Abstract
Hypoxia-induced apoptosis is an inevitable problem in cyanotic congenital heart disease. In the present study, we investigated effects of melatonin on hypoxic cardiomyocytes in vitro and in vivo, and explored its underlying mechanism. H9C2 cells were subjected to hypoxia for 48 hours. Mice were subjected to hypoxia treatment (10% O2) for 4 weeks. Cell viability was detected by the cell counting kit-8 assay. Cellular apoptosis was assessed by Annexin V/7 AAD assay. Western blotting was employed to determine the expression of Bcl-2, Bax, cleaved caspase 3, phosphorylation of PI3K, and AKT. Melatonin increased cell viability and alleviated apoptosis in hypoxic H9C2 cells and cardiomyocytes of hypoxia-treated mice. Melatonin pretreatment increased Bcl-2 and decreased cleaved caspase 3 and Bax levels. Moreover, melatonin activated the PI3K/Akt pathway. The protective effects of melatonin were abolished by a PI3K/Akt-inhibitor, LY294002. Our results demonstrated that melatonin confers cardioprotection by inhibiting apoptosis through the activation of PI3K/Akt signaling pathway in hypoxic cardiomyocytes.
Keywords: Melatonin, hypoxia, apoptosis, PI3K/Akt signaling, myocardial
Introduction
Cyanotic congenital heart disease (CHD), including tetralogy of Fallot, is a type of innate malformation causing systemic chronic hypoxemia. Impairment in oxygen delivery in CHD patients is the consequence of abnormal anatomy of the heart. Hypoxia increases cardiomyocyte apoptosis [1], which can result in myocardial cell loss. Abundant studies have reported that inhibition of apoptosis could decrease myocardial damage, alleviating cardiomyocyte removal caused by hypoxic injury [2]. Hypoxic stress is an inevitable problem in cardiomyocytes of CHD patients. Therefore, inhibition of hypoxia-induced apoptosis in cardiomyocytes might be of great importance in treating CHD.
Melatonin, a biorhythm modifier secreted by the pineal gland, is well known for its antioxidant and anti-inflammatory properties [3,4]. Several studies have reported that melatonin exhibits beneficial effects against cardiac dysfunction induced by ischemia/reperfusion injury [5,6]. In addition, it has been reported that melatonin exhibits protective effects against cardiac injuries induced by chronic intermittent hypoxia in rats by mitigating the SR-Ca2+ homeostasis, decreasing antioxidant enzymes and NADPH oxidase, and inducing autophagy [7,8]. Furthermore, there is evidence that melatonin protects against hypoxia-induced deterioration of cardiac dysfunction, which may be associated with the calcium handling protein SERCA expression preservation [9]. However, the mechanism of melatonin in protecting cardiomyocytes from hypoxia requires further investigation.
Phosphatidylinositol 3-kinase (PI3K)/kinase B (Akt) signaling pathway has been documented to be an intracellular signaling pathway that regulates cell survival, growth, and apoptosis [10]. Activation of the PI3K/Akt pathway has been demonstrated to promote cellular survival of cardiomyocytes and protect the heart from ischemia/reperfusion injury [11,12]. Moreover, activation of PI3K/Akt has been demonstrated to alleviate hypoxic injury in cardiomyocytes [2,13]. However, the role of PI3K/Akt pathway in melatonin’s protective effect in hypoxia-induced myocardial injury is not clear. The present study is designed to investigate cardioprotective effects of melatonin against hypoxic injury in vivo and in vitro and the underlying mechanisms.
Materials and methods
Ethics statement
All animals received humane care in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health (NIH Publication No.85e 23, revised 1996). All experiments were performed according to the protocols approved by the Institute Animal Care Committee, Third military Medical University. All efforts have been made to minimize the suffering of mice during experiments.
Animals
Male C57BL/6j mice (20-25 g, 8 to 10 weeks old) were obtained from the Experimental Animal Center of the Xinqiao Hospital. Mice in the hypoxia group were kept in a chamber (Baker Ruskinn InvivO2-1000, Ruskinn Technology, UK) for normobaric hypoxia and had free access to chow and water. The oxygen fraction in the chamber was maintained at 10 ± 0.5%. The hypoxic mice were exposed to hypoxia for four weeks. The normoxic mice were kept in room air in the same housing and maintenance as the hypoxic groups.
