Abstract
Background: Although the absolute number of postoperative positive lymph nodes has been considered as one of the most important predictors for the survival of rectal cancer patients, many researchers have suggested that the lymph node ratio (LNR) may have a better predictive effect. After chemoradiotherapy for rectal cancer, the number of lymph nodes harvested is often reduced, and the predictive value of the postoperative LNR after chemoradiotherapy is still unclear. We performed a retrospective study to analyze the survival predictive value of the LNR after preoperative chemoradiotherapy for rectal cancer. Methods: Between January 2012 and December 2014, a total of 133 patients with locally advanced rectal cancer who underwent preoperative chemoradiotherapy followed by total mesorectal excision were reviewed. According to the postoperative pathological LNR, patients were divided into 3 groups, i.e., LNR = 0, 0 < LNR ≤ 0.15, and 0.15 < LNR ≤ 1, and patients with lymph node metastasis were divided into 2 groups, and the overall survival (OS) and disease-free survival (DFS) of each group were analyzed and evaluated. Results: The median follow-up time of this study was 40 months, and the 5-year OS and DFS rates were 86.3% and 74.8% for the whole cohort, respectively. According to the postoperative pathological LNR, patients were divided into 3 groups, LNR = 0, 0 < LNR ≤ 0.15, 0.15 < LNR ≤ 1, the respective 5-year OS and DFS rates for the LNR 3 groups were 96.7%, 94.7% and 42.9% (P < 0.001) and 84.9%, 73%, and 30.7% (P < 0.001), respectively. Multivariate analysis revealed that only the LNR was the independent prognostic factor for these patients. For patients with lymph node metastasis, it was also revealed that only the LNR was an independent prognostic factor. Subgroup analysis demonstrated that there was a significant correlation between the LNR and patient survival both in the number of lymph nodes harvested, less than 12 and more than 12 subgroups (P < 0.05). Conclusion: The postoperative positive lymph node ratio (LNR) is an independent prognostic factor for patients with locally advanced rectal cancer who undergo preoperative chemoradiotherapy followed by total mesorectal excision. Both in the number of lymph nodes harvested less than 12 and more than 12 subgroups, the LNR is significantly correlated with patient survival.
Keywords: Rectal cancer, lymph node, chemoradiotherapy
Introduction
In the past 20 years, the incidence of colorectal cancer has been increasing rapidly with economic development and improvement in the average life span in China. Moreover, more than half of the rectal cancer patients were diagnosed with locally advanced rectal cancer (LARC) at the time of diagnosis, which poses many challenges to colorectal surgeons in China.
Preoperative chemoradiotherapy (CRT) followed by total mesorectal excision (TME) and postoperative chemotherapy are now considered the standard strategies for treating patients with LARC [1]. The number of positive lymph nodes is one of the most important factors in determining the prognosis of patients with colorectal cancer, and it is also a major determinant of the need for adjuvant therapy [2]. The number of positive lymph nodes is not only significantly associated with the severity of the disease but also relies on the total number of retrieved lymph nodes [3]. Thus, this method disregards the number of lymph nodes harvested, which has been identified as an important prognostic factor in colorectal cancer. Many researchers have found that preoperative CRT decreases the number of lymph nodes harvested in rectal cancer patients. In addition, the American Joint Committee on Cancer (AJCC) recommendations have revealed that < 12 retrieved lymph nodes are frequently found in patients with LARC treated with preoperative CRT [3]. For this reason, the lymph node ratio (LNR), the ratio of the positive lymph node numbers and the total harvested lymph node number has been regarded as a key prognostic factor in rectal cancer and may be used as a better prognostic indicator in patients with LARC who received preoperative CRT [4,5].
In fact, some studies have shown that LNR is a more accurate stratification system than the current staging system based on the number of metastatic lymph nodes for colorectal cancer [4,5]. However, few studies have focused on the prognostic value of LNR in patients with LARC who received preoperative CRT followed by TME and postoperative chemotherapy. Therefore, the purpose of this study was to evaluate the impact of the LNR on prognosis in patients with LARC treated with preoperative CRT followed by TME and postoperative chemotherapy.
Methods
Patients and data collection
We performed a retrospective review of all patients who underwent preoperative CRT followed by TME and postoperative chemotherapy from January 2012 to December 2014. The basic information of the patients was taken from the medical records in our hospital. This study was approved by the ethics committee of Sir Run Run Shaw Hospital Zhejiang University College of Medicine, and all patients signed the informed consent.
The study inclusion criteria were histological examination confirming adenocarcinoma of the middle and lower rectum (10 cm above the anal verge) and the location of the tumor being defined as the distance between the distal margin of the tumor and the anal verge, measured by digital examination and colonoscopy; patients being diagnosed with a T3 or T4 tumor with/or stages N1 or N2 and M0 according to preoperative evaluation; and patients who received a preoperative long-course CRT and postoperative chemotherapy.
