Abstract
Objective: To investigate the potential prognostic value of osteopontin (OPN) in hepatocellular carcinoma (HCC) patients after hepatectomy. Methods: A total of 384 HCC specimens with paired adjacent non-tumorous tissues from liver resections were collected to construct a tissue microarray for immunohistochemistry (IHC) analysis to detect the expression of OPN. OPN expression was semi-quantified with scores according to IHC staining. The potential correlations between OPN expression and neoplastic features of HCC were analyzed. The survivals in patients stratified with different OPN expression levels were calculated and compared. Uni- and multi-variate analysis were conducted to identify the potential prognostic value of OPN in HCC patients received liver resections. Results: The OPN in HCC tissues was 0.82 ± 0.68, significantly higher than that in matched nontumorous tissue (0.45 ± 0.59; P < 0.001). Higher OPN expression was associated with vascular invasion (P = 0.019). Patients with high OPN expression had worse overall survival (P < 0.001), disease-free survival (P = 0.045) and higher probability of recurrence (P = 0.048), compared with the patients of low OPN expression. Multivariate analysis indicated that OPN expression was an independent risk factor for overall survival (P = 0.045), disease-free survival (P = 0.048), and HCC recurrence (P = 0.044). Conclusion: OPN expression was elevated in HCC tissues. High OPN expression was correlated with HCC vascular invasion. High OPN expression in HCC tissues is an independent factor for DFS and OS. OPN could be a predictor of HCC patients and a potential therapeutic target for the disease.
Keywords: Osteopontin, prognosis, hepatocellular carcinoma, biomarke
Introduction
HCC is a common malignancy in liver ranked as the third highest mortality among all malignancies [1,2], especially in Asian-Pacific region, which is a high prevalence region for hepatitis B virus infection [3-5]. Although surgical treatment had improved the prognosis of HCC patients, the overall survival of HCC is still poor for metastasis. The recurrence rate of HCC after liver resection is still as high as 60% to 70% [6,7]. Thus, early diagnosis is critical for potential curative resections to acquire better prognosis of HCC.
Osteopontin (OPN) is a secreted calcium-binding phosphorylated glycoprotein [8]. Studies found that OPN expression increased in various tumors, includes gastric cancer, rectal cancer, ovarian cancer, etc. [9-11]. High OPN expression is correlated with tumor metastasis and recurrence [12,13]. Several studies indicated that OPN can be implicated for tumor diagnosis and prognosis prediction [14,15]. However, the OPN expression in HCC and its relationship with tumor invasion and metastasis remained to be explored.
In our study, we investigated the role of OPN in human HCC. We detected OPN expression in HCC tissues, and analyzed the potential association between OPN expression and prognosis of HCC patients.
Subjects and methods
Subjects
We collected a total of 384 paraffin-embedded HCC tissues resected in HCC liver surgeries from January 2002 to December 2012. None of the patients received any chemotherapy or radiotherapy before surgeries. The follow-up period was defined as the time interval between the date of operation and the date of death or the last follow-up. The study was approved by the medical ethics committee of Tianjin First Central Hospital, First Central Clinical College of Tianjin Medical University. Since all specimens used were anonymous, the Medical Ethics Committee exempted patients from the need for informed consent.
Tissue microarray construction and immunohistochemistry
We constructed the tissue microarray (TMA). Each tissue core (diameter: 0.6 mm) is perforated and re-embedded from the labeled area by using a tissue array (MiniCore; Excilone, UK). The specimens were fixed with 4% paraformaldehyde. The biotin blocking Kit (Dark, Germany) was closed. After closure, the tissues were incubated with OPN antibodies (ab8448, 1:1000; Abcam, Cambridge, MA, USA) in a humid chamber at 4°C overnight. The tissues were washed with PBS three times and incubated with biotinylated goat anti-rabbit antibodies for one hour. Finally, the slices were stained with hematoxylin and observed under a microscope.