Experimental protocols in vivo
To study the role of melatonin in hypoxia-induced apoptosis: 32 mice were randomly divided into four groups (N=8 each group): (1) Normoxia + vehicle; (2) Hypoxia + vehicle; (3) Hypoxia + melatonin; (4) Hypoxia + melatonin + LY294002. Mice in hypoxia + melatonin group were intraperitoneally injected with melatonin at a dose of 20 mg/kg body weight per day as previously reported [14,15]. LY294002 was intraperitoneally injected at a dose of 50 mg/kg per mouse per day [16]. The same volume of saline was intraperitoneally injected in those of the Normoxia + vehicle group and the Hypoxia + vehicle group.
Melatonin was dissolved in absolute ethanol and further dilution with normal saline; the final concentration of ethanol was 1%. LY294002 was dissolved in dimethyl sulfoxide (DMSO), and further diluted with normal saline and the final concentration of DMSO was 1%.
Cell culture
The rat cardiac cell line, H9C2, was purchased from the American Type Culture collection (ATCC; Rockville, MD, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (GIBCO, USA) supplemented with 10% (v/v) fetal bovine serum (GIBCO, USA), 100 U/mL penicillin, 100 µg/mL streptomycin (Beyotime Institute of Biotechnology, China). Cells in the hypoxic group were pretreated with indicated drugs for 2 hours (h), followed by 48 h hypoxia treatment in a hypoxic chamber (Ruskinn Technology, UK) with 1% O2, 94% N2, and 5% CO2. Control cells were maintained in a humidified incubator with 21% O2 at 37°C. To determine the role of melatonin, H9C2 cardiac myocytes were pretreated with melatonin (Sigma, St. Louis, MO, USA) for 2 h before hypoxia for 48 h. In some experiments, H9C2 cells were pretreated with LY294002 (20 µM, Sigma, St. Louis, MO, USA) before melatonin stimulation.
Cell viability assay
Cell viability was measured by cell counting Kit-8 (CCK-8; Wuhan Bioengineering institute, Wuhan, China) assays according to the manufacturer’s protocols. After the stimulation with hypoxia in the presence or absence of melatonin (1-1000 μM), the cells in 96-well plate were treated with 10 µl CCK-8 solution and 100 µl complete medium. The absorbance of the media was measured at 450 nm with a microplate reader.
Western blot
H9C2 cells and mice heart tissues were used to extract proteins by lysis buffer, and then were separated by SDS-PAGE gels and transferred to PVDF membranes (Roche, Germany). Membranes were incubated with the following primary antibodies overnight at 4°C: p-PI3K, PI3K, p-Akt Ser473, Akt, cleaved Caspase 3, and β-actin (Cell Signaling Technology, USA); Bcl-2, Bax (Abcam, USA). The membranes were further incubated with secondary antibody in TBST solution for 1 h at room temperature, and detected by ECL (Beyotime Institute of Biotechnology, China). Densitometric analyses were analyzed by Image J software.
Apoptosis assayed by annexin V and 7AAD flow cytometry
Cell apoptosis was detected by flow cytometry. H9C2 cells were harvested, washed, and resuspended in 500 µl binding buffer. Cells were then incubated with 5 µl annexin V and 5 µl 7-amino-actino-mycin D (7AAD) (BD Biosciences, San Jose, CA, USA) in the dark at room temperature for 15 mins. Then, the samples were analyzed by flow cytometry (Beckman Coulter, USA).
TUNEL assay
Myocardial apoptosis was assessed by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay using an in-situ cell death detection kit (Roche, Germany). Briefly, mice heart sections were incubated with 50 µl TUNEL reaction mixture for 1 h in a dark and humidified atmosphere. Then the sections were washed by PBS three times (5 min per time). TUNEL positive cells are apoptotic cells. DAPI (Beyotime Institute of Biotechnology, China) was used to quantitate the whole myocardial cell nuclei. The apoptotic cells in five random fields were photographed by confocal microscopy. Apoptosis was detected as the ratio of the TUNEL-positive nuclei to that of DAPI-positive nuclei.