The study exclusion criteria were synchronous distant metastases to the lung, liver, bones, or other organs; patients whose postoperative pathology showed melanoma, neuroendocrine carcinoma or other non-adenocarcinoma; patients whose pathological lymph node stage was N1c due to the existence of cancer nodules; and patients who refused to receive preoperative CRT or postoperative chemotherapy.
Based on the inclusion and exclusion criteria, a total of 133 patients were eligible for this study. Measures such as age, gender, tumor location, type of operation, tumor pathological stage (ypTNM), depth of invasion (ypT), number of metastatic lymph nodes (ypN), number of lymph nodes harvested, and tumor differentiation were evaluated. Recurrences and distant metastases were also documented.
Preoperative evaluation and chemoradiotherapy
All patients received a digital rectal examination, colonoscopy, and computed tomography (CT) of the chest, abdomen and pelvic region. Transrectal ultrasonography or pelvic magnetic resonance imaging was performed for preoperative staging of rectal cancer. The clinical stages were scored according to the AJCC stage classification system (7th edition).
The patients with T3/T4 stage and/or metastatic lymph nodes received preoperative CRT. A total dose of 45-50 Gy was delivered in 25 days. During the period of radiotherapy, all patients received concomitant chemotherapy. Preoperative chemotherapy began on the 1st day of pelvic radiotherapy. Capecitabine at 825 mg/m2 twice a day was given concurrently with radiotherapy.
Surgery and postoperative chemotherapy
All patients received curative resection followed by preoperative CRT, and colorectal specialists performed TME for all rectal cancer cases. The interval between preoperative CRT and surgery ranged from 6 to 8 weeks. Surgical procedures included an abdominoperineal resection (APR), Hartmann operation, low anterior resection (LAR), with or without a terminal diverting ileostomy.
Approximately 2 to 4 weeks after surgery, all patients received postoperative adjuvant chemotherapy. Two different chemotherapy regimens were used: mFOLFOX6 and Capox.
Pathological analysis
After gross examination by the surgeon in the operating room, each specimen was sent to pathology. The pathological stage of the tumor was determined according to the AJCC (7th edition) staging system and marked as ypTNM because of the preoperative CRT. We assessed and calculated the histological type, depth of invasion (ypT), number of metastatic lymph nodes (ypN), number of lymph nodes harvested and the tumor differentiation for each patient.
Follow-up
Patients were reviewed at the hospital or contacted through telephone or mail every 3 months within the first 2 years after the operation, every 6 months for the next 3 years, and annually thereafter in according with the NCCN Guidelines. Patients received a series of follow-up evaluations that included complete serum CEA and CA-199 measurements, and digital rectal examination. Liver color Doppler ultrasound, abdominal and pelvic CT and chest X-rays were also performed.
The median follow-up time was 40 months. Overall survival (OS) was defined as the time between the date of surgery and date of death or last follow-up. Disease-free survival (DFS) was defined as the time between the date of the surgery and date of the local recurrence or metastasis.
Statistical analysis
Continuous variables are reported as the mean and SD, whereas categorical variables are reported as the number of patients and percentages. Correlation analysis between each group was performed with Student’s t-tests and Chi-squared tests. Patients were divided into 3 groups, i.e., LNR = 0, 0 < LNR ≤ 0.15, and 0.15 < LNR ≤ 1. These values were used because the median LNR for the entire cohort was 0 and we referred to previous studies and used the mean LNR (0.15) of stage ypIII patients as a cutoff. Survival analyses were performed using the Kaplan-Meier method, and the log-rank test was used to compare variables. A Cox proportional hazard model was used for multivariate analysis, adjusted hazard ratios and their 95% CIs were calculated. All statistical tests were two-sided, and a P value of less than 0.05 was considered statistically significant. Data were analyzed with SPSS17.0.
Results
Patient population and basic clinical information
In all, 133 patients with locally advanced rectal cancer who received preoperative chemoradiotherapy, total mesorectal excision, and postoperative chemotherapy were enrolled in this study. Table 1 contains the detailed clinical information. The median age of the included patients was 60 years. Among the patients, 87 (65.4%) underwent lower anterior resection, 35 (26.3%) underwent abdominoperineal resection, and 11 (8.3%) underwent the Hartmann procedure. The median follow-up time was 40 months.
Table 1.
