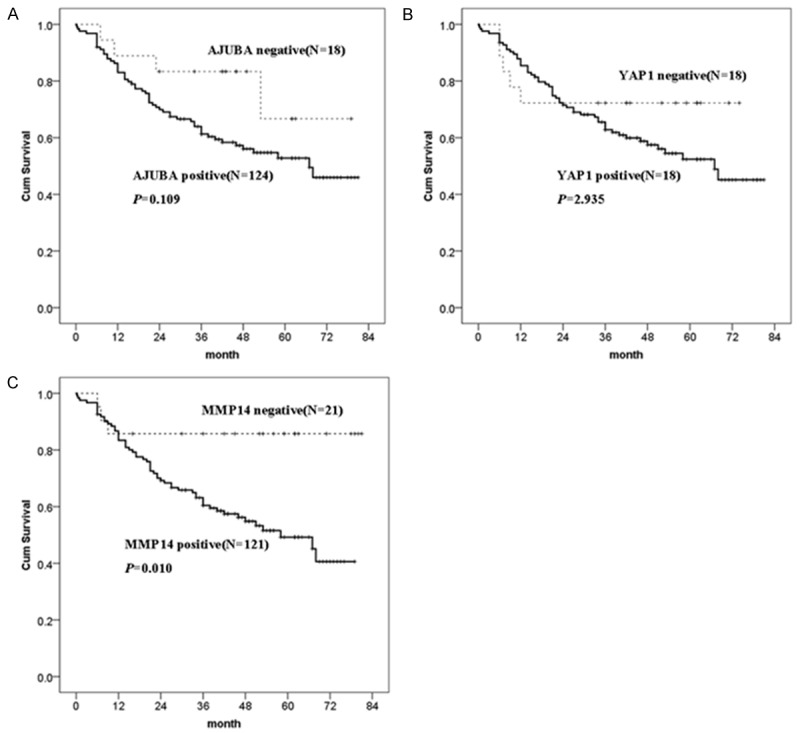
Survival curves of AJUBA, YAP1, and MMP14 expression. The x-axis shows the months after surgery, while the y-axis shows the cumulative survival rate (%).
Discussion
AJUBA belongs to the zinc finger protein family in the LIM domain protein family and mediates a wide range of biological functions, including regulation of cell cycle, cell adhesion and ligation, cell migration, cell proliferation and apoptosis, and cell differentiation. AJUBA can inhibit epithelial cell adhesion molecule E-cadherin by recruiting a protein isomer with a 14-3-3 motif and protein arginine methyltransferase 5 (PRMT5) complex in mouse teratoma cells [22] and human breast cancer cells [23]. In this study, we found that AJUBA expression was elevated in ESCC and related to tumor TNM stage, indicating that it may contribute to tumor invasion in ESCC. Acting as one of the various factors modulating the Hippo pathway, AJUBA can inhibit Hippo pathway signaling by binding to LATS1/2. Hippo signaling is crucial for controlling organ size and proliferation and thus may be involved in tumorigenesis.
Acting as a nuclear effector of the Hippo pathway, YAP1 is overexpressed in numerous tumors, such as oral squamous carcinoma [24], liver cancer [25], and breast cancer [26]. Many studies have focused on the role of YAP1 in tumor apoptosis resistance [27] and metastasis [28]. In this study, we found that expression of AJUBA was positively correlated with YAP1 expression, indicating the potential for AJUBA to regulate YAP1 in ESCC. Survival analysis showed that high expression of AJUBA and YAP1 was related to poor survival outcomes among ESCC patients. However, whether this phenomenon was related to the binding of AJUBA to LATS1/2 requires further study; we are thus planning future in vitro experiments to explore this molecular mechanism. We also observed that high expression of YAP1 in ESCC tumor tissues was related to lymph node metastasis, indicating that YAP1 may contribute to tumor invasion, consistent with the findings of Pei et al. [29]. Similar findings have been observed in other cancers by Muramatsu et al. [30] and Tanaka et al. [15]. However, Yuan et al. [31] found that YAP1 acted as a tumor suppressor gene in breast cancer, suggesting that the role of YAP1 in various cancers remains controversial. We found that YAP1 served as a protective factor in multivariable Cox regression analysis (HR = 0.341, 95% CI = 0.117-0.989, P = 0.048; Table 3).
Next, we assessed MMP14 expression in ESCC and found that elevated expression of MMP14 was related to lymph node metastasis and TNM stage. Bouchard et al. [32] found that MMP14 promoted tumor metastasis in their mouse lung metastasis model. In addition, MMP14 expression was positively correlated with YAP1 expression, indicating that YAP1 may regulate MMP14 expression in ESCC.
