Abstract
The present study investigates the effects of perfluorooctanoic acid (PFOA) exposure on reproductive toxicity and uterine apoptosis in pregnant mice. Sixty pregnant mice were randomly divided into 6 groups (groups A, B, C, D, E and F). In control group (A), the mice received distilled water at 10 mg/kg body weight per day on gestation days (GD) 1 to 17. The mice in group B, C, D, E and F were treated with PFOA solution at 1, 5, 10, 20 and 40 mg/kg body weight respectively from GD1 to GD17. The mice were sacrificed on GD18. The distribution and expression of Fas, FasL, Bcl-2, Bax, and Caspase-3 in uterine cells were detected by immunohistochemistry. The apoptosis of uterine cells was detected by TdT-mediated dUTP Nick-End Labeling (TUNEL). Results showed that the expression of Fas, FasL, and Caspase 3 in uterus increased significantly after PFOA was applied. The expression of Bcl-2 was decreased significantly and the expression of Bax was increased significantly. The ratio of Bcl-2/Bax decreased significantly compared with the control group (P<0.01). PFOA exposure increased the number of apoptotic uterine cells in a dose-dependent manner. The results indicated that PFOA could accelerate the apoptosis of uterine cells, and lead to slow embryo development or abortion by regulating the expression of Fas, FasL, Bax, Bcl-2 and Caspase-3 in uterine cells.
Keywords: Perfluorooctanoic acid, pregnancy, mice, apoptosis
Introduction
Perfluorinated compounds (PFCs) are considered a new type of persistent organic pollutant in addition to the organochlorine pesticides, dioxins and polychlorinated biphenyls [1,2]. PFCs are very stable in physical and chemical properties and are characterized as lipophilic and hydrophobic. Thus, PFCs are widely used in civil and industrial applications [3,4] such as automobile inner decoration, inner wall painting, stain-resistant coatings for clothing and other textiles and in oil-resistant coatings for food wrapping materials [5], clothes, and furniture coatings, firefighting foams, paints, metal plating, aviation hydraulic fluids, lubricants and pesticides [6]. Because of massive usages of PFCs, existence of pollutants was detected in environmental media, crowds, wild animals, and even remote polar regions [7-10]. PFCs mainly accumulate in blood, liver, and kidney of living organisms due to their high water-solubility, in comparison with other well-studied organic pollutants that are of adipose-accumulative toxicity [11-13].
Perfluorooctanoic acid (PFOA) is one of the perfluorooctanoic compounds (PFCs), which exhibits serious hepatotoxicity, glucose metabolism disturbance, developmental toxicity, endocrine disruption, neurotoxicity, and reproductive toxicity through the enrichment of the food chain [14-18]. Maternal concentrations of PFOA during pregnancy have been previously associated with lower offspring birth weight in systematic reviews of human evidence [19]. PFOA exposure during pregnancy can lead to early miscarriage, reduced survival rate of newborns, or serious liver injury in pregnant mice [20,21]. Dixon [22] et al. found that mild pathologic changes appeared in mice uterus, cervix, and vagina after taking 0.01 mg/kg PFOA per day. PFOA has no anti-estrogenic potential. Some data indicate that PFOA does not activate mouse or human estrogen receptors (ER) [23]. With the wide application of PFOA, the pollution problem is becoming more serious worldwide. How PFOA causes toxicity is complex. Research data about causing reproductive toxicity are also not completely understood, and its complex mechanism process is unclear so far. In this study, the effect of PFOA exposure on reproductive toxicity and uterine apoptosis in pregnant mice were detected by immunohistochemistry and TUNEL, so as to provide basic scientific research data for effective control of PFOA and minimize the undesired impacts to the environment.
Materials and methods
Reagents
PFOA was purchased from Sigma Aldrich Corporation (Saint Louis, USA) with a purity of 99.2%. PFOA mother solution of 10 mg/ml was prepared with 100 mg PFOA dissolved in 10 ml distilled water and heated at 37°C. When used, PFOA stock solution was diluted with distilled water to the final concentrations of 0.1, 0.5, 1, 2 and 4 mg/ml, respectively.