Clinical characteristics of this study
| Variable | Characteristics | Patients N = 133 (%) |
|---|---|---|
| Sex | Male | 88 (66.2) |
| Female | 45 (33.8) | |
| Tumor location | 0 cm-5 cm | 60 (45.1) |
| > 5 cm | 73 (54.9) | |
| Type of operation | LAR | 87 (65.4) |
| APR | 35 (26.3) | |
| Hartmann | 11 (8.3) | |
| Surgical approach | Open | 28 (21.1) |
| Laparoscopy | 105 (78.9) | |
| Pathological stage (ypTNM) | yp0 | 26 (19.5) |
| ypI | 22 (16.6) | |
| ypII | 46 (34.6) | |
| ypIII | 39 (29.3) | |
| Depth of invasion (ypT) | ypT0 | 27 (20.3) |
| ypT1-ypT2 | 26 (19.5) | |
| ypT3-ypT4 | 80 (60.2) | |
| No. of metastatic lymph nodes (ypN) | ypN0 | 94 (70.7) |
| ypN1 | 32 (24.0) | |
| ypN2 | 7 (5.3) | |
| No. of lymph nodes harvested | < 12 | 55 (41.4) |
| ≥ 12 | 78 (58.6) | |
| Differentiation | Poor/Anaplastic | 21 (15.8) |
| High/Moderate | 45 (33.8) | |
| Unknown | 40 (30.1) | |
| No residual tumor | 27 (20.3) |
Lymph node ratio (LNR) and survival time
To evaluate the extent to which the LNR predicts the survival of the enrolled patients with locally advanced rectal cancer, we divided the patients with an LNR > 0 into two groups. First, we tested six different LNR cutoff values (Table 2). A univariate analysis showed that among these cutoff values, the median LNR (0.15) for patients with stage III rectal cancer was an appropriate cutoff value for the evaluation of patient survival. Thus, patients were divided into two groups: the LNR ≤ 0.15 group and the LNR > 0.15 group. The age, sex, tumor location, type of operation, surgical approach, and tumor differentiation of the two groups showed no significant difference.
Table 2.
Univariate analysis for the effect of lymph node ratio (LNR) influencing overall and disease-free survival
| LNR | No. of patients | 5-year OS | p | 5-year DFS | P |
|---|---|---|---|---|---|
| ≤ 0.05 | 4 | 100 | 0.282 | 75.0 | 0.441 |
| > 0.05 | 35 | 63.9 | 48.8 | ||
| ≤ 0.1 | 16 | 93.3 | 0.037 | 72.7 | 0.026 |
| > 0.1 | 23 | 52.6 | 36.9 | ||
| ≤ 0.15 | 20 | 94.7 | 0.004 | 73.0 | 0.002 |
| > 0.15 | 19 | 42.9 | 30.7 | ||
| ≤ 0.2 | 24 | 85.9 | 0.040 | 67.8 | 0.002 |
| > 0.2 | 15 | 47.4 | 25.0 | ||
| ≤ 0.25 | 28 | 84.1 | 0.014 | 61.6 | 0.014 |
| > 0.25 | 11 | 36.8 | 24.2 | ||
| ≤ 0.3 | 32 | 79.3 | 0.047 | 53.6 | 0.419 |
| > 0.3 | 7 | 35.7 | 42.9 |
LNR: Lymph Node Ratio; OS: Overall Survival; DFS: Disease-Free Survival.
Overall prognostic factors
The median follow-up time was 40 months, during which 13 patients (9.8%) died. The 5-year OS and 5-year DFS rates were 86.3% and 74.8%, respectively. For patients with postoperative stage yp0, ypI, ypII and ypIII disease, the 5-year OS rates were 96%, 100%, 95.6%, and 67.0%, respectively, while the 5-year DFS rates were 96.0%, 100%, 72.0% and 51.4%, respectively (Table 3). A univariate analysis and Cox proportional hazard model were used to analyze the clinical variables, which included pathological stage, depth of invasion (ypT), number of metastatic lymph nodes (ypNs), number of lymph nodes harvested, tumor differentiation, and LNR.
Table 3.
Univariate analysis of prognostic factors for overall and disease-free survival in whole population
| Variables | Characteristics | 5-year OS (%) | P | 5-year DFS (%) | P |
|---|---|---|---|---|---|
| Pathological stage (ypTNM) | yp0 | 96.0 | 0.003 | 96.0 | < 0.001 |
| ypI | 100 | 100 | |||
| ypII | 95.6 | 72.0 | |||
| ypIII | 67.0 | 51.4 | |||
| Depth of invasion (ypT) | ypT0 | 96.2 | 0.207 | 96.2 | 0.001 |
| ypT1-ypT2 | 95.0 | 92.3 | |||
| ypT3-ypT4 | 80.9 | 62.7 | |||
| No. of metastatic lymph nodes (ypN) | ypN0 | 96.7 | < 0.001 | 84.9 | < 0.001 |
| ypN1 | 82.4 | 59.6 | |||
| ypN2 | 28.6 | 14.3 | |||
| No. of lymph nodes harvested | < 12 | 79.1 | 0.005 | 69.9 | 0.126 |
| ≥ 12 | 92.0 | 78.7 | |||
| Differentiation | Poor/Anaplastic | 75.6 | 0.143 | 52.4 | 0.001 |
| High/Moderate | 92.2 | 67.5 | |||
| Unknown | 77.7 | 76.6 | |||
| No residual tumor | 96.2 | 96.2 | |||
| LNR | 0 | 96.7 | < 0.001 | 84.9 | < 0.001 |
| 0-0.15 | 94.7 | 73.0 | |||
| 0.15-1 | 42.9 | 30.7 |
LNR: Lymph Node Ratio.
