Abstract
Objective: The aim of this study was to explain the effects of curcumin to depress gastric cancer biological activity by regulation miRNA-21 an in in vitro study. Methods: Collecting 30 pairs of adjacent and cancer tissues to measure miRNA-21 expression by ISH, evaluating pathology by H&E staining and measuring PTEN protein expression by IHC. Evaluating curcumin anti-tumor and correlation between curcumin and miRNA-21 in gastric cancer cell line (AGS) biological activities by CCK-8, flow cytometry, transwell, scratch test, transmission electron microscope, and western blot. Results: Compared with adjacent normal tissues, the miRNA-21 and PTEN expressions of gastric cancer tissues were significantly different (P < 0.001, respectively). By cell experiments, compared with NC group, the AGS cell proliferation was significantly depressed with significantly increasing cell apoptosis by keeping cell cycle in G1 phase (P < 0.001, respectively), and AGS cell invasion and migration were significantly down-regulated (P < 0.001, respectively) in Cur and Cur+BL groups. However, with miRNA-21 supplementation, the AGS cell biological activities were significantly recovered (P < 0.001, respectively). By western blot, compared with the NC group, the PTEN and P21 proteins expressions were significantly up-regulated (P < 0.001, respectively) and the PI3K, AKT, MMP-2 and MMP-9 proteins expressions were significantly down-regulated (P < 0.001, respectively). PTEN, PI3K, AKT, P21, MMP-2 and MMP-9 proteins were significantly decreased with miRNA-21 supplementation (P < 0.001, respectively). Conclusion: Curcumin had anti-tumor effects to gastric cancer via ion of miRNA-21 by regulation of the PTEN/PI3K/AKT pathway.
Keywords: Curcumin, gastric cancer, miRNA-21, PTEN, PI3K, AKT, P21, MMP-2, MMP-9
Introduction
The mortality of malignant tumors is second among human diseases, only lower than that of the cerebrovascular diseases. Gastric cancer is a common malignant tumor in the world and the second leading cause of cancer death [1]. The incidence rate of gastric cancer in China remains high, and the number of gastric cancer deaths accounts for half of the world’s gastric cancer deaths [2], and the reason is that most patients have missed the best operative period at the time of diagnosis. miRNA is a conserved non-coding single-stranded small molecule RNA with a length of 22-28 nt, which can degrade target mRNA by interacting with the untranslated region (3’-UTR) of target gene mRNA3’ to regulate gene expression. Several teams have found nearly a thousand similar small molecules in a variety of eukaryotic cells, which are collectively called miRNAs and play an important role in cell activity, histogenesis, and tumorigenesis [3]. In recent years, miRNAs have become a hot topic in the field of cancer, closely related to the occurrence and development of many kinds of tumors. miRNA-21 has many physiological activities, and its over-expression can inhibit the expression of tumor suppressor PTEN. Furthermore, miRNA-21 participates in the occurrence and development of many kinds of malignant tumors [4-7]. Curcumin is a major component extracted from turmeric plants. Curcumin has many functions such as in anti-inflammation, anti-atherosclerosis, anti-oxidation, anti-cancer and anti-tumor metastasis [8,9]. Some studies have demonstrated that curcumin (30 μmol/L) could effectively inhibit the biological activity of gastric cancer cells [10]. However, the mechanism of curcumin in inhibiting the biological activity of gastric cancer cells by regulating miRNA is unclear.
In this study, the expression of miRNA-21 and PTEN in adjacent normal tissues and gastric cancer tissues was detected first, and then the effect of curcumin on the biological activity of AGS gastric cancer cells by regulating the expression of miRNA-21 was investigated by cell experiments to further explore the specific mechanism of curcumin.
Materials and methods
Clinical samples
The tumor tissues and adjacent normal tissues of 30 patients with primary gastric adenocarcinoma were collected from September 2010 to July 2016 in our hospital. The samples were put into 10% polymethanol for 1 hour after resection, and then they were embedded in paraffin wax, sliced and stained with H&E staining.