Semi-quantitative IHC was used to detect OPN protein expression levels according to the following criteria: “0” (negative staining), “1” (weak staining), “2” (moderate staining) and “3” (strong staining). The final score was calculated as the percentage of positive expression multiplied by the intensity score. The median IHC score was used as a cut-off value for high and low levels of expression.
Statistical analysis
Statistical analysis was performed using SPSS software (version 13; SPSS Inc., Chicago, IL, USA). Student’s t test and Chi square test were used to examine the correlation between OPN expression and clinical and pathological variables. The Kaplan-Meier method (logarithmic rank test) was used to construct the survival curve. Multivariate Cox proportional hazards regression model was used to assess the independence of OPN in prediction results. A P value < 0.05 was considered significant.
Results
Expression of OPN in the HCC TMA
We used an HCC TMA (n = 384) to detect OPN expression. OPN was expressed mainly in the cytoplasm of HCC cells (Figure 1). The OPN IHC score for HCC tissue was 0.82 ± 0.68, significantly higher than that in matched nontumorous tissue (0.45 ± 0.59; P < 0.001) (Figure 2).
Figure 1.
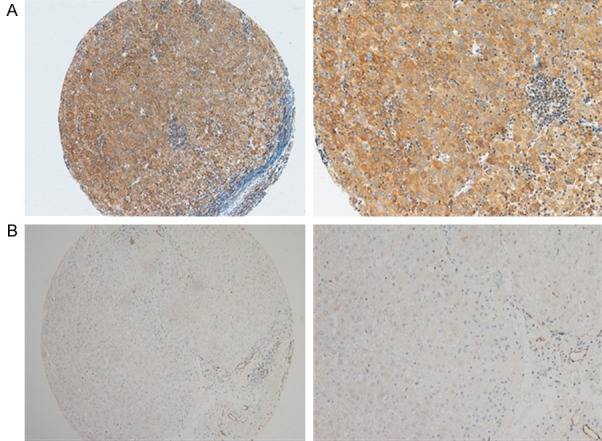
Immunohistochemical staining of osteopontin. OPN was expressed mainly in the cytoplasm of HCC cells. Representative images of hepatocellular carcinoma (HCC) tissues showing OPN expression (A). Representative images of OPN expression in a non-tumorous sample (B) (Left panel: magnification 100X; Right panel: magnification 400X).
Figure 2.
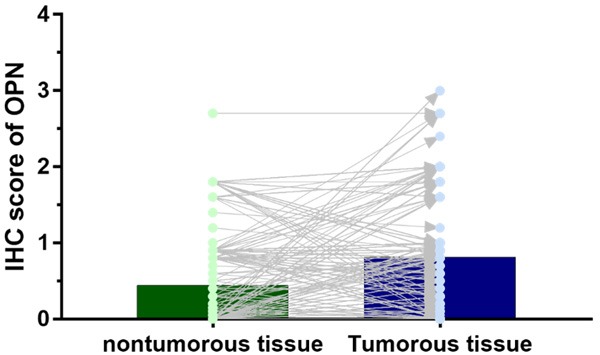
Expression of OPN in HCC tissues. The OPN IHC score for HCC tissue was 0.82 ± 0.68, being significantly higher than that for matched nontumorous tissue (0.45 ± 0.59; P < 0.001).
Association of cytoplasmic OPN with HCC clinical features
To explore the potential clinical significance of OPN in HCC, the relationship between OPN and the clinical features of patients with HCC was analyzed. Using the OPN median IHC score (0.8) of the tumorous tissues, patients were divided into high and low OPN expression group. Patients with high OPN expression had more vascular invasion (P = 0.019), as shown in Table 1.
Table 1.