The univariate analysis showed that pathological stage, number of metastatic lymph nodes, and the LNR were significantly correlated with patient survival (P < 0.05). The patients were divided into three groups on the basis of LNR as follows: LNR = 0, 0 < LNR ≤ 0.15, and 0.15 < LNR ≤ 1. The 5-year OS rates of these three groups were 96.7%, 94.7% and 42.9%, respectively, while the 5-year DFS rates were 84.9%, 73.0% and 30.7%, respectively (Table 3; Figure 1).
Figure 1.
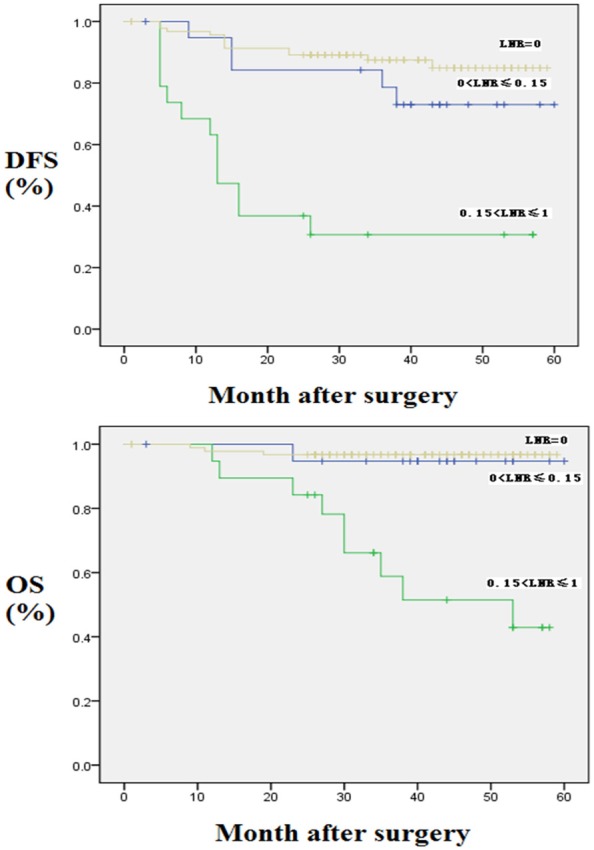
Kaplan-Meier curves for OS and DFS according to LNR in whole population. DFS: Disease-Free Survival; OS: Overall Survival; LNR: Lymph Node Ratio.
According to the results of the univariate analysis, we performed a multivariate analysis with the following four clinical variables: pathological stage, number of metastatic lymph nodes, LNR, and tumor differentiation. The results showed that the LNR was the only independent prognostic factor for patient survival (Table 4).
Table 4.
Multivariate analysis of LNR for overall and disease-free survival in whole population
| Variables | OS | DFS | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| LNR = 0 | 1 | 0.003 | 1 | 0.001 | ||
| 0 < LNR ≤ 0.15 | 2.125 | 0.126-35.728 | 1.536 | 0.319-7.389 | ||
| 0.15 < LNR ≤ 1 | 15.632 | 2.728-89.563 | 5.803 | 1.942-17.339 | ||
LNR: Lymph Node Ratio; CI: Confidence Interval; HR: Hazard Ratio.
Prognostic factors for node-positive patients
For node-positive patients after surgery, we performed a univariate analysis with the following five clinical variables: depth of invasion (ypT), number of metastatic lymph nodes (ypN), number of lymph nodes harvested, LNR, and tumor differentiation (Table 5). The results showed that number of metastatic lymph nodes and LNR were significantly correlated with the survival of node-positive patients (P < 0.05). According to the results of the univariate analysis and the potential risk factors for patient survival, we performed a multivariate analysis and found that the LNR was the only independent prognostic factor for the survival of node-positive patients (Table 6).
Table 5.
Univariate analysis of prognostic factors for overall and disease-free survival in node-positive rectal cancer
| Variables | Characteristics | 5-year OS (%) | P | 5-year DFS (%) | P |
|---|---|---|---|---|---|
| No. of metastatic lymph nodes (ypN) | ypN1 | 82.4 | 0.007 | 59.6 | 0.003 |
| ypN2 | 28.6 | 14.3 | |||
| No. of lymph nodes harvested | < 12 | 53.4 | 0.048 | 38.6 | 0.200 |
| ≥ 12 | 75.2 | 61.1 | |||
| Differentiation | Poor/Anaplastic | 51.9 | 0.287 | 33.3 | 0.126 |
| High/Moderate | 85.9 | 61.9 | |||
| Unknown | 48.1 | 46.7 | |||
| No residual tumor | 100 | 100 | |||
| LNR | 0-0.15 | 94.7 | 0.004 | 73.0 | 0.002 |
| 0.15-1 | 42.9 | 30.7 | |||
| Depth of invasion (ypT) | ypT0 | 100 | 0.723 | 100 | 0.550 |
| ypT1-ypT2 | 66.7 | 33.3 | |||
| ypT3-ypT4 | 66.7 | 51.9 |
LNR: Lymph Node Ratio.