Statistical analysis
Data are shown as means ± SEM. The significant differences among the groups were analyzed with one-way ANOVA. All statistical tests were performed with GraphPad Prism 5 (San Diego, CA, USA). p-value <0.05 was considered significant.
Results
Melatonin inhibits hypoxia-induced apoptosis of H9C2 cardiomyoblasts
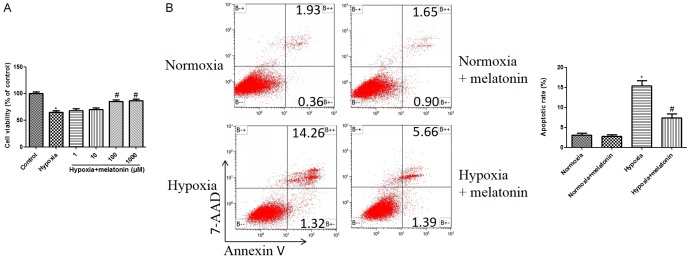
To determine the direct cardio-protective effects of melatonin against hypoxic injury, we treated H9C2 cells with various dose of melatonin (1, 10, 100, 1000 μM) 2 h before hypoxia treatment, and CCK-8 was used to assess cardiomyocytes injury. 48 h hypoxia treatment significantly decreased cell viability, which was suppressed by melatonin in a dose dependent manner. 100 μM and 1000 μM melatonin treatment significantly increased cell viability, compared to the hypoxia control group (P<0.05, Figure 1A). According to the experimental results and previous articles [15,17], the concentration of melatonin at 100 μM is selected for later study.
Figure 1.
Protective effects of melatonin on hypoxia induced apoptosis in H9C2 cardiomyocytes. A. Effects of different doses of melatonin (1, 10, 100, and 1000 μM) on cell viability in hypoxia treated H9C2 cells. Cell viability of H9C2 cells was detected by CCK-8 assay. B. Melatonin (100 μM) reduced cardiomyocyte apoptosis in response to hypoxia. Cell apoptosis was tested by Annexin V-PE/7-AAD flow cytometry. The normoxia group was used as control. Data are shown as the means ± SEM (n=3). *P<0.05 compared with control group, #P<0.05 compared with hypoxia group.
Flow cytometry was used to analyze the cell apoptosis (early + late apoptotic rate). The apoptosis rate of cells in the hypoxia group was higher than that of the control group (Figure 1B), while melatonin remarkably decreased the percentages of apoptotic cell compared to the hypoxia group (Figure 1B).
Melatonin regulates PI3K/Akt signaling in hypoxic H9C2 cells
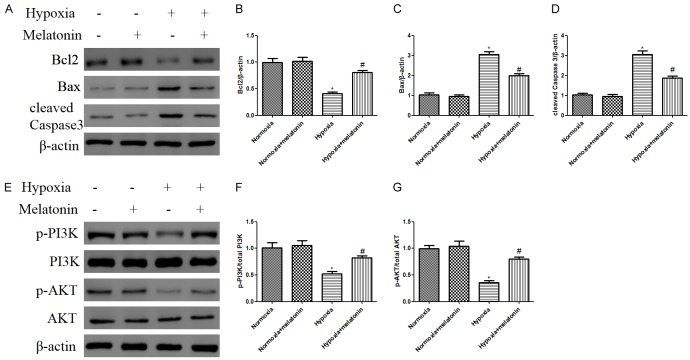
To further assess the anti-apoptotic effects of melatonin, we detected the expression of anti-apoptotic Bcl-2, and pro-apoptotic Bax and cleaved Caspase 3 (Figure 2A). Protein expressions of Bax and cleaved Caspase 3 (Figure 2C, 2D, P<0.05) in the hypoxia group were increased while the anti-apoptotic Bcl-2 expression (Figure 2B, P<0.05) was decreased compared to that of normoxia group. In addition, pretreatment with melatonin increased the expression of Bcl-2 and decreased cleaved Caspase 3 and Bax (Figure 2B-D).
Figure 2.