Clinical variables difference in different OPN expression
| Variable | OPN expression | P value | |
|---|---|---|---|
|
| |||
| Low expression | High expression | ||
| Sample size | 254 | 130 | |
| Age, years | 0.115 | ||
| > 50 | 123 (48.4%) | 74 (56.9%) | |
| ≤ 50 | 131 (51.6%) | 56 (43.1%) | |
| Gender | 0.709 | ||
| Male | 226 (89.0%) | 114 (87.7%) | |
| Female | 28 (11.0%) | 16 (12.3%) | |
| AFP, ng/mL | 0.530 | ||
| < 20 | 62 (24.4%) | 28 (21.5%) | |
| ≥ 20 | 192 (75.6%) | 102 (78.5%) | |
| Cirrhosis | 0.845 | ||
| Yes | 213 (83.9%) | 108 (83.1%) | |
| No | 41 (16.1%) | 22 (16.9%) | |
| Tumor size, cm | 0.268 | ||
| < 5 | 53 (20.9%) | 21 (16.2%) | |
| ≥ 5 | 201 (79.1%) | 109 (83.8%) | |
| Differentiation | 0.278 | ||
| Well-moderate | 33 (13.0%) | 12 (9.2%) | |
| Poor-undifferentiated | 221 (87.0%) | 118 (90.8%) | |
| TNM stage | 0.790 | ||
| I-II | 115 (45.3%) | 57 (43.8%) | |
| III-IV | 139 (54.7%) | 73 (56.2%) | |
| Vascular invasion | 0.019 | ||
| Yes | 41 (16.1%) | 34 (26.2%) | |
| No | 213 (83.9%) | 96 (73.8%) | |
Association of OPN expression with survival in patients with HCC
To determine the prognostic effect of OPN expression on patients with HCC, we conducted Kaplan-Meier survival analysis to compare both the DFS and OS between patients with high OPN expression and low OPN expression. For the patients with high OPN expression, Kaplan-Meier analysis revealed that they had worse disease-free survival (P = 0.045) and overall survival (P < 0.001), in comparison with those of patients with low OPN expression. Moreover, the probability of recurrence in patients with high OPN expression was also higher than that of patients with low OPN expression (P = 0.048), as shown in Figure 3.
Figure 3.
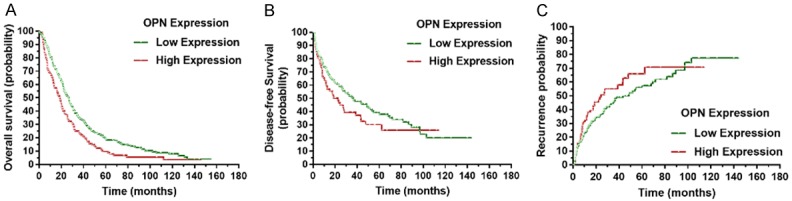
The overall survival, disease-free survival, and recurrence between groups with high and low OPN expression. Kaplan-Meier analysis revealed significantly worse outcomes in terms of overall survival (P < 0.001). In addition, compared toHCC patients with low OPN expression, those with high OPN expression had a significantly worse disease-free survival (P = 0.045) and a higher probability of recurrence (P = 0.048).
Univariate and multivariate analyses of prognostic variables in HCC
To evaluate whether OPN expression was an independent risk factor for outcomes in HCC, both univariate and multivariate analyses were conducted. Univariate analysis indicated that tumor differentiation, TNM stage, vascular invasion and OPN expression were prognostic factors for overall survival in patients with HCC. In the multivariate analysis, vascular invasion (P < 0.001) and OPN expression (P = 0.045) were found to be independent prognostic variables for overall survival (Table 2).
Table 2.