Hybridization in situ
The collected tissue samples were extracted, dehydrated conventionally, soaked in wax, embedded, and sliced with a thickness of 4 μm. Paraffin sections were baked in a 65°C constant temperature oven to form liquid, and then dewaxed to water, and then the slices were removed and dried. Endogenous enzymes were inactivated by dripping the proper amount of 3% H2O2 on tissues. The slices were digested at 37°C with 3% pepsin for 15 min to expose mRNA nucleic acid fragment; 1% paraformaldehyde/0.1 mol/L PBS (pH = 7.2-7.6) was used to fix the tissue samples at room temperature for 10 minutes (min). The slices were wiped to a dry state. The tissue samples were incubated with 20 μL pre-hybridization solution at 42°C for 3 hours (h) to dry the sections. The hybridization solution of 10 μL (miRNA probe: hybridization solution = 1:250) (Exiqon, USA) was added in the samples, and the slides were covered with cover glass for hybridization in a hybridization oven overnight at 55°C. The samples were washed in 2 × SSC solution, 0.5 × SSC solution, and 0.2 × SSC solution at 37°C for 15 min. After dripping blocking solution, the samples were incubated at 37°C for 30 min. Biotinylated mouse against digoxin solution was dripped in the samples for incubation at room temperature for 2 h. The samples were washed with PBST 4 times with 5 min for each washing. After adding DAB chromogenic solution to the sections, the sections were observed under the microscope. When the positive sections appeared brown, the sections were immediately put into water to stop developing. After washing thoroughly, hematoxylin was employed for counterstaining. Finally, neutral gum was used for sealing the sections, and observed under the microscope.
Cells and cell culture
Human gastric adenocarcinoma AGS cells were provided by American Type Culture Collection (ATCC). The cells were cultured with DMEM containing 10% fetal bovine serum and 1% penicillin and streptomycin. The cells were cultured in a constant temperature incubator containing 5% CO2 at 37°C.
Cell grouping and cell culture
AGS cells were divided into normal control group (NC), curcumin-treated group (Cur), curcumin + empty package carrier group (Cur+BL) and curcumin + miRNA group (Cur+miRNA). AGS cells in the NC group were cultured normally. The AGS cells in the Cur group were treated with curcumin with a concentration of 30 μmol/L (Beijing Union Pharmaceutical Factory, Beijing, China). AGS cells of the Cur+BL group were treated with curcumin with a concentration of 30 μmol/L after transfection of the empty package vector (Kaiji Biotechnology Company, Nanjing, China). AGS cells of the Cur+miRNA group were treated with curcumin with a concentration of 30 μmol/L after transfection of miRNA-21 (Kaiji Biotechnology Company, Nanjing, China).
Detection of cell proliferation by CCK-8 colorimetry method
Gastric cancer cells in each group were treated with the corresponding treatments as described above. After digestion with trypsin, the pre-treated cells were inoculated in a 96-well plate with a number of cells of about 3 × 105 for each well. The cells were cultured in an incubator containing 5% CO2 r for 24 h, 48 h, and 72 h, respectively. After adding 10 μL CCK-8 reagent, the cells were mixed evenly and kept in the incubator for 1 h. The absorbance (A) at 450 nm was measured by enzyme-linked immunosorbent assay reader, and the growth curve was plotted. Three multiple wells were set up in each group.
Detection of cell cycle and apoptosis by flow cytometry
After 48 h of treatment with AGS cells in each group, the AGS cells in each group with a cell number of about 1 × 106 were collected and washed twice with pre-cooled PBS. The cells were fixed with 70% ethanol and then placed overnight in the refrigerator at 4°C. The cells were additionally washed with pre-cooled PBS for two times, and PI dyeing solution (20 ×) and RNase A (50 ×) were added to each well with cells. After incubation at 37°C in the dark for 0.5 h, cell cycle was detected by flow cytometry.
After 48 h of treatment, the AGS cells were collected and digested with trypsin without EDTA. Digestion of cells was terminated with supernatant cell culture medium. The cells were collected by centrifugation for 5 min at 1000 r/min. After washing with PBS twice, about (1-5) × 105 cells were collected and resuspended with 500 μL 1 × binding buffer. Finally, 5 μL Annexin V-FITC and 10 μL PI were added to the cells, respectively. After incubation at room temperature for 5 min, flow cytometry was used to detect the apoptosis rate of each group.