Melatonin alleviated apoptosis and activated PI3K/Akt pathway in H9C2 cells exposed to hypoxia. H9C2 cells were pretreated with melatonin 100 μM for 2 h prior exposure to hypoxia for 48 h. A. Representative western blotting of Bcl2, Bax, and cleaved caspase 3 with β-actin determined as the internal control. B-D. Histogram representing relative protein levels of Bcl2, Bax, and cleaved caspase 3. E. Phosphorylation of PI3K and Akt as analyzed by immunoblotting. F, G. Relative levels of p-PI3K versus total PI3K and p-AKT versus total AKT as determined by blot densitometry. Data are shown as the means ± SEM (n=3). *P<0.05 compared with control group, #P<0.05 compared with hypoxia group.
Next, we analyzed the effect of melatonin on the regulation of PI3K/Akt signaling pathway by measuring the expression levels of p-PI3K and p-Akt (Figure 2E). Expressions of p-PI3K and p-Akt were significantly decreased after 48 h hypoxia compared with that in normoxia group (Figure 2F, 2G). However, the expression levels of p-PI3K and p-Akt were significantly increased by melatonin compared to the hypoxia group (Figure 2F, 2G), indicating that melatonin promoted that activation of PI3K/Akt pathway in hypoxic H9C2 cells.
Melatonin suppresses hypoxia induced apoptosis in H9C2 cardiomyoblasts via PI3K/Akt pathway
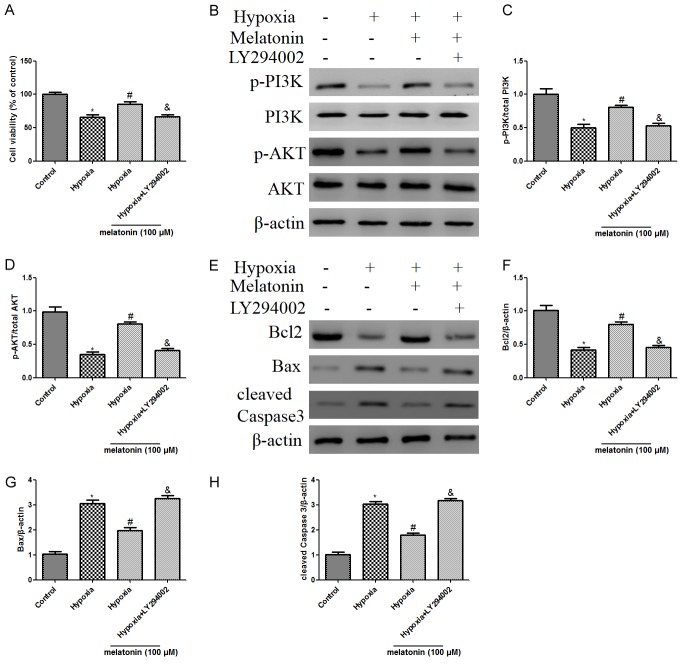
To explore whether PI3K/Akt is involved with melatonin mediated cardio-protective mechanisms, the PI3K/Akt-inhibitor LY294002 (20 µM) was used, and then the effects of melatonin on cell viability and apoptosis were detected. Melatonin + LY294002 treatment significantly lowered cell viability compared with melatonin alone administration in hypoxic H9C2 cells (Figure 3A). Moreover, co-treatment of LY294002 blocked melatonin-increased PI3K phosphorylation and Akt phosphorylation (Figure 3B-D). Furthermore, the decrease in Bax and cleaved Caspase 3 expressions and increase in Bcl-2 were blocked by administration of LY294002, compared with that in melatonin alone group (Figure 3E-H).
Figure 3.
Melatonin treatment ameliorated hypoxic injury by reducing cardiomyocyte apoptosis, and activating PI3K/Akt signaling, while these protective effects were attenuated by LY294002 treatment. H9C2 cardiac cells were treated with LY294002 (20 μM) for 1 h prior to stimulation with melatonin, followed by 48 h hypoxia treatment. A. Effects of melatonin on cell viability determined by CCK-8 in cultured H9C2 cells. B. Representative western blot of phosphorylation of PI3K and Akt in different groups with or without LY294002. C. Ratio of phosphorylated PI3K/ total PI3K. D. Ratio of phosphorylated Akt/total Akt. E. Representative western blotting of Bcl2, Bax, and cleaved Caspase 3. β-actin was used as the internal control. F-H. The relative levels of Bcl2, Bax, and cleaved Caspase 3. The results are expressed as the means ± SEM, n=3. *P<0.05 versus control group, #p<0.05 versus hypoxia group, &P<0.05 versus hypoxia + melatonin group.