Table 6.
Multivariate analysis of LNR for overall and disease-free survival in node-positive rectal cancer
| Variables | OS | DFS | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| 0 < LNR ≤ 0.15 | 1 | 0.048 | 1 | 0.039 | ||
| 0.15 < LNR ≤ 1 | 9.355 | 1.024-85.464 | 3.456 | 1.067-11.200 | ||
LNR: Lymph Node Ratio; CI: Confidence Interval; HR: Hazard Ratio.
Subgroup survival analysis according to number of lymph nodes harvested
Among the whole population, the postoperative pathological examination showed that the median number of lymph nodes harvested was 12 and less than 12 lymph nodes were harvested in 55 patients (41.4%). The patients were therefore divided into two groups according to the number of lymph nodes harvested: < 12 and ≥ 12. A significant difference between two groups was observed in the 5-year OS rate (P < 0.05) but not in the 5-year DFS rate (Table 3).
As LNR was an independent prognostic factor for OS and DFS in the whole population with preoperative CRT and node-positive rectal cancer patients as found above, we further evaluated the prognostic value of the LNR in different subgroups (Table 7A and 7B; Figure 2). The results showed that the LNR was significantly correlated with patient survival, i.e., whether the number of lymph nodes harvested was < 12 or ≥ 12. Moreover, a higher LNR was correlated with lower 5-year OS and 5-year DFS rates regardless of the number of lymph nodes harvested (P < 0.05).
Table 7A.
Subgroup analysis of LNR for overall and disease-free survival in the number of lymph nodes harvested < 12 patients
| Variables | OS | DFS | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 5-year (%) | HR | 95% CI | P | 5-year (%) | HR | 95% CI | P | |
| LNR=0 | 92.1 | 1 | 0.033 | 84.2 | 1 | 0.009 | ||
| 0 < LNR ≤ 0.15 | 75.0 | 2.874 | 0.298-27.679 | 50.0 | 3.015 | 0.608-14.957 | ||
| 0.15 < LNR ≤ 1 | 43.9 | 6.349 | 1.585-25.435 | 36.9 | 5.293 | 1.821-15.383 | ||
LNR: Lymph Node Ratio; CI: Confidence Interval; HR: Hazard Ratio.
Table 7B.
Subgroup analysis of LNR for overall and disease-free survival in the number of lymph nodes harvested ≥ 12 patients
| Variables | OS | DFS | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 5-year (%) | HR | 95% CI | P | 5-year (%) | HR | 95% CI | P | |
| LNR = 0 | 100 | 1 | < 0.001 | 85.7 | 1 | < 0.001 | ||
| 0 < LNR ≤ 0.15 | 100 | 1 | --- | 79.4 | 1.682 | 0.419-6.760 | ||
| 0.15 < LNR ≤ 1 | 33.3 | --- | --- | 16.7 | 21.020 | 5.956-74.187 | ||
LNR: Lymph Node Ratio; CI: Confidence Interval; HR: Hazard Ratio.
Figure 2.
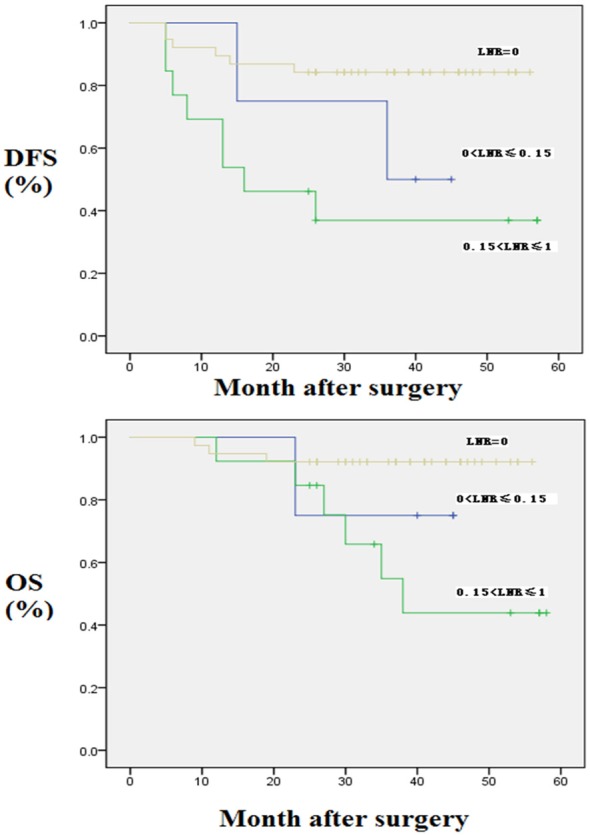
Kaplan-Meier curves for OS and DFS according to LNR in the number of lymph nodes harvested < 12 patients. DFS: Disease-Free Survival; OS: Overall Survival; LNR: Lymph Node Ratio.