Transwell cell invasion assay
The basement membrane at the bottom of the cell culture chamber was coated with 50-100 μl Matrigel diluted with the dilution solution at a ratio of 1:20 and air-dried at 4°C. The basement membrane was hydrated by adding 100 μl RPMI 1640 medium to each cell chamber of the transwell apparatus at 37°C for 1 h. The cells were washed with serum-free medium and digested with 0.25% trypsin. After centrifugation at 1200 r/min for 5 min, the cells were resuspended with the RPMI 1640 medium containing 1% serum to a concentration of 1 × 105 cells/ml. The liquid in the transwell chamber was discarded. A total of 900 μl RPMI 1640 medium containing 10% serum was added to the small cell culture chamber, and 200 μl cells were added into the chamber with a number of cells of about 1 × 105. After 24-48 h of conventional cell culture, the transwell chamber was washed with PBS and fixed with 4% paraformaldehyde for 30 min. After the membrane at the bottom of the chamber was air-dried and stained with crystal violet dye for 20 min, the cells were observed and photographed under the microscope with a magnification of 400 x. Five visual fields were randomly selected in each group for counting.
Scratch test
A straight line was drawn crossing wells on the back of the 6-well plate every 0. 5-0.1 cm approximately on the first day by using a marker pen. At least 5 lines passed through each well. Cells with a density of 5 × 105 cells/ml were cultured routinely until monolayer cells were formed. The “1” zigzag scratches were made on monolayer cultured cells by using sterilized 100 μl yellow tip to form an acellular growth area (i.e. scratch). The tip was as perpendicular as possible, and the width of the scratch was about 500 μm. The relative distance of the scratch area was recorded. The dropped cells by scratching were then washed off with serum-free medium. The serum-free medium was changed for the cells and mitomycin was added to inhibit cell division at the same time. The cells were incubated in the incubator of 5% CO2 at 37°C. After culturing for 0 h, 24 h, and 48 h, the dropped cells by scratching were washed with PBS, and the relative distance of cell migration from the wound area was measured under the inverted microscope with a magnification of 200 times.
Observation of morphological changes of cells in each group under transmission electron microscope
AGS cells of logarithmic growth phase were inoculated in 6-well plates with 1 × 105 cells/well. The cells were treated with Imiquimod (100 μg/ml) for 24 h and digested with 0.25% trypsin. After centrifugation at 1500 r/min for 15 min, 1% osmium tetroxide was added for fixation overnight, followed by dehydration, embedding, polymerization, and dyeing. The ultrastructure of cells was observed under transmission electron microscope.
Detection of the expression of the related proteins in AGS cells by western blot
The AGS cells in each group were collected by removing the cell culture medium and washed with ice-cold PBS for two times. The cells were lysed with 250 μl cell lysis buffer on ice for 1 h, and the total protein was extracted. The protein quantification was conducted by using the BCA method. The loading protein was prepared with 20-30 μg for each hole. After 90 min of SDS-PAGE gel electrophoresis of 8% concentration, the proteins were transferred to the PVDF membrane and the 5% skim milk was used for blocking for 2 h. The rabbit anti-PTEN antibody (1:200) (Abcam, Cambridge, UK) and the antibodies against PI3K (1:200) (Abcam, Cambridge, UK), AKT (1:200) (Abcam, Cambridge, UK), P21 (1:200) (Abcam, Cambridge UK), MMP-9 (1:200) (Abcam, Cambridge UK) and MMP-2 (1:200) (Abcam, Cambridge UK) were incubated overnight at 4°C. After washing with TBST for 3 times, the sheep anti-rabbit secondary antibody (1:3000) was added and incubated with the samples at room temperature for 2 h. Finally, after washing with TBST for 3 times and washing with PBS once, the medium ratio of chemiluminescence solution A and B was added to the dark chamber for 3 min, and the gel imaging system was used for photographing.
Statistical analysis
All the data were expressed as mean ± standard deviation. The data were analyzed by SPSS 20.0 statistical software. The independent sample mean t-test was used for the comparison between two groups. The comparison between multiple groups was conducted by LSD t-test after analysis of variance. P < 0.05 indicated a difference that was significant.
Results
Clinical data and analysis
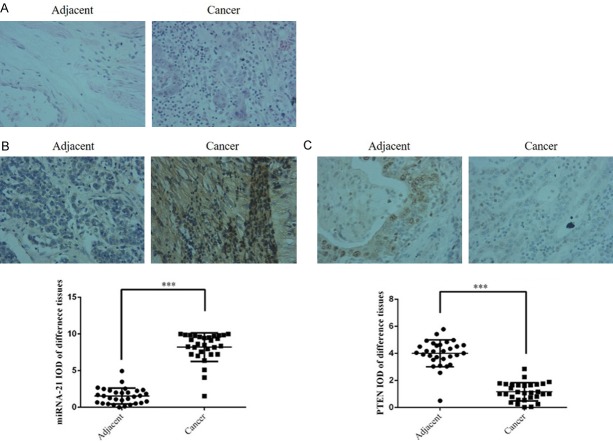
Compared with adjacent normal tissues, Figure 1A found the cancer tissues invasion and migration increased by H&E staining; Figure 1B was shown that miRNA-21 expression was significantly up-regulated (P < 0.001) and Figure 1C found PTEN protein expression was significantly suppressed (P < 0.001) in gastric cancer tissues.