In conclusion, our study revealed that AJUBA, YAP1, and MMP14 expression were elevated in ESCC cancer. These proteins may work together to contribute to metastasis in ESCC. MMP14 may also serve as independent risk factors for predicting ESCC patient outcome. Thus, AJUBA, YAP1 and MMP14 may represent novel treatment targets for ESCC.
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Shi X, Chen Z, Hu X, Luo M, Sun Z, Li J, Shi S, Feng X, Zhou C, Li Z, Yang W, Li Y, Wang P, Zhou F, Gao Y, He J. AJUBA promotes the migration and invasion of esophageal squamous cell carcinoma cells through upregulation of MMP10 and MMP13 expression. Oncotarget. 2016;7:36407–36418. doi: 10.18632/oncotarget.9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, Kuo CJ, Camargo FD. Aurora-A: the maker and breaker of spindle poles. J Cell Sci. 2007;120:2987–2996. doi: 10.1242/jcs.013136. [DOI] [PubMed] [Google Scholar]
- 4.Marie H, Pratt SJ, Betson M, Epple H, Kittler JT, Meek L, Moss SJ, Troyanovsky S, Attwell D, Longmore GD, Braga VM. The LIM protein Ajuba is recruited to cadherin-dependent cell junctions through an association with alpha-catenin. J Biol Chem. 2003;278:1220–1228. doi: 10.1074/jbc.M205391200. [DOI] [PubMed] [Google Scholar]
- 5.Kisseleva M, Feng Y, Ward M, Song C, Anderson RA, Longmore GD. The LIM protein Ajuba regulates phosphatidylinositol 4,5-bisphosphate levels in migrating cells through an interaction with and activation of PIPKI alpha. Mol Cell Biol. 2005;25:3956–3966. doi: 10.1128/MCB.25.10.3956-3966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer EM, Feng Y, Zhaoyuan H, Rauscher FJ 3rd, Kroll KL, Longmore GD. Ajuba LIM proteins are snail/slug corepressors required for neural crest development in xenopus. Dev Cell. 2008;14:424–436. doi: 10.1016/j.devcel.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng Y, Longmore GD. The LIM protein Ajuba influences interleukin-1-induced NF-kappaB activation by affecting the assembly and activity of the protein kinase czeta/p62/TRAF6 signaling complex. Mol Cell Biol. 2005;25:4010–4022. doi: 10.1128/MCB.25.10.4010-4022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai M, Ni J, Shen S, Huang Q, Wu J, Le Y, Yu L. Aurora-A kinase-inactive mutants disrupt the interaction with Ajuba and cause defects in mitotic spindle formation and G2/M phase arrest in HeLa cells. BMB Rep. 2014;47:631–636. doi: 10.5483/BMBRep.2014.47.11.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nola S, Daigaku R, Smolarczyk K, Carstens M, Martin-Martin B, Longmore G, Bailly M, Braga VM. Ajuba is required for Rac activation and maintenance of E-cadherin adhesion. J Cell Biol. 2011;195:855–871. doi: 10.1083/jcb.201107162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang XH, Zhang GX, Zeng YB, Yang HF, Li WH, Liu QL, Tang YL, He WG, Huang YN, Zhang L, Yu LN, Zeng XC. LIM protein JUB promotes epithelial-mesenchymal transition in colorectal cancer. Cancer Sci. 2014;105:660–666. doi: 10.1111/cas.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun D, Li X, He Y, Li W, Wang Y, Wang H, Jiang S, Xin Y. YAP1 enhances cell proliferation, migration, and invasion of gastric cancer in vitro and in vivo. Oncotarget. 2016;7:81062–81076. doi: 10.18632/oncotarget.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Yu Z, Chen SS, Li F, Lei CY, Chen XX, Bao JM, Luo Y, Lin GZ, Pang SY, Tan WL. The YAP1 oncogene contributes to bladder cancer cell proliferation and migration by regulating the H19 long noncoding RNA. Urol Oncol. 2015;33:427. doi: 10.1016/j.urolonc.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Kim HR, Hwang SJ, Shin CH, Choi KH, Ohn T, Kim HH. SRSF3-regulated miR-132/212 controls cell migration and invasion by targeting YAP1. Exp Cell Res. 2017;358:161–170. doi: 10.1016/j.yexcr.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, Longmore GD. Ajuba LIM proteins are negative regulators of the hippo signaling pathway. Curr Biol. 2010;20:657–662. doi: 10.1016/j.cub.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka I, Osada H, Fujii M, Fukatsu A, Hida T, Horio Y, Kondo Y, Sato A, Hasegawa Y, Tsujimura T, Sekido Y. LIM-domain protein AJUBA suppresses malignant mesothelioma cell proliferation via hippo signaling cascade. Oncogene. 2015;34:73–83. doi: 10.1038/onc.2013.528. [DOI] [PubMed] [Google Scholar]