Melatonin alleviated myocardial apoptosis is in hypoxic mice
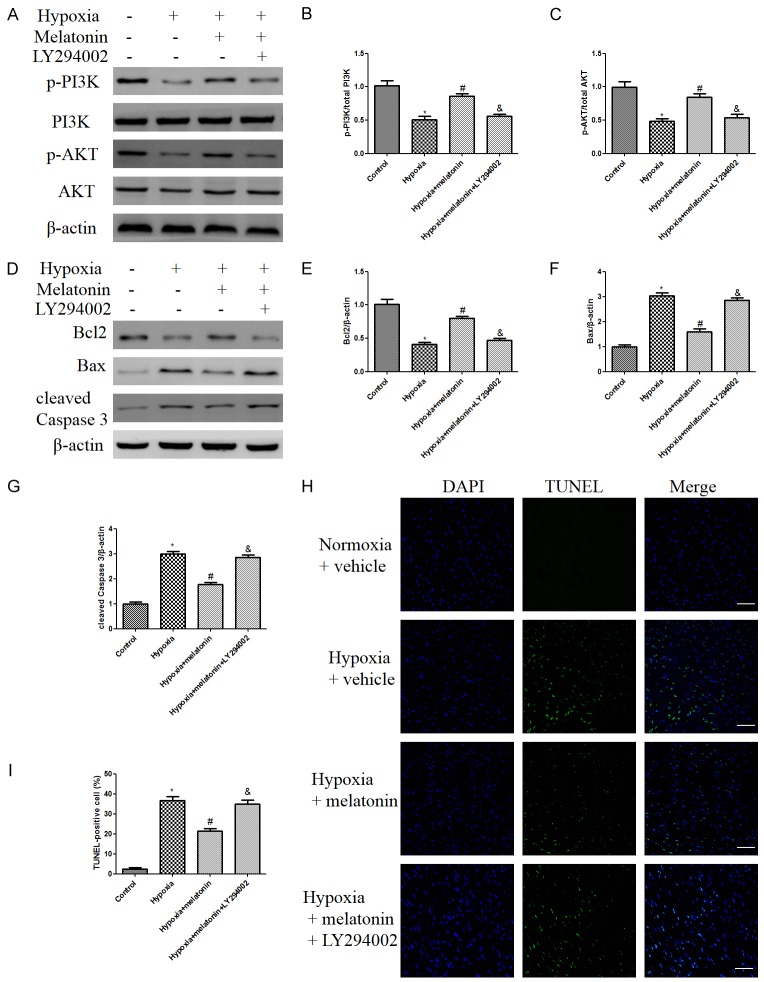
Melatonin treatment enhanced PI3K and Akt phosphorylation, compared to those in the hypoxia group. However, the activation of PI3K/Akt by melatonin was abolished when LY294002 was co-administrated with melatonin (Figure 4A-C). Moreover, hypoxia exposure induced a robust increase in cleaved Caspase 3 and Bax expression relative to the normoxia group. Compared with the hypoxia + vehicle group, melatonin treatment significantly decreased myocardial apoptosis as evidenced by increased Bcl-2 level and decreased cleaved Caspase 3 and Bax expressions (Figure 4D-G). However, these beneficial effects were abolished by LY294002 treatment (Figure 4D-G).
Figure 4.
Melatonin reduced myocardial apoptosis index in hypoxic mice, while these effects were abolished by LY294002 treatment. A. Representative western blot of p-PI3K, PI3K, p-AKT, AKT in different groups. B, C. Relative protein expression levels of p-PI3K and p-AKT. D. The expressions of apoptosis-related proteins were measured by western blot. E-G. Relative levels of Bcl2, Bax, and cleaved Caspase 3. H, I. Heart sections were stained with TUNEL (green) and DAPI (blue). Representative images of TUNEL staining (scale bar indicates 50 μm). The results are expressed as the means ± SEM, n=8. *P<0.05 versus Normoxia + vehicle group, #p<0.05 versus Hypoxia + vehicle group, &P<0.05 versus Hypoxia + melatonin group.