Figure 1.
Clinical data and analysis. A. Pathology of gastric cancer and adjacent normal tissues by H&E staining (200 ×). B. The miRNA-21 expression of different tissues by ISH (200 ×). C. The PTEN protein expression of different tissues by IHC (200 ×). Adjacent: Gastric cancer adjacent normal tissue; Cancer: Gastric cancer tissues. ***: P < 0.001 vs. Adjacent. Adjacent: Gastric cancer-adjacent normal tissue; Cancer: Gastric cancer tissues.
AGS cell proliferation, apoptosis and cell cycle in difference groups
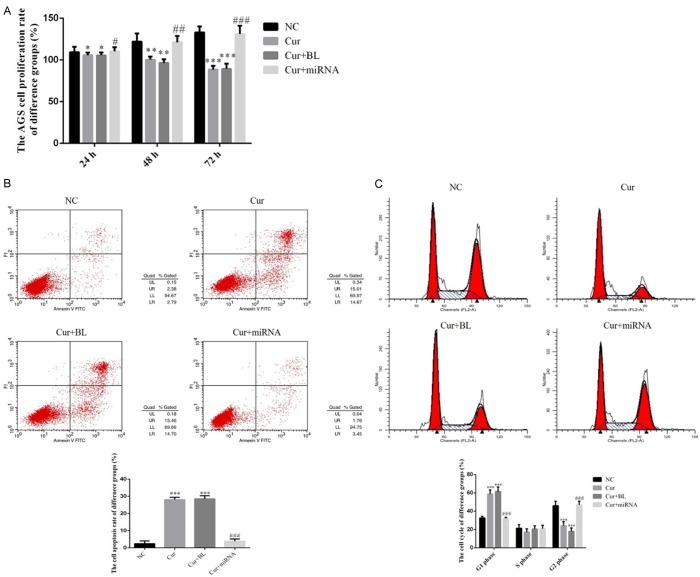
By CCK-8 assay, the result was shown that AGS cell proliferation rates of Cur and Cur+BL groups were significantly suppressed compared with NC group in 24 h, 48 h and 72 h (P < 0.05, P < 0.01 or P < 0.001, respectively, Figure 2A). However, the cell proliferation rate of Cur+miRNA group was significantly increased compared with that of Cur group with miRNA-21 supplement in 24 h, 48 h and 72 h (P < 0.05, P < 0.01 or P < 0.001, Figure 2A). By flow cytometty, Figure 2B shows that the cell apoptosis rate of Cur and Cur+BL groups were significantly up-regulated compared with that of NC group (P < 0.001, respectively). With miRNA-21 transfection, the AGS cell apoptosis rate was significantly suppressed compared with Cur group (P < 0.001, Figure 2B). We also found that the G1 phase rates were significantly up-regulated and G2 phase rates were significantly down-regulated in Cur and Cur+BL groups compared with that of NC group (P < 0.001, respectively, Figure 2C). With miRNA-21 supplementation, the G1 phase rate was significantly down-regulated and the G2 phase rate was significantly up-regulated in Cur+miRNA-21 group compared with that of Cur group (P < 0.001, respectively, Figure 2C).
Figure 2.
AGS cell proliferation, apoptosis and cell cycle in different groups. A. AGS cell proliferation rate of different groups by CCK-8 assay. NC: normal control group; Cur: AGS cell treated by curcumin group; Cur+BL: AGS cell transfected by empty vector treated with curcumin group; Cur+miRNA: AGS cells transfected by miRNA-21 treated with curcumin group. *: P < 0.05, **: P < 0.01, ***: P < 0.001 vs. NC; #: P < 0.05, ##: P < 0.01, ###: P < 0.001 vs. Cur. B. AGS cell apoptosis by flow cytometry. NC: normal control group; Cur: AGS cells treated by curcumin group; Cur+BL: AGS cells transfected by empty vector treated with curcumin group; Cur+miRNA: AGS cells transfected by miRNA-21 treated with curcumin group. ***: P < 0.001 vs. NC; ###: P < 0.001 vs. Cur. C. AGS cell cycle of different groups by flow cytometry. NC: normal control group; Cur: AGS cells treated by curcumin group; Cur+BL: AGS cells transfected by empty vector treated with curcumin group; Cur+miRNA: AGS cells transfected by miRNA-21 treated with curcumin group. ***: P < 0.001 vs. NC; ###: P < 0.001 vs. Cur.