Univariate and multivariate analyses of variables for overall survival
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | 1.004 | 0.812-1.242 | 0.970 | |||
| Sex | 0.821 | 0.578-1.165 | 0.268 | |||
| AFP | 1.247 | 0.973-1.598 | 0.081 | |||
| Cirrhosis | 0.873 | 0.655-1.163 | 0.352 | |||
| Tumor size, cm | 1.252 | 0.955-1.642 | 0.104 | |||
| Differentiation | 1.596 | 1.146-2.222 | 0.001 | |||
| TNM stage | 1.466 | 1.182-1.820 | 0.001 | |||
| Vascular invasion | 2.514 | 1.925-3.282 | < 0.001 | 1.939 | 1.437-2.616 | < 0.001 |
| OPN expression | 1.460 | 1.167-1.827 | 0.001 | 1.272 | 1.006-1.609 | 0.045 |
Abbreviations: OPN: Osteopontin; AFP: alpha-fetoprotein.
We also explored the risk factors associated with disease-free survival (Table 3) and HCC recurrence (Table 4). Univariate analysis showed that age, serum AFP level, vascular invasion, and OPN expression were risk factors associated with disease-free survival. In the multivariate analysis, vascular invasion (P = 0.045) and OPN expression (P = 0.048) were independent risk factors associated with disease-free survival. For HCC recurrence, serum AFP (P = 0.031) and OPN expression (P = 0.044) were independent risk factors.
Table 3.
Univariate and multivariate analyses for disease-free survival
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | 0.931 | 0.698-1.241 | 0.625 | |||
| Sex | 0.732 | 0.450-1.192 | 0.210 | |||
| AFP | 1.413 | 1.001-1.995 | 0.049 | |||
| Cirrhosis | 0.907 | 0.619-1.328 | 0.616 | |||
| Tumor size, cm | 0.940 | 0.668-1.322 | 0.722 | |||
| Differentiation | 1.187 | 0.788-1.789 | 0.411 | |||
| TNM stage | 0.863 | 0.647-1.151 | 0.315 | |||
| Vascular invasion | 1.384 | 1.063-1.910 | 0.027 | 1.365 | 1.076-2.219 | 0.045 |
| OPN expression | 1.349 | 1.095-1.830 | 0.034 | 1.317 | 1.006-1.609 | 0.048 |
Abbreviations: OPN: Osteopontin; AFP: alpha-fetoprotein.
Table 4.
Univariate and multivariate analyses of variables for recurrence
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | 0.992 | 0.979-1.006 | 0.260 | |||
| Sex | 0.590 | 0.339-1.026 | 0.061 | |||
| AFP | 1.484 | 1.062-1.990 | 0.020 | 1.416 | 1.070-2.067 | 0.031 |
| Cirrhosis | 0.861 | 0.566-1.312 | 0.487 | |||
| Tumor size, cm | 0.818 | 0.565-1.184 | 0.287 | |||
| Differentiation | 0.988 | 0.629-1.554 | 0.959 | |||
| TNM stage | 0.707 | 0.498-1.523 | 0.152 | |||
| Vascular invasion | 1.463 | 0.906-2.363 | 0.120 | |||
| OPN expression | 1.310 | 1.040-1.825 | 0.031 | 1.349 | 1.059-1.830 | 0.044 |
Abbreviations: OPN: Osteopontin; AFP: alpha-fetoprotein.
Subgroup analyses of the prognostic value of OPN expression in the cytoplasm in HCC
A stratified survival analysis with the clinical characteristics of patients was conducted to further reveal the prognostic significance of OPN expression among patients with HCC. Kaplan-Meier survival analysis showed that OPN expression was associated with overall survival in both younger and older HCCs (younger HCCs: P = 0.023; older HCCs: P = 0.014), in serum AFP-positive and -negative HCCs (AFP-negative HCCs: P = 0.005; AFP-positive HCCs: P = 0.044), TNM stage I-II HCCs and stage III-IV HCCs (stage I-II HCCs: P = 0.010; stage III-IV HCCs: P = 0.042), as shown in Figure 4.
Figure 4.