As illustrated in Figure 4H, 4-week hypoxia treatment resulted in significant myocardial damage, as evidenced by increased myocardial apoptosis. Melatonin treatment significantly decreased the apoptotic rate in the hypoxia + melatonin group, compared with that of hypoxia group. However, the anti-apoptotic effects were abolished by LY294002 treatment (Figure 4H, 4I), suggesting that melatonin mediated protection against hypoxia induced myocardial apoptosis is at least partially through PI3K/Akt activation. Together with the results of western blotting, these results demonstrated that melatonin reduced myocardial apoptosis in hypoxic mice probably through PI3K/Akt signaling.
Discussion
In the current study, the effects and mechanisms of melatonin in modulating hypoxia-induced injury in cardiomyocytes were explored. We demonstrated that melatonin exerts a protective effect on hypoxia-induced apoptosis in H9C2 cells and cardiomyocytes of mice subjected to hypoxia exposure through the activation of the PI3K/Akt pathway.
Melatonin, an extractive from the pineal gland, has been widely accepted as a cardioprotective agent in ischemia/reperfusion injury [6,17], cardiac hypertrophy [14,18], heart failure [19]. And these effects were attributed to its free radical scavenger and antioxidant properties, and its anti-inflammatory effects. Moreover, melatonin has been reported to be effective agent that protects cardiomyocytes against apoptosis in the context of myocardial infarction [20]. However, the mechanisms by which melatonin exerts cardioprotection against hypoxia remain to be elucidated.
Chronic hypoxia is a basic pathophysiologic feature of cyanotic CHD. Exploring the potential mechanism of the protective role of melatonin in adaption of cardiomyocytes to hypoxia is necessary. Of note, several animal studies demonstrated that melatonin is cardio-protective in rat exposed to chronic hypoxia [9] and chronic intermittent hypoxia [7,8]. It has been reported that Ca2+ handling ability was decreased in hypoxic heart, and melatonin administration alleviated oxidative stress and Ca2+ overload in the cardiomyocytes of hypoxic rats [9]. Moreover, cardiac hypertrophy and LDH release were significantly lowered by melatonin treatment in the hypoxic rats. Several studies demonstrated that sustained severe hypoxia is detrimental [22-24]. A recent study reported that 24 h severe hypoxia (FiO2=0.09) disrupts circadian rhythm of systolic blood pressure, temperature, heart rate and activity in mice [25]. Like previous results, we report that melatonin exerted protective effects in hypoxia induced myocardial injury. Previous studies have shown that hypoxia increases apoptosis in cardiomyocytes [1,2,13]. A recent study has reported that pro-apoptotic proteins were increased and cardioprotective molecules were decreased in mice subjected to chronic hypoxia treatment (FiO2=8, 10 days) [23], confirming that apoptosis is increased in chronic hypoxia. Consistent with this study, we found that 4 weeks hypoxia treatment (FiO2=10) increased cardiac apoptosis. Apoptosis has been demonstrated to play an important role in hypoxic cardiomyocytes [1]. The balance between the pro-apoptotic (Bax) and anti-apoptotic (Bcl2) protein expression determines whether the cells either undergo apoptosis or survive. This study determined that melatonin could decrease cell death by downregulating the pro-apoptotic cleaved caspase 3 and Bax proteins. Melatonin upregulated Bcl-2 expression, which was downregulated by hypoxia.
The PI3K/Akt pathway has been proven to be cardioprotective [11]. Moreover, when Akt is activated, it may exert its anti-apoptotic effects. In the present study, the phosphorylated PI3K and Akt increased in cardiomyocytes treated with melatonin, and hypoxia-induced apoptosis was reduced. Furthermore, our study showed that the activation of AKT was required for the cardioprotection of melatonin, in that inhibition of PI3K/Akt by LY294002 significantly decreased the anti-apoptotic effects of melatonin on hypoxic cardiomyocytes. Our results showed that the protective effects of melatonin in inhibiting hypoxia-induced apoptosis is at least partially mediated by the PI3K/Akt signaling pathway.