- 16.Reddy BV, Irvine KD. Regulation of Hippo signaling by EGFR-MAPK signaling through ajuba family proteins. Dev Cell. 2013;24:459–471. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu YH, Fu L, Chen L, Qin YR, Liu H, Xie F, Zeng T, Dong SS, Li J, Li Y, Dai Y, Xie D, Guan XY. Downregulation of the novel tumor suppressor DIRAS1 predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Res. 2013;73:2298–2309. doi: 10.1158/0008-5472.CAN-12-2663. [DOI] [PubMed] [Google Scholar]
- 18.Zhu YH, Fu L, Chen L, Qin YR, Liu H, Xie F, Zeng T, Dong SS, Li J, Li Y, Dai Y, Xie D, Guan XY. Downregulation of LGI1 promotes tumor metastasis in esophageal squamous cell carcinoma. Carcinogenesis. 2014;35:1154–1161. doi: 10.1093/carcin/bgu040. [DOI] [PubMed] [Google Scholar]
- 19.Augoff K, Hryniewicz-Jankowska A, Tabola R, Czapla L, Szelachowski P, Wierzbicki J, Grabowski K, Sikorski AF. Upregulated expression and activation of membraneassociated proteases in esophageal squamous cell carcinoma. Oncol Rep. 2014;31:2820–2826. doi: 10.3892/or.2014.3162. [DOI] [PubMed] [Google Scholar]
- 20.Akanuma N, Hoshino I, Akutsu Y, Murakami K, Isozaki Y, Maruyama T, Yusup G, Qin W, Toyozumi T, Takahashi M, Suito H, Hu X, Sekino N, Matsubara H. MicroRNA-133a regulates the mRNAs of two invadopodia-related proteins, FSCN1 and MMP14, in esophageal cancer. Br J Cancer. 2014;110:189–198. doi: 10.1038/bjc.2013.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naoi Y, Miyoshi Y, Taguchi T, Kim SJ, Arai T, Maruyama N, Tamaki Y, Noguchi S. Connexin26 expression is associated with aggressive phenotype in human papillary and follicular thyroid cancers. Cancer Lett. 2008;262:248–256. doi: 10.1016/j.canlet.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Hou Z, Peng H, White DE, Wang P, Lieberman PM, Halazonetis T, Rauscher FJ 3rd. 14-3-3 binding sites in the snail protein are essential for snail-mediated transcriptional repression and epithelial-mesenchymal differentiation. Cancer Res. 2010;70:4385–4393. doi: 10.1158/0008-5472.CAN-10-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou Z, Peng H, Ayyanathan K, Yan KP, Langer EM, Longmore GD, Rauscher FJ 3rd. The LIM protein AJUBA recruits protein arginine methyltransferase 5 to mediate SNAIL-dependent transcriptional repression. Mol Cell Biol. 2008;28:3198–3207. doi: 10.1128/MCB.01435-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snijders AM, Schmidt BL, Fridlyand J, Dekker N, Pinkel D, Jordan RC, Albertson DG. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene. 2005;24:4232–4242. doi: 10.1038/sj.onc.1208601. [DOI] [PubMed] [Google Scholar]
- 25.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, Mu D, Lucito R, Powers S, Lowe SW. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cottini F, Hideshima T, Sattler M, Caligaris-Cappio F, Anderson KC, Tonon G. The role of the ABL1/YAP1/P73 axis in prevention of DNA damage-mediated apoptosis in multiple myeloma. Blood. 2015;120:725–725. [Google Scholar]
- 28.Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. Cellular energy stress induces AMPK-mediated regulation of YAP and the hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei T, Li Y, Wang J, Wang H, Liang Y, Shi H, Sun B, Yin D, Sun J, Song R, Pan S, Sun Y, Jiang H, Zheng T, Liu L. YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget. 2015;6:17206–17220. doi: 10.18632/oncotarget.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muramatsu T, Imoto I, Matsui T, Kozaki K, Haruki S, Sudol M, Shimada Y, Tsuda H, Kawano T, Inazawa J. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011;32:389–398. doi: 10.1093/carcin/bgq254. [DOI] [PubMed] [Google Scholar]
- 31.Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, Gangeswaran R, Manson-Bishop C, Smith P, Danovi SA, Pardo O, Crook T, Mein CA, Lemoine NR, Jones LJ, Basu S. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 32.Bouchard G, Therriault H, Geha S, Bujold R, Saucier C, Paquette B. Radiation-induced lung metastasis development is MT1-MMP-dependent in a triple-negative breast cancer mouse model. Br J Cancer. 2017;116:479–488. doi: 10.1038/bjc.2016.448. [DOI] [PMC free article] [PubMed] [Google Scholar]