Anti-FAS antibody, Anti-FasL (mouse) Monoclonal Antibody (A11), Anti-Bcl-2 Antibody, Anti-Bax Antibody, Anti-Caspase-3 antibody were purchased from Boster Biological Technology (Pleasanton, CA, USA), and ready-to-use SABC immunohistochemical kit from Boster Biological Technology Co Ltd (Wuhan, China). Metal Enhanced DAB Substrate Kit and Proteinase K were purchased from Pierce Biotechnology (Rockford, IL, USA). TUNEL test kit was purchased from Roche Company (Mannheim, Germany).
Treatment of animals
Eight-week-old female and male Kunming mice were obtained from the Sibeifu Animals Biotech Co. Ltd. (Beijing, China), license number SCXK (Beijing) 2011-0004. Animals were housed in polypropylene cages and provided pellet chow and tap water ad lib. The mice were acclimatized for one week in the laboratory under standard conditions of temperature (23±2°C), humidity (55±5%) and kept under a 12 h/12 h light-dark cycle. Female mice were mated with males overnight and the next morning was considered as gestation day (GD) 0 if a vaginal plug was detected. The pregnant mice were randomly divided into 6 groups of 10 mice in each group. The mice in group A were given distilled water at 10 ml/kg as the control group on GD 1-17; mice in groups B, C, D, E, and F received a gavage of PFOA solution respectively at 1, 5, 10, 20, and 40 mg/kg body weight (10 ml/kg) on GD 1-17. The body weight of each group was taken daily. All gravid mice were sacrificed by cervical dislocation at GD 18, and the liver and uterus samples collected. The uterine tissue was fixed in 4% paraformaldehyde solution. After 48 h immobilization, paraffin sections were prepared. All animals’ studies were approved by the Council for Animal Care in Hebei province.
Immunohistochemistry detection of Fas, FasL, Bax, Bcl-2 and caspase-3 in uterine cells
Sections routinely were dewaxed in gradient alcohols down to water, each gradient for 5 min. The sections were then brought to 3% H2O2 and allowed to react for 10 min at room temperature and wished 3 times with distilled water. SABC method was conducted according to the manufacturer’s instructions. At last the sections were visualized with DAB after washed with PBS for 4 times.
The expression of the target protein was observed under microscope (magnification 40× for the objective lens). The cells with brown granules were regarded as positive cells. Positive cells in campus visualis were counted and pooled from 50 fields.
TUNEL assay
The tissue sections were hydrated in gradient alcohols and incubated with 20 μg/ml Proteinase K at 37°C for 20 min, then washed with PBS twice. Then the TUNEL assay was carried out in accordance with the manufacturer’s instructions.
The images were collected under the microscope, 5 slices in each group and 8 fields in each section were selected, and the positive cells were counted. Each test consisted of 10 slices to be tested, two negative control sections and one positive control section.
Statistical analysis
The test data were recorded in SPSS 19.0 system and the results were expressed as average ± variance. Significant differences were compared among groups by one-way analysis of variance (ANOVA), and significant differences of embryo survival rate were compared by χ2 test. The significance levels were P<0.05 or P<0.01. The experimental data were recorded into GraphPad Prism 5.0 system for analyzing and drawing line chart and column chart.
Results
Body weight changes of pregnant mice exposed to PFOA
The body weight of the mice in the control group, 1 mg/kg (group B) and 5 mg/kg groups (group C) increased continuously during the period of GD1-17. In the 10 mg/kg (group D) and 20 mg/kg (group E) groups, the mice weight decreased suddenly, then gradually rose. Body weights of 40 mg/kg group (group F) began to decline from GD 5 until the end of pregnancy. See Figure 1.
Figure 1.
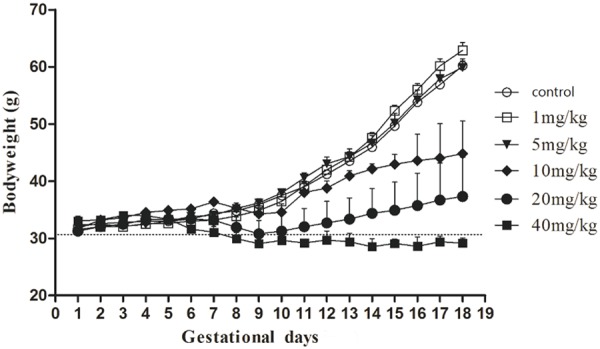
Body weight during pregnancy in each group.