AGS cell invasion, migration, and mitochondrial morphology in different groups
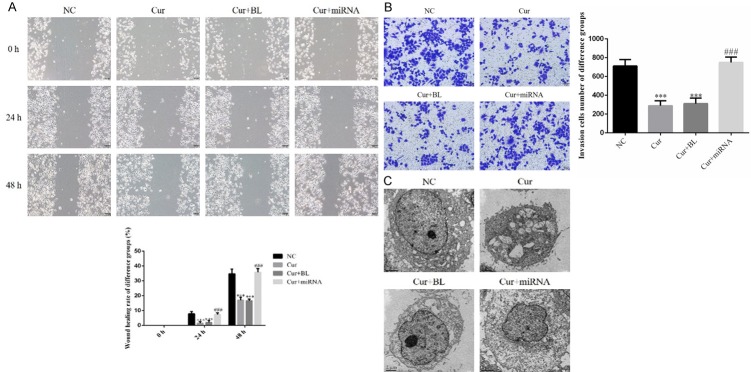
By scratch test, the wound healing rates of Cur and Cur+BL groups were significantly depressed compared with those of NC group in 24 h and 48 h (P < 0.001, respectively, Figure 3A); however, the wound healing rates of Cur+miRNA group were significantly increased with miRNA-21 transfection compared with those of Cur group in 24 h and 48 h (P < 0.001, respectively, Figure 3A). By transwell assay, the invasion AGS cell number of Cur and Cur+BL groups were significantly depressed compared with that of NC group (P < 0.001, Figure 3B), the AGS cell invasion ability was significantly recovered with miRNA-21 supplement (P < 0.001, Figure 3B). By transmission electron microscope, we found the mitochondrion was damaged in Cur and Cur+BL groups. We also found the mitochondrion was recovered with miRNA-21 transfection in Figure 3C.
Figure 3.
AGS cell invasion, migration and mitochondrial morphology in different groups. A. Wound healing rate of difference groups by Scratch test. NC: normal control group; Cur: AGS cells treated by curcumin group; Cur+BL: AGS cells transfected by empty vector treated with curcumin group; Cur+miRNA: AGS cells transfected by miRNA-21 treated with curcumin group. ***: P < 0.001 vs. NC; ###: P < 0.001 vs. Cur. B. The invasion cell number of difference groups by transwell assay. NC: normal control group; Cur: AGS cells treated by curcumin group; Cur+BL: AGS cells transfected by empty vector treated with curcumin group; Cur+miRNA: AGS cells transfected by miRNA-21 treated with curcumin group. ***: P < 0.001 vs. NC; ###: P < 0.001 vs. Cur. C. Mitochondrial morphology of different groups by transmission electron microscope. NC: normal control group; Cur: AGS cells treated by curcumin group; Cur+BL: AGS cells transfected by empty vector treated with curcumin group; Cur+miRNA: AGS cells transfected by miRNA-21 treated with curcumin group.
Relative proteins expressions of difference groups by WB assay
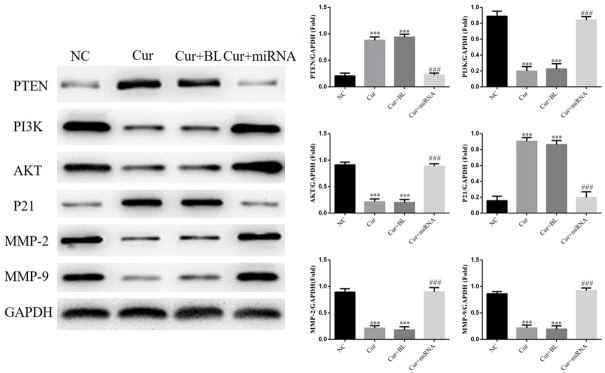
Compared with NC group, PTEN and P21 proteins expressions of Cur and Cur+BL groups were significantly increased compared with those of NC group (P < 0.001, respectively). The PI3K, AKT, MMP-2 and MMP-9 proteins expressions of Cur and Cur+BL groups were significantly depressed compared with those of NC group (P < 0.001, respectively). With miRNA-21 transfection, PTEN and P21 proteins expressions were significantly suppressed and PI3K, AKT, MMP-2 and MMP-9 proteins expressions were significantly stimulated in Cur+miRNA group (P < 0.001, respectively). The relative data are shown in Figure 4.