Based on our results, we speculated that melatonin protects the cardiomyocytes from hypoxia induced apoptosis are likely partly mediated through activating PI3K/Akt signaling pathway. The findings indicate that melatonin may have therapeutic value in protecting cardiomyocytes against hypoxic injury.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No: 81370004, 81270228, and 81471408).
Disclosure of conflict of interest
None.
References
- 1.He S, Liu P, Jian Z, Li J, Zhu Y, Feng Z, Xiao Y. miR-138 protects cardiomyocytes from hypoxia-induced apoptosis via MLK3/JNK/c-jun pathway. Biochem Biophys Res Commun. 2013;441:763–769. doi: 10.1016/j.bbrc.2013.10.151. [DOI] [PubMed] [Google Scholar]
- 2.Kanazawa H, Imoto K, Okada M, Yamawaki H. Canstatin inhibits hypoxia-induced apoptosis through activation of integrin/focal adhesion kinase/Akt signaling pathway in H9c2 cardiomyoblasts. PLoS One. 2017;12:e0173051. doi: 10.1371/journal.pone.0173051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García JJ, López-Pingarrón L, Almeida-Souza P, Tres A, Escudero P, García-Gil FA, Tan DX, Reiter RJ, Ramírez JM, Bernal-Pérez M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J Pineal Res. 2014;56:225–237. doi: 10.1111/jpi.12128. [DOI] [PubMed] [Google Scholar]
- 4.Patel R, Rathwa N, Palit SP, Ramachandran AV, Begum R. Association of melatonin &MTNR1B variants with type 2 diabetes in gujarat population. Biomed Pharmacother. 2018;103:429–434. doi: 10.1016/j.biopha.2018.04.058. [DOI] [PubMed] [Google Scholar]
- 5.Liu LF, Qin Q, Qian ZH, Shi M, Deng QC, Zhu WP, Zhang H, Tao XM, Liu Y. Protective effects of melatonin on ischemia-reperfusion induced myocardial damage and hemodynamic recovery in rats. Eur Rev Med Pharmacol Sci. 2014;18:3681–3686. [PubMed] [Google Scholar]
- 6.Yu L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai M, Pei H, Wang X, Zhang H, Meng Q, Zhang Y, Yu S, Duan W. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res. 2014;57:228–238. doi: 10.1111/jpi.12161. [DOI] [PubMed] [Google Scholar]
- 7.Yeung HM, Hung MW, Lau CF, Fung ML. Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J Pineal Res. 2015;58:12–25. doi: 10.1111/jpi.12190. [DOI] [PubMed] [Google Scholar]
- 8.Xie S, Deng Y, Pan YY, Wang ZH, Ren J, Guo XL, Yuan X, Shang J, Liu HG. Melatonin protects against chronic intermittent hypoxia-induced cardiac hypertrophy by modulating autophagy through the 5’ adenosine monophosphate-activated protein kinase pathway. Biochem Biophys Res Commun. 2015;464:975–981. doi: 10.1016/j.bbrc.2015.06.149. [DOI] [PubMed] [Google Scholar]
- 9.Yeung HM, Hung MW, Fung ML. Melatonin ameliorates calcium homeostasis in myocardial and ischemia-reperfusion injury in chronically hypoxic rats. J Pineal Res. 2008;45:373–382. doi: 10.1111/j.1600-079X.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 10.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 13.Lin KH, Kuo WW, Jiang AZ, Pai P, Lin JY, Chen WK, Day CH, Shen CY, Padma VV, Huang CY. Tetramethylpyrazine ameliorated hypoxia-induced myocardial cell apoptosis via HIF-1alpha/JNK/p38 and IGFBP3/BNIP3 inhibition to upregulate PI3K/Akt survival signaling. Cell Physiol Biochem. 2015;36:334–344. doi: 10.1159/000374076. [DOI] [PubMed] [Google Scholar]