Discussion
The tumor node metastasis (TNM) staging system provides an accurate prediction that can guide the postoperative treatment strategy and follow-up [6,7]. Currently, the TNM system, which is the most commonly used staging system for colorectal cancer, predicts a prognosis on the basis of the depth of primary tumor invasion, lymph node metastasis, and distant metastasis. A high number of positive nodes and an advanced stage are correlated with a worse prognosis. However, the number of positive lymph nodes is not only significantly associated with the severity of disease but also relies on the total number of retrieved lymph nodes. Therefore, the number of lymph nodes harvested plays a key role in determining the postoperative pathological stage and in guiding the adjuvant chemotherapy strategy [8]. Thus, the old method disregards the number of lymph nodes harvested, which is identified to be an important prognostic factor in colorectal cancer.
Preoperative chemoradiotherapy followed by total mesorectal excision (TME) and postoperative chemotherapy is currently the standard strategy for the treatment of locally advanced rectal cancer [1]. The number of positive nodes is an important factor in the prognosis of colorectal cancer and the key determinant for postoperative adjuvant therapy [2]. Currently, the American Joint Committee on Cancer (AJCC) guidelines recommend that at least 12 lymph nodes should be dissected during colorectal cancer surgery for accurate staging [9]. However, not all surgical colorectal cancer samples meet this criterion, as the number of lymph nodes dissected is affected by many factors, including age, sex, comorbidities, tumor size, tumor site, tumor differentiation, lymph node response, and preoperative chemoradiotherapy [10,11]. To overcome the limitations of the current node staging system and to provide more accurate postoperative staging and prognostic information, the LNR is considered a better predictor that can replace the original staging system of colorectal cancer [12,13].
In 2005, Berger et al. first reported the use of the LNR as a prognostic factor for colon cancer. They analyzed patients with stage II and stage III colon cancer who underwent adjuvant chemotherapy and found that the LNR was an important factor for the prognosis of colon cancer [14]. In 2008, Peng et al. first reported the value of the LNR in the prediction of the survival of patients with node-positive rectal cancer [15]. In their study, the patients were divided into three groups according to the LNR, i.e., < 0.14, 0.14-0.49, and 0.5-1. The 5-year DFS rates of the patients in the three groups were 72.57%, 58.54% and 34.75% (P < 0.0001), respectively, and the 5-year OS rates were 72.19%, 61.92% and 38.47% (P < 0.002), respectively. Likewise, in 2010, Huh et al. divided 514 patients with confirmed node-positive colorectal cancer into four groups on the basis of LNR quartiles, i.e., < 0.09, 0.09-0.18, 0.19-0.34 and 0.35-1. The median follow-up time was 48.5 months. The 5-year OS rates of the patients in the four groups were 79%, 72%, 62% and 55%, respectively, and the 5-year DFS rates were 73%, 67%, 54% and 42%, respectively. All these results were significant, and the LNR was determined to be a significant factor in both subgroups; that is, the less than 12 or more than 12 numbers of lymph nodes harvested subgroups [16].
For patients with rectal cancer, preoperative chemoradiotherapy affects the postoperative lymph node status. According to the NCCN guidelines, preoperative chemoradiotherapy followed by total mesorectal excision (TME) and postoperative chemotherapy are now considered the standard strategies for treating patients with LARC. However, preoperative chemoradiotherapy may affect the number of lymph nodes dissected. Several studies have shown that the total number of retrieved LNs is often fewer than 12 in-patients who undergo preoperative CRT, because of lymph node atrophy, fibrosis, and lymphocyte depletion caused by chemoradiotherapy. The updated 2017 NCCN guidelines indicate that, in only 20% of the cases, the number of lymph nodes dissected for postoperative pathological examination was 12 or more after preoperative chemoradiotherapy. Previous studies have shown that, although preoperative chemoradiotherapy reduces the number of lymph nodes dissected, preoperative chemoradiotherapy has no effect on patient survival. In this study, the number of lymph nodes dissected was 12 or more in only 58.6% of the patients, consistent with the results of other reports [17,18].