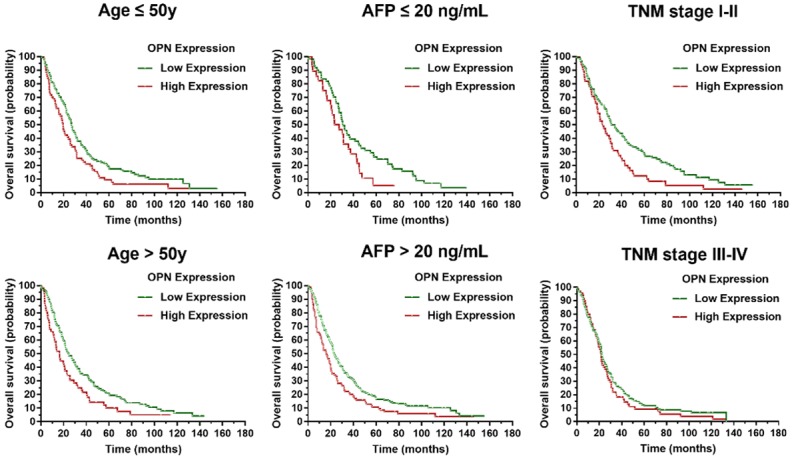
Subgroup analysis of OPN prognostic value in HCC. OPN expression was associated with overall survival in both younger and older age HCCs (younger HCCs: P = 0.023; older HCCs: P = 0.014), in serum AFP-positive and -negative HCCs (AFP-negative HCCs: P = 0.005; AFP-positive HCCs: P = 0.044), TNM stage I-II HCCs and stage III-IV HCCs (stage I-II HCCs: P = 0.010; stage III-IV HCCs: P = 0.042).
Discussion
HCC is the most common malignant tumor that occurs in the liver [7,16,17]. The pathogenesis of HCC is complex with heterogeneity due to the various etiologies including chronic virus infection, alcohol abuse, and metabolic abnormalities [18-21]. In vitro studies showed that OPN was associated with the proliferation, invasion, and metastasis of HCC [22]. According to our results, we confirm that the expression of OPN is elevated in HCC tissues. In addition, we found that the OPN expression is significantly associated with vascular invasion. Moreover, high OPN expression in HCC tissues is an independent risk factor for both DFS and OS. Our studies indicate that OPN can be a potential therapeutic target and a promising biomarker for prognosis of HCC.
According to previous studies, OPN is involved in the infiltration, distant metastasis, and recurrence of several malignant tumors [8,10,13]. There are two important types of receptors for OPN [23,24]. One is the integrin receptor, which recognizes the RGD sequence binding site, thereby promoting the formation of cell-to-cell adhesion and enhancing the invasiveness of tumor cells. The other one is hyaluronic acid receptor (CD44), which is one of the chemokines that help tumor cell adhesion and is closely related to tumor invasion and metastasis. Bandiera et al found that the ovarian cancer cell lines with high expression of OPN by transfections would acquire higher invasion ability [25]. OPN can induce local neovascularization and inhibit the apoptosis of tumor cells induced by modifying the extracellular microenvironment, thus playing a critical role in tumor invasion and metastasis [10,23]. In one study of breast cancer, OPN induced the expression of intercellular adhesion molecule-1, thereby activating the NF-κB pathway, enhancing the transcription of MMP-9, and promoting the growth and metastasis of tumor cells [26]. Kumar et al [27] also proved that OPN can activate the ERK2 pathway to enhance the proliferation and metastasis ability of tumor cells, change the composition of extracellular matrix and weaken the adhesion to tumor cells. In our study, we found that highly expression of OPN in HCC is associated with vascular invasion, which indicated that OPN might be involved in the metastasis of HCC. However, further study is needed.
High serum OPN level is also correlated with the prognosis of HCC. El-Din et al [28] showed that OPN in serum of patients with HCC is related to tumor differentiation, TNM stage and tumor size. According our results, we found that OPN expression in HCC tissue had prognostic value. Although AFP is a widely used prognostic factor for HCC, AFP in some HCC patients is negative [29,30]. According to our results, OPN can be used as a prognostic indicator regardless of AFP status. Therefore, OPN alone or in combination with AFP can be a better predictor for prognosis of HCC, which will be our next study.