Changes in liver index, uterine index, uterine weight, and embryo survival rate of pregnant mice exposed to PFOA
The liver index of 1 mg/kg PFOA treated group was increased significantly compared to that of the control group (P<0.05). The liver indices in the 5 mg/kg, 10 mg/kg, 20 mg/kg, and 40 mg/kg PFOA treated groups were increased significantly compared to the control group (P<0.01). There was no significant difference in uterine index, mean uterine weight, and mean embryo weight (P>0.05) in 1 mg/kg PFOA group. In the 5 mg/kg, 10 mg/kg, 20 mg/kg, and 40 mg/kg PFOA treatment groups, uterine indices were decreased significantly compared to that of the control group (P<0.01) and the average uterine weights were reduced significantly versus the control group (P<0.01). Compared with the control group, the average embryo weight in the 5 mg/kg PFOA group significantly decreased (P<0.05), and the average embryo weight in the 10 mg/kg, 20 mg/kg, and 40 mg/kg PFOA groups was significantly lower (P<0.01). All the embryos in the 40 mg/kg group were absorbed. Therefore, the average embryo survival rate was 0. The average survival rate of the other PFOA treated groups was lower than that of the control group in varying degrees. See Table 1.
Table 1.
Changes in liver index, uterus index, weight of uterus and embryo, and embryo survival rate
| Groups | Liver index (‰) | Uterus index (‰) | Uterus weight (g) | Embryo weight (g) | Embryo survival rate |
|---|---|---|---|---|---|
| A | 40.06±1.34 | 382.59±7.25 | 24.22±1.67 | 1.52±0.012 | 94.43% |
| B | 59.76±2.93* | 374.83±6.03 | 22.76±0.72 | 1.45±0.01 | 94.02% |
| C | 99.56±2.42** | 312.41±16.39** | 18.63±2.12** | 1.34±0.01* | 87.83% |
| D | 152.87±11.95** | 217.80±12.25** | 13.12±1.51** | 0.99±0.10** | 71.25%** |
| E | 166.83±12.33** | 170.583±19.57** | 10.79±1.17** | 0.85±0.05** | 67.19%** |
| F | 229.70±2.28** | 1.48±5.19** | 0.28±0.43** | 0.00±0.00** | 0.00%** |
Notes: Group A was the control group treated with distilled water. Mice in groups B, C, D, E and F were treated with 1 mg/kg, 5 mg/kg, 10 mg/kg, 20 mg/kg and 40 mg/kg PFOA respectively. Compared with the cntrol group.
Shows p<0.05;
Shows p<0.01.
Expression of fas, fasl and caspase-3 in the uterus
Fas (Figure 2), FasL (Figure 3) and Caspase-3 (Figure 4) in the uterus were positively stained with brown color or brown granules, mainly expressed in the decidual tissue, uterine ring muscle and longitudinal muscle; and there was less expression in intimal tissues. Positive particles were mainly expressed in the cytoplasm, and some cells stained positive in the nucleus.
Figure 2.
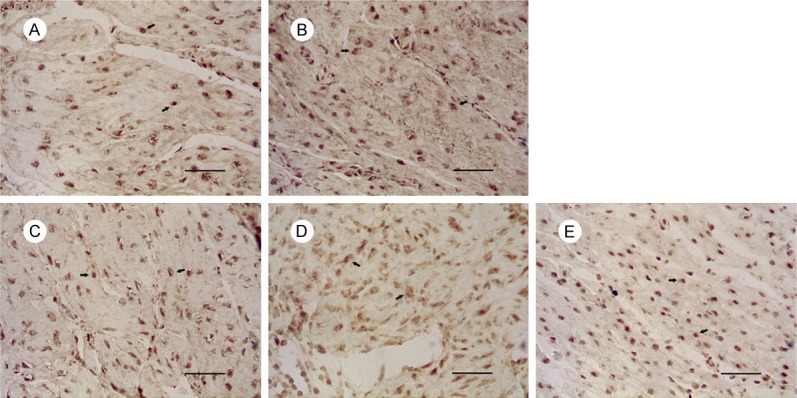
Expression of Fas in uterus of mice on gestational day 18. Notes: Expression of Fas in: (A) the control group, (B) 1 mg/kg PFOA group, (C) 5 mg/kg PFOA group, (D) 10 mg/kg PFOA group, (E) 20 mg/kg PFOA group. The arrows indicate positive expression. Bar = 50 μm.