Figure 4.
The relative protein expressions of different groups by western blot. NC: normal control group; Cur: AGS cell treated by curcumin group; Cur+BL: AGS cells transfected by empty vector treated with curcumin group; Cur+miRNA: AGS cells transfected by miRNA-21 treated with curcumin group. ***: P < 0.001 vs. NC; ###: P < 0.001 vs. Cur.
Discussion
Curcumin is an active component extracted from traditional Chinese medicine turmeric. Some researchers have carried out phase I/II clinical study of curcumin. The results demonstrated that curcumin combined with other chemotherapeutic drugs could lighten the toxic and side effects of chemotherapeutic drugs, reduce the dosage of chemotherapeutic drugs, improve the quality of life of patients and prolong the survival time. Furthermore, curcumin has no obvious side effect, and the patients have a good tolerance, so it can be used as a long-term preventive treatment for colorectal cancers [11]. The results of this study confirmed that curcumin could effectively inhibit the biological activity of AGS gastric cancer cells. In order to explore the specific anti-cancer mechanism of curcumin, we have conducted a corresponding cell level study.
miRNA can be fully or partially paired with the 3’-UTR region of the target gene mRNA, duplicating multiple times, thus amplifying the activity of miRNA gene regulation [12]. About 60% of the genes that encode proteins in humans are at risk of binding to mRNA. Different tumors have different miRNA expression profiles [13-15]. miRNAs can promote and inhibit tumor growth. For a particular miRNA, there are usually multiple target genes, which makes the regulatory mechanism of the targeting genes of miRNA very complex [16]. miRNA-21 promoted the growth of many kinds of tumors, including breast cancer, pancreatic cancer, colorectal cancer, head and neck cancer, esophageal cancer, malignant glioma, fibroblastoma, cholangiocarcinoma, lung cancer and gastric cancer [17-19]. In cervical cancer, a study [20] showed that over-expression of miRNA-21 in HeLa cells could promote cell proliferation and down-regulate the expression of PDCD4. In squamous carcinoma of the cervix, research [21] showed that miRNA-21 could promote cell proliferation by regulating the expression of chemokine CCL20. The results of this study showed that miRNA-21 was highly expressed in gastric cancer tissues. Curcumin could effectively inhibit the biological activity of AGS gastric cancer cells. However, when miRNA-21 was transfected into AGS cells, the inhibitory effect of curcumin on gastric cancer was significantly decreased. The results suggest that curcumin’s inhibitory effect on gastric cancer may be closely related to the reduction of miRNA-21 expression.
PTEN is a tumor suppressor gene located mainly in mitochondria, and its expression is decreased in blood system tumor, digestive tract tumor, gynecological and urinary tumors. Some studies [22] have shown that abnormal PTEN may lead to the activation of PI3K/AKT signaling pathway, resulting in the occurrence and development of the tumor. P21 gene is significantly expressed at a low level in many tumors, and it has a wide range of cell cycle-dependent kinase inhibitory activity. It can specifically inhibit the formation of Cyclin D1-CDK6 complex and make the cells stay in G1 phase to inhibit the growth of the tumor. It has an obvious anti-cancer effect [23,24]. In this study, we found that curcumin did not change the morphology of AGS cells by transmission electron microscope, but it caused some changes in the shape of mitochondria, which may be related to the changes of PTEN protein in mitochondria. This study also revealed that curcumin could effectively enhance the expression of PTEN, thereby inhibiting the activity of PI3K/AKT and activate the activity of the P21 protein, leading to a large number of AGS cells arrested in G1 phase to inhibit the proliferation of AGS cells and promote the apoptosis of AGS cells.