There are some limitations to this study. First, the sample size is relatively small, which restricted our further analysis. The data collected in this study came from a single center and may lead to some enrollment bias. A multicenter prospective study is needed for further validation of the role of OPN in HCC and its potential prognosis prediction value.
In summary, our study results demonstrate that OPN plays a critical role in the development of HCC. Our data revealed that OPN expression was increased in HCC samples, and such elevation was significantly correlated with vascular invasion. High OPN expression was significantly correlated with poor prognosis of HCC patients after liver resection. Our data suggests that OPN is a promising biomarker for the prognosis of patients with HCC and a potential target in HCC treatment.
Acknowledgements
The Institutional Review Board of Tianjin First Central Hospital, First Central Clinical College of Tianjin Medical University had approved this study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and the Helsinki Declaration of 1975, as revised in 2008.
Disclosure of conflict of interest
None.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 3.Cai S, Cao J, Yu T, Xia M, Peng J. Effectiveness of entecavir or telbivudine therapy in patients with chronic hepatitis B virus infection pre-treated with interferon compared with de novo therapy with entecavir and telbivudine. Medicine (Baltimore) 2017;96:e7021. doi: 10.1097/MD.0000000000007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao YB, Cai SH, Liu LL, Yang X, Yun JP. Decreased expression of peroxisome proliferator-activated receptor alpha indicates unfavorable outcomes in hepatocellular carcinoma. Cancer Manag Res. 2018;10:1781–1789. doi: 10.2147/CMAR.S166971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng J, Cai S, Liu J, Xue X, Wu X, Zheng C. Dynamic changes in liver stiffness measured by transient elastography predict clinical outcomes among patients with chronic hepatitis B. J Ultrasound Med. 2017;36:261–268. doi: 10.7863/ultra.15.12054. [DOI] [PubMed] [Google Scholar]
- 6.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, Khin MW, Koo WH. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian oncology summit 2009. Lancet Oncol. 2009;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 8.Wei R, Wong J, Kwok HF. Osteopontin -- a promising biomarker for cancer therapy. J Cancer. 2017;8:2173–2183. doi: 10.7150/jca.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastos A, Blunck CB, Emerenciano M, Gimba E. Osteopontin and their roles in hematological malignancies: splice variants on the new avenues. Cancer Lett. 2017;408:138–143. doi: 10.1016/j.canlet.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Castello LM, Raineri D, Salmi L, Clemente N, Vaschetto R, Quaglia M, Garzaro M, Gentilli S, Navalesi P, Cantaluppi V, Dianzani U, Aspesi A, Chiocchetti A. Osteopontin at the crossroads of inflammation and tumor progression. Mediators Inflamm. 2017;2017:4049098. doi: 10.1155/2017/4049098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018;59:17–24. doi: 10.1016/j.clinbiochem.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Briones-Orta MA, Avendano-Vazquez SE, Aparicio-Bautista DI, Coombes JD, Weber GF, Syn WK. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochim Biophys Acta. 2017;1868:93–108. doi: 10.1016/j.bbcan.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Ramchandani D, Weber GF. Interactions between osteopontin and vascular endothelial growth factor: implications for cancer. Biochim Biophys Acta. 2015;1855:202–222. doi: 10.1016/j.bbcan.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Hu ZD, Wei TT, Yang M, Ma N, Tang QQ, Qin BD, Fu HT, Zhong RQ. Diagnostic value of osteopontin in ovarian cancer: a meta-analysis and systematic review. PLoS One. 2015;10:e126444. doi: 10.1371/journal.pone.0126444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpinsky G, Fatyga A, Krawczyk MA, Chamera M, Sande N, Szmyd D, Izycka-Swieszewska E, Bien E. Osteopontin: its potential role in cancer of children and young adults. Biomark Med. 2017;11:389–402. doi: 10.2217/bmm-2016-0308. [DOI] [PubMed] [Google Scholar]