Figure 3.
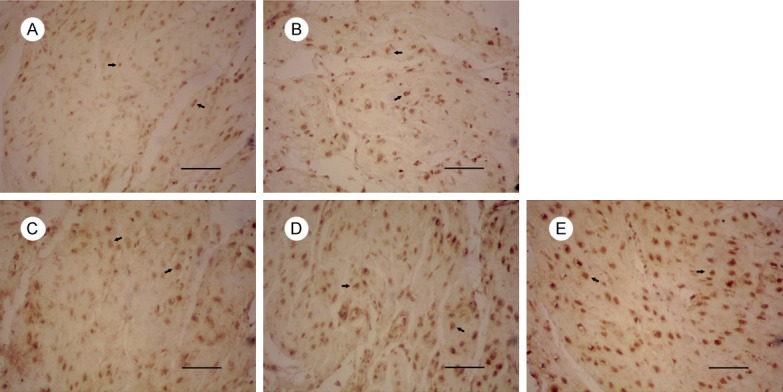
Expression of FasL in uterus of mice on gestational day 18. Notes: Expression of FasL in: (A) the control group, (B) 1 mg/kg PFOA group, (C) 5 mg/kg PFOA group, (D) 10 mg/kg PFOA group, (E) 20 mg/kg PFOA group. The arrows indicate positive expression. Bar = 50 μm.
Figure 4.
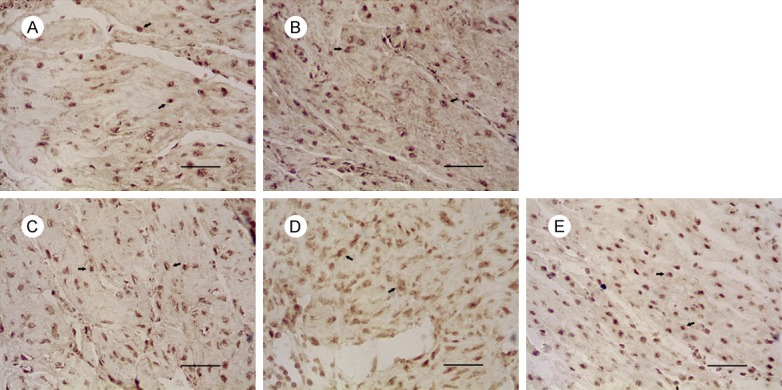
Expression of Caspase-3 in uterus of mice on gestational day 18. Notes: Expression of Caspase-3 in: (A) the control group, (B) 1 mg/kg PFOA group, (C) 5 mg/kg PFOA group, (D) 10 mg/kg PFOA group, (E) 20 mg/kg PFOA group. The arrows indicate positive expression. Bar = 50 μm.
The results are shown in Table 2. The expressions of Fas in 5 mg/kg, 10 mg/kg, and 20 mg/kg groups were increased significantly compared to control group (P<0.01). There was no significant difference between 10 mg/kg and 20 mg/kg group (P>0.05) in the Fas expression, and the positive expression of FasL and Caspase-3 in the uterus was significantly different between the 10 mg/kg and 20 mg/kg groups (P<0.01).
Table 2.
The expression of Fas, FasL and Caspase-3 in the uterus of mice in later pregnancy
| Groups | Fas | FasL | Caspase-3 |
|---|---|---|---|
| A | 90.48±7.06 | 82.83±11.39 | 89.14±9.20 |
| B | 93.53±11.91 | 95.48±6.30** | 97.38±9.78** |
| C | 105.59±11.48** | 106.65±10.85** | 111.35±12.13** |
| D | 126.00±13.71** | 115.38±8.81** | 125.06±13.97** |
| E | 126.10±12.66** | 124.50±10.97** | 132.06±10.66** |
Notes: Group A was the control group. Mice in groups B, C, D and E were treated with 1 mg/kg, 5 mg/kg, 10 mg/kg, 20 mg/kg PFOA respectively. Compared with the cntrol group.
Shows p<0.01.
Expression of Bax and Bcl-2 in mice uterus at late pregnancy
The expressions of Bax and Bcl-2 were observed in the uterus cells at late pregnancy. The positive results were expressed in decidual tissue and myometrium, which were located in the cell membrane and cytoplasm, showing brown or brown particles, as shown in Figures 5 and 6.
Figure 5.