In 2011, Klos et al. first investigated the value of the LNR in predicting the postoperative survival of patients with rectal cancer who received preoperative chemoradiotherapy. The results showed that the LNR was a better independent staging method than the number of positive nodes [19]. Kim et al. evaluated the value of the LNR in predicting the survival of 232 node-positive patients who received preoperative chemoradiotherapy and found that the higher LNR was associated with the worst survival [20]. Our study enrolled 133 patients with locally advanced rectal cancer and included 39 node-positive patients. The 5-year OS and 5-year DFS rates were 86.3% and 74.8%, respectively. According to the postoperative pathological LNR, patients were divided into 3 groups, i.e., LNR = 0, 0 < LNR ≤ 0.15, and 0.15 < LNR ≤ 1, and the respective 5-year OS and DFS rates for the 3 groups were 96.7%, 94.7% and 42.9% (P < 0.001) and 84.9%, 73% and 30.7% (P < 0.001), respectively. The multivariate analysis showed that the LNR was the only independent prognostic factor for patient survival. For node-positive patients, the multivariate analysis also showed that the LNR was an independent prognostic factor for survival. These results indicated that the LNR was a better predictor of survival than the number of metastatic lymph nodes in patients with locally advanced rectal cancer who underwent radical resection after preoperative chemoradiotherapy, whether the number of lymph nodes harvested was < 12 or ≥ 12.
This study has shown the potential value of the LNR in predicting the survival of patients with locally advanced rectal cancer who undergo TME after preoperative neoadjuvant chemoradiotherapy. However, this study has some limitations. First, this was a retrospective study, and the sample size from a single center is small, thus large cohort studies are needed to validate the results. Second, different surgical teams performed TME, which may have affected the number of lymph nodes dissected during surgery. Third, some pathologyu reports are not standardized, and this might have affected the analysis and statistics. Finally, although the value of the LNR in predicting survival has been proven, the optimal cutoff value is still unknown, which has limited its clinical application. Previous studies used different methods to determine the optimal cutoff value, which included 0, 0.05, 0.10, 0.15, 0.20, 0.25, 0.40, 0.50 and 0.75; some of these values were based on quartiles while some were based on the mean or median LNR [21]. In this study, we referenced other reports and used the median LNR (0.15) in patients with stage III rectal cancer as the cutoff value, because the median LNR was 0 for the entire cohort. So far, different studies have proposed different cutoff values. Large comprehensive studies are needed to determine the cutoff value for the LNR in patients with rectal cancer who have undergone preoperative chemoradiotherapy. In addition, besides the clinical and pathological features discussed in this study, molecular genetic changes such as tumor microsatellite instability (MSI) [22] and BRAF mutations have also been reported to correlate with the prognosis of patients with rectal cancer. Therefore, future research is needed to carefully evaluate the relationship between clinical and pathological features and the patient’s molecular genetic profile as well as their effect on the survival of patients with colorectal cancer.
Conclusion
The postoperative positive lymph node ratio (LNR) is an independent prognostic factor for patients with locally advanced rectal cancer who undergo preoperative chemoradiotherapy followed by total mesorectal excision. Both in the number of lymph nodes harvested less than 12 and more than 12 subgroups, the LNR is significantly correlated with patient survival.
Acknowledgements
This work was supported by the Zhejiang University College of Medicine. Our special thanks are due to Prof. He Chao for his helpful discussion with preparing the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 2.Harisi R, Schaff Z, Flautner L, Winternitz T, Jaray B, Nemeth Z, Kupcsulik P, Weltner J. Evaluation and comparison of the clinical, surgical and pathological TNM staging of colorectal cancer. Hepatogastroenterology. 2008;55:66–72. [PubMed] [Google Scholar]
- 3.Zeng WG, Zhou ZX, Wang Z, Liang JW, Hou HR, Zhou HT, Zhang XM, Hu JJ. Lymph node ratio is an independent prognostic factor in node positive rectal cancer patients treated with preoperative chemoradiotherapy followed by curative resection. Asian Pac J Cancer Prev. 2014;15:5365–5369. doi: 10.7314/apjcp.2014.15.13.5365. [DOI] [PubMed] [Google Scholar]