- 16.Cai SH, Lu SX, Liu LL, Zhang CZ, Yun JP. Increased expression of hepatocyte nuclear factor 4 alpha transcribed by promoter 2 indicates a poor prognosis in hepatocellular carcinoma. Therap Adv Gastroenterol. 2017;10:761–771. doi: 10.1177/1756283X17725998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou H, Cai S, Liu Y, Xia M, Peng J. A noninvasive diagnostic model to assess nonalcoholic hepatic steatosis in patients with chronic hepatitis B. Therap Adv Gastroenterol. 2017;10:207–217. doi: 10.1177/1756283X16681707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai SH, Lv FF, Zhang YH, Jiang YG, Peng J. Dynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHB. BMC Infect Dis. 2014;14:85. doi: 10.1186/1471-2334-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Cai S, Li Z, Zheng C, Xue X, Zeng J, Peng J. Potential effects of telbivudine and entecavir on renal function: a systematic review and meta-analysis. Virol J. 2016;13:64. doi: 10.1186/s12985-016-0522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai S, Ou Z, Liu D, Liu L, Liu Y, Wu X, Yu T, Peng J. Risk factors associated with liver steatosis and fibrosis in chronic hepatitis B patient with component of metabolic syndrome. United European Gastroenterol J. 2018;6:558–566. doi: 10.1177/2050640617751252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai S, Yu T, Jiang Y, Zhang Y, Lv F, Peng J. Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week result. Clin Exp Med. 2016;16:429–436. doi: 10.1007/s10238-015-0373-2. [DOI] [PubMed] [Google Scholar]
- 22.Wen Y, Jeong S, Xia Q, Kong X. Role of osteopontin in liver diseases. Int J Biol Sci. 2016;12:1121–1128. doi: 10.7150/ijbs.16445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Regan A, Berman JS. Osteopontin: a key cytokine in cell-mediated and granulomatous inflammation. Int J Exp Pathol. 2000;81:373–390. doi: 10.1046/j.1365-2613.2000.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 25.Bandiera E, Zanotti L, Fabricio AS, Bucca E, Squarcina E, Romani C, Tassi R, Bignotti E, Todeschini P, Tognon G, Romagnolo C, Gion M, Sartori E, Maggino T, Pecorelli S, Ravaggi A. Cancer antigen 125, human epididymis 4, kallikrein 6, osteopontin and soluble mesothelin-related peptide immunocomplexed with immunoglobulin M in epithelial ovarian cancer diagnosis. Clin Chem Lab Med. 2013;51:1815–1824. doi: 10.1515/cclm-2013-0151. [DOI] [PubMed] [Google Scholar]
- 26.Mi Z, Bhattacharya SD, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011;32:477–487. doi: 10.1093/carcin/bgr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Sharma P, Kumar D, Chakraborty G, Gorain M, Kundu GC. Functional characterization of stromal osteopontin in melanoma progression and metastasis. PLoS One. 2013;8:e69116. doi: 10.1371/journal.pone.0069116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Din BS, Elwan NM, Suliman GA, El-Shourbagy SH. Clinical significance of plasma osteopontin level in Egyptian patients with hepatitis C virus-related hepatocellular carcinoma. Arch Med Res. 2010;41:541–547. doi: 10.1016/j.arcmed.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Cai S, Li Z, Yu T, Xia M, Peng J. Serum hepatitis B core antibody levels predict HBeAg seroconversion in chronic hepatitis B patients with high viral load treated with nucleos(t)ide analogs. Infect Drug Resist. 2018;11:469–477. doi: 10.2147/IDR.S163038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim TS, Kim DY, Han KH, Kim HS, Shin SH, Jung KS, Kim BK, Kim SU, Park JY, Ahn SH. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol. 2016;51:344–353. doi: 10.3109/00365521.2015.1082190. [DOI] [PubMed] [Google Scholar]