MMP-2/9 are the two key genes [25] downstream of the PI3K/AKT signaling pathway, and they are also tumor migration-related factors participating in migration and invasion of tumors [26]. MMPs are the major rate-limiting enzymes involved in regulation of intercellular matrix (ECM) metabolism. Due to the need for metal ions Ca2+ and Zn2+ as auxiliary factors, MMPs can specifically degrade ECM, and participate in many physiological and pathophysiological processes, and include a large family. Among them, MMP-2 and MMP-9 could degrade gelatin, laminin, and type IV collagen, and play a vital role in the invasion and metastasis of various malignant tumors [27,28]. Under the intervention of curcumin, the invasion and migration of AGS cells were significantly reduced and the expressions of MMP-2 and MMP-9 were significantly decreased. However, after the transfection of miRNA-21 into AGS cells, AGS, AGS cell invasion and migration ability and MMP-2/9 protein expression were restored, indicating that curcumin suppresses cancer by inhibiting the expression of miRNA-21.
In conclusion, curcumin activates the activity of PTEN by inhibiting the expression of miRNA-21 and inhibits the activity of the PI3K/AKT signaling pathway which in turn inhibits the biological activity of gastric cancer cells.
Disclosure of conflict of interest
None.
References
- 1.Kim SG, Ji SM, Lee NR, Park SH, You JH, Choi IJ, Lee WS, Park SJ, Lee JH, Seol SY, Kim JH, Lim CH, Cho JY, Kim GH, Chun HJ, Lee YC, Jung HY, Kim JJ. Quality of life after endoscopic submucosal dissection for early gastric cancer: a prospective multicenter cohort study. Gut Liver. 2017;11:87–92. doi: 10.5009/gnl15549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strong VE, Wu AW, Selby LV, Gonen M, Hsu M, Song KY, Park CH, Coit DG, Ji JF, Brennan MF. Differences in gastric cancer survival between the U.S. and China. J Surg Oncol. 2015;112:31–37. doi: 10.1002/jso.23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan HL, Wang T, Zhang KH. MicroRNAs as potential biomarkers for diagnosis, therapy and prognosis of gastric cancer. Onco Targets Ther. 2018;11:3891–3900. doi: 10.2147/OTT.S156921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Xu B, Miu X, Deng Z, Liao H, Hao L. Inhibition of miRNA-21 attenuates the proliferation and metastasis of human osteosarcoma by upregulating PTEN. Exp Ther Med. 2018;15:1036–1040. doi: 10.3892/etm.2017.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X, Chen Y, Tian R, Li J, Li H, Lv T, Yao Q. miRNA-21 enhances chemoresistance to cisplatin in epithelial ovarian cancer by negatively regulating PTEN. Oncol Lett. 2017;14:1807–1810. doi: 10.3892/ol.2017.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang H, Xie J, Zhang M, Zhao Z, Wan Y, Yao Y. miRNA-21 promotes proliferation and invasion of triple-negative breast cancer cells through targeting PTEN. Am J Transl Res. 2017;9:953–961. [PMC free article] [PubMed] [Google Scholar]
- 7.Luo M, Tan X, Mu L, Luo Y, Li R, Deng X, Chen N, Ren M, Li Y, Wang L, Wu J, Wan Q. MiRNA-21 mediates the antiangiogenic activity of metformin through targeting PTEN and SMAD7 expression and PI3K/AKT pathway. Sci Rep. 2017;7:43427. doi: 10.1038/srep43427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson JL, Hill R, Yaffe PB, Greenshields A, Walsh M, Lee PW, Giacomantonio CA, Hoskin DW. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. 2010;297:1–8. doi: 10.1016/j.canlet.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Waghela BN, Sharma A, Dhumale S, Pandey SM, Pathak C. Curcumin conjugated with PLGA potentiates sustainability, anti-proliferative activity and apoptosis in human colon carcinoma cells. PLoS One. 2015;10:e0117526. doi: 10.1371/journal.pone.0117526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang F, Yao Y, Wu J, Liu Q, Zhang J, Pu X, Zhang Q, Xia L. Curcumin inhibits gastric cancer-derived mesenchymal stem cells mediated angiogenesis by regulating NF-κB/VEGF signaling. Am J Transl Res. 2017;9:5538–5547. [PMC free article] [PubMed] [Google Scholar]