- 4.Kim YW, Kim NK, Min BS, Lee KY, Sohn SK, Cho CH. The infl uence of the number of retrieved lymph nodes on staging and survival in patients with stage II and III rectal cancer undergoing tumor-specific mesorectal excision. Ann Surg. 2009;249:965–972. doi: 10.1097/SLA.0b013e3181a6cc25. [DOI] [PubMed] [Google Scholar]
- 5.Moug SJ, Saldanha JD, McGregor JR, Balsitis M, Diament RH. Positive lymph node retrieval ratio optimizes patient staging in colorectal cancer. Brit J Cancer. 2009;100:1530–1533. doi: 10.1038/sj.bjc.6605049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noura S, Ohue M, Kano S, Shingai T, Yamada T, Miyashiro I, Ohigashi H, Yano M, Ishikawa O. Impact of metastatic lymph node ratio in node-positive colorectal cancer. World J Gastrointest Surg. 2010;2:70–77. doi: 10.4240/wjgs.v2.i3.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madbouly KM, Abbas KS, Hussein AM. Metastatic lymph node ratio in stage III rectal carcinoma is a valuable prognostic factor even with less than 12 lymph nodes retrieved: a prospective study. Am J Surg. 2014;207:824–831. doi: 10.1016/j.amjsurg.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Greene FL. Current TNM staging of colorectal cancer. Lancet Oncol. 2007;8:572–573. doi: 10.1016/S1470-2045(07)70185-7. [DOI] [PubMed] [Google Scholar]
- 9.McDonald JR, Renehan AG, O’Dwyer ST, Haboubi NY. Lymph node harvest in colon and rectal cancer: current considerations. World J Gastrointest Surg. 2012;4:9–19. doi: 10.4240/wjgs.v4.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorn CC, Woodcock NP, Scott N, Verbeke C, Scott SB, Ambrose NS. What factors affect lymph node yield in surgery for rectal cancer? Colorectal Dis. 2004;6:356–361. doi: 10.1111/j.1463-1318.2004.00670.x. [DOI] [PubMed] [Google Scholar]
- 11.Mekenkamp LJ, van Krieken JH, Marijnen CA, van de Velde CJ, Nagtegaal ID Pathology Review Committee and the Co-operative Clinical Investigators. Lymph node retrieval in rectal cancer is dependent on many factors-the role of the tumor, the patient, the surgeon, the radiotherapist, and the pathologist. Am J Surg Pathol. 2009;33:1547–1553. doi: 10.1097/PAS.0b013e3181b2e01f. [DOI] [PubMed] [Google Scholar]
- 12.Peschaud F, Benoist S, Julie C, Beauchet A, Penna C, Rougier P, Nordlinger B. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg. 2008;248:1067–1073. doi: 10.1097/SLA.0b013e31818842ec. [DOI] [PubMed] [Google Scholar]
- 13.Dekker JW, Peeters KC, Putter H, Vahrmeijer AL, van de Velde CJ. Metastatic lymph node ratio in stage III rectal cancer; prognostic significance in addition to the 7th edition of the TNM. Eur J Surg Oncol. 2010;36:1180–1186. doi: 10.1016/j.ejso.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J. Clin. Oncol. 2005;23:8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 15.Peng J, Xu Y, Guan Z, Zhu J, Wang M, Cai G, Sheng W, Cai S. Prognostic significance of the metastatic lymph node ratio in node-positive rectal cancer. Ann Surg Oncol. 2008;15:3118–3123. doi: 10.1245/s10434-008-0123-8. [DOI] [PubMed] [Google Scholar]
- 16.Huh JW, Kim YJ, Kim HR. Ratio of metastatic to resected lymph nodes as a prognostic factor in node-positive colorectal cancer. Ann Surg Oncol. 2010;17:2640–6. doi: 10.1245/s10434-010-1015-2. [DOI] [PubMed] [Google Scholar]
- 17.Rullier A, Laurent C, Capdepont M, Vendrely V, Belleannée G, Bioulac-Sage P, Rullier E. Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol. 2008;32:45–50. doi: 10.1097/PAS.0b013e3180dc92ab. [DOI] [PubMed] [Google Scholar]
- 18.Lee SD, Kim TH, Kim DY, Baek JY, Kim SY, Chang HJ, Park SC, Park JW, Oh JH, Jung KH. Lymph node ratio is an independent prognostic factor in patients with rectal cancer treated with preoperative chemoradiotherapy and curative resection. Eur J Surg Oncol. 2012;38:478–483. doi: 10.1016/j.ejso.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Klos CL, Bordeianou LG, Sylla P, Chang Y, Berger DL. The prognostic value of lymph node ratio after neoadjuvant chemoradiation and rectal cancer surgery. Dis Colon Rectum. 2011;54:171–175. doi: 10.1007/DCR.0b013e3181fd677d. [DOI] [PubMed] [Google Scholar]
- 20.Kim YS, Kim JH, Yoon SM, Choi EK, Ahn SD, Lee SW, Kim JC, Yu CS, Kim HC, Kim TW, Chang HM. Lymph node ratio as a prognostic factor in patients with stage III rectal cancer treated with total meso-rectal excision followed by chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;74:796–802. doi: 10.1016/j.ijrobp.2008.08.065. [DOI] [PubMed] [Google Scholar]
- 21.Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol. 2010;17:2847–55. doi: 10.1245/s10434-010-1158-1. [DOI] [PubMed] [Google Scholar]
- 22.Soreide K, Nedrebo BS, Soreide JA, Slewa A, Kørner H. Lymph node harvest in colon cancer: infl uence of microsatellite instability and proximal tumor location. World J Surg. 2009;33:2695–2703. doi: 10.1007/s00268-009-0255-4. [DOI] [PubMed] [Google Scholar]