- 14.Zhai M, Liu Z, Zhang B, Jing L, Li B, Li K, Chen X, Zhang M, Yu B, Ren K, Yang Y, Yi W, Yang J, Liu J, Yi D, Liang H, Jin Z, Reiter RJ, Duan W, Yu S. Melatonin protects against the pathological cardiac hypertrophy induced by transverse aortic constriction through activating PGC-1β: In vivo and in vitro studies. J Pineal Res. 2017;63 doi: 10.1111/jpi.12433. [DOI] [PubMed] [Google Scholar]
- 15.Zhai M, Li B, Duan W, Jing L, Zhang B, Zhang M, Yu L, Liu Z, Yu B, Ren K, Gao E, Yang Y, Liang H, Jin Z, Yu S. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017;63 doi: 10.1111/jpi.12419. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Yang Q, Zhu LH, Liu J, Deng KQ, Zhu XY, Liu Y, Gong J, Zhang P, Li S Xia H, She ZG. Carboxyl-terminal modulator protein ameliorates pathological cardiac hypertrophy by suppressing the protein kinase B signaling pathway. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu L, Liang H, Lu Z, Zhao G, Zhai M, Yang Y, Yang J, Yi D, Chen W, Wang X, Duan W, Jin Z, Yu S. Membrane receptor-dependent Notch1/Hes1 activation by melatonin protects against myocardial ischemia-reperfusion injury: in vivo and in vitro studies. J Pineal Res. 2015;59:420–433. doi: 10.1111/jpi.12272. [DOI] [PubMed] [Google Scholar]
- 18.Mogulkoc R, Baltaci AK, Oztekin E, Aydin L, Sivrikaya A. Melatonin prevents oxidant damage in various tissues of rats with hyperthyroidism. Life Sci. 2006;79:311–315. doi: 10.1016/j.lfs.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Sehirli AO, Koyun D, Tetik S, Ozsavci D, Yiginer O, Cetinel S, Tok OE, Kaya Z, Akkiprik M, Kilic E, Şener G. Melatonin protects against ischemic heart failure in rats. J Pineal Res. 2013;55:138–148. doi: 10.1111/jpi.12054. [DOI] [PubMed] [Google Scholar]
- 20.Sallinen P, Manttari S, Leskinen H, Vakkuri O, Ruskoaho H, Saarela S. Long-term postinfarction melatonin administration alters the expression of DHPR, RyR2, SERCA2, and MT2 and elevates the ANP level in the rat left ventricle. J Pineal Res. 2008;45:61–69. doi: 10.1111/j.1600-079X.2008.00556.x. [DOI] [PubMed] [Google Scholar]
- 21.Hu J, Zhang L, Yang Y, Guo Y, Fan Y, Zhang M, Man W, Gao E, Hu W, Reiter RJ, Wang H, Sun D. Melatonin alleviates postinfarction cardiac remodeling and dysfunction by inhibiting Mst1. J Pineal Res. 2017;62 doi: 10.1111/jpi.12368. [DOI] [PubMed] [Google Scholar]
- 22.Sheedy W, Thompson JS, Morice AH. A comparison of pathophysiological changes during hypobaric and normobaric hypoxia in rats. Respiration. 1996;63:217–222. doi: 10.1159/000196548. [DOI] [PubMed] [Google Scholar]
- 23.Vigano A, Vasso M, Caretti A, Bravata V, Terraneo L, Fania C, Capitanio D, Samaja M, Gelfi C. Protein modulation in mouse heart under acute and chronic hypoxia. Proteomics. 2011;11:4202–4217. doi: 10.1002/pmic.201000804. [DOI] [PubMed] [Google Scholar]
- 24.Simpson JA, Iscoe S. Hypoxia, not hypercapnia, induces cardiorespiratory failure in rats. Respir Physiol Neurobiol. 2014;196:56–62. doi: 10.1016/j.resp.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Allwood MA, Edgett BA, Eadie AL, Huber JS, Romanova N, Millar PJ, Brunt KR, Simpson JA. Moderate and severe hypoxia elicit divergent effects on cardiovascular function and physiological rhythms. J Physiol. 2018;596:3391–3410. doi: 10.1113/JP275945. [DOI] [PMC free article] [PubMed] [Google Scholar]