- 11.Irving GR, Howells LM, Sale S, Kralj-Hans I, Atkin WS, Clark SK, Britton RG, Jones DJ, Scott EN, Berry DP, Hemingway D, Miller AS, Brown K, Gescher AJ, Steward WP. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration-a clinical pilot study including assessment of patient acceptability. Cancer Prev Res (Phila) 2013;6:119–128. doi: 10.1158/1940-6207.CAPR-12-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philippidou D, Schmitt M, Moser D, Margue C, Nazarov PV, Muller A, Vallar L, Nashan D, Behrmann I, Kreis S. Signatures of microRNAs and selected microRNA target genes in human melanoma. Cancer Res. 2010;70:4163–4173. doi: 10.1158/0008-5472.CAN-09-4512. [DOI] [PubMed] [Google Scholar]
- 14.Conti A, Aguennouz M, La Torre D, Tomasello C, Cardali S, Angileri FF, Maio F, Cama A, Germanò A, Vita G, Tomasello F. MiR-21 and 22 up-regulation and miR-181b downregulation in human grade II-IV astrocytic tumor. J Neurooncol. 2009;93:325–332. doi: 10.1007/s11060-009-9797-4. [DOI] [PubMed] [Google Scholar]
- 15.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet. 2007;39:1278–1284. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- 16.Sempere LF, Christensen M, Silahtaroglu A, Bak M, Heath CV, Schwartz G, Wells W, Kauppinen S, Cole CN. Altered microRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res. 2007;67:11612–11620. doi: 10.1158/0008-5472.CAN-07-5019. [DOI] [PubMed] [Google Scholar]
- 17.Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171–2176. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, Liu CG, Calin GA, Croce CM, Harris CC. MicroRNA expression profiles associated with profiles associated with prognosis and therapeutic outcome in colon adenocamoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao T, Lin Z. MiR-21 is involved in cervical squamous cell tumorigenesis and regulates CCL20. Biochim Biophys Acta. 2012;1822:248–260. doi: 10.1016/j.bbadis.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun. 2009;388:539–542. doi: 10.1016/j.bbrc.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Jhawer M, Goel S, Wilson AJ, Montagna C, Ling YH, Byun DS, Nasser S, Arango D, Shin J, Klampfer L, Augenlicht LH, Perez-Soler R, Mariadason JM. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008;68:1953–1961. doi: 10.1158/0008-5472.CAN-07-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan B, Zou M, Zhao Y, Zhang K, Sun Y, Peng X. Up-Regulation of miR-130b-3p activates the PTEN/PI3K/AKT/NF-κB pathway to defense against Mycoplasma gallisepticum (HS Strain) infection of chicken. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19082172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye D, Luo H, Lai Z, Zou L, Zhu L, Mao J, Jacob T, Ye W, Wang L, Chen L. ClC-3 chloride channel proteins regulate the cell cycle by up-regulating cyclin D1-CDK4/6 through suppressing p21/p27 expression in nasopharyngeal carcinoma cells. Sci Rep. 2016;6:30276. doi: 10.1038/srep30276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen P, Luo X, Che Z, Zhang W, Liu F, Hou D, Yang D, Liu J. Targeting of the C-Jun/BCL-XL/P21 axis accelerates the switch from senescence to apoptosis upon ROC1 knockdown in gastric cancer cells. Cell Physiol Biochem. 2018;48:1123–1138. doi: 10.1159/000491979. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Wang Z, Wang J, Wang Y, Liu L, Xu X. LncRNA MEG3 has anti-activity effects of cervical cancer. Biomed Pharmacother. 2017;94:636–643. doi: 10.1016/j.biopha.2017.07.056. [DOI] [PubMed] [Google Scholar]
- 26.Giganti MG, Tresoldi I, Sorge R, Melchiorri G, Triossi T, Masuelli L, Lido P, Albonici L, Foti C, Modesti A, Bei R. Physical exercise modulates the level of serum MMP-2 and MMP-9 in patients with breast cancer. Oncol Lett. 2016;12:2119–2126. doi: 10.3892/ol.2016.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao CL, Chu YL, Lin HY, Chen CY, Hsu MJ, Liu KC, Lai KC, Huang AC, Chung JG. Bisdemethoxycurcumin suppresses migration and invasion of human cervical cancer hela cells via inhibition of NF-ĸB, MMP-2 and -9 pathways. Anticancer Res. 2018;38:3989–3997. doi: 10.21873/anticanres.12686. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Li K, Liang Q, Zheng G, Zhang S, Lao X, Liang Y, Liao G. Elevated hydrostatic pressure promotes ameloblastoma cell invasion through upregulation of MMP-2 and MMP-9 expression via Wnt/β-catenin signalling. J Oral Pathol Med. 2018;47:836–846. doi: 10.1111/jop.12761. [DOI] [PubMed] [Google Scholar]