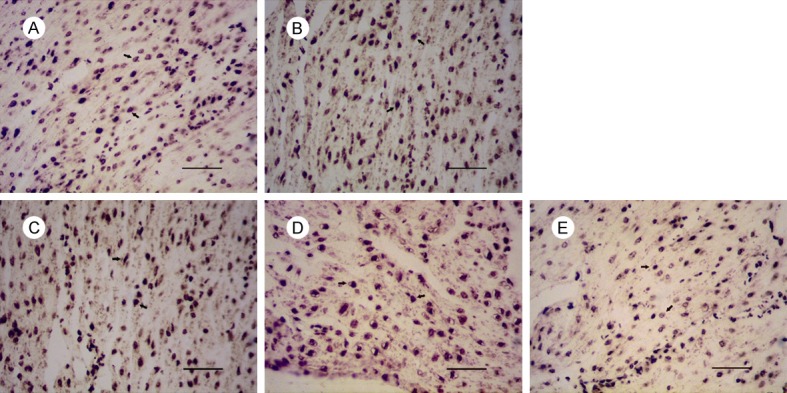
Expression of Bcl-2 in uterus of mice on gestational day 18. Notes: Expression of Bcl-2 in: (A) the control group, (B) 1 mg/kg PFOA group, (C) 5 mg/kg PFOA group, (D) 10 mg/kg PFOA group, (E) 20 mg/kg PFOA group. The arrows indicate positive expression. Bar = 50 μm.
Figure 6.
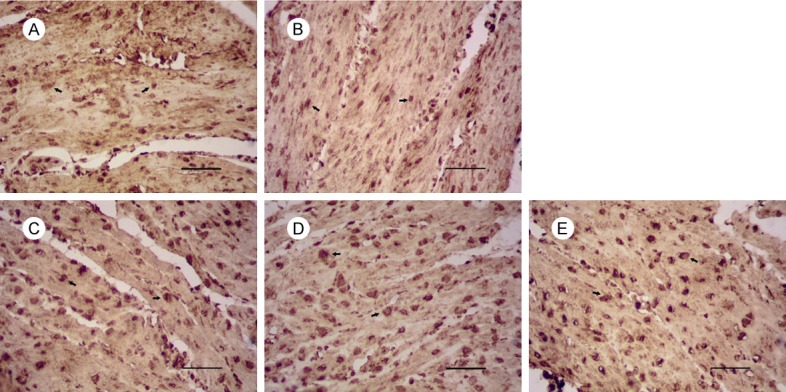
Expression of Bax in uterus of mice on gestational day 18. Notes: Expression of Bax in: (A) the control group, (B) 1 mg/kg PFOA grop, (C) 5 mg/kg PFOA group, (D) 10 mg/kg PFOA group, (E) 20 mg/kg PFOA group. The arrows indicate positive expression. Bar = 50 μm.
The results showed that thedegree of positive expression of Bcl-2 protein was significantly different between the two groupsexcept the 20 mg/kg group (P<0.01). There was no significant difference between the 10 mg/kg and 20 mg/kg groups (P>0.05). Compared with the control group, there was no significant difference in the expression of Bax protein in the 1 mg/kg group (P>0.05), but the difference was significant at 5 mg/kg, 10 mg/kg and 20 mg/kg (P<0.01). The ratio of Bcl-2/Bax in each PFOA-treated group was found to be significantly decreased compared with that of the control group (p<0.01), and the difference between each PFOA-treated group and the control group was significant (P<0.01) (Table 3).
Table 3.
The expression of Bcl-2 and Bax in the uterus of mice in late pregnancy
| Bcl-2 | Bax | Bcl-2/Bax | |
|---|---|---|---|
| A | 151.00±20.09 | 93.29±8.83 | 1.64±0.22 |
| B | 132.25±14.90** | 100.56±12.34* | 1.37±0.22** |
| C | 115.97±17.82** | 117.50±9.72** | 1.01±0.19** |
| D | 100.91±10.54** | 116.53±11.73** | 0.86±0.12** |
| E | 96.77±15.07** | 131.00±13.84** | 0.75±0.11** |
Notes: Group A was the control group. Mice in groups B, C, D, E were treated with 1 mg/kg, 5 mg/kg, 10 mg/kg, 20 mg/kg PFOA respectively. Compared with the cntrol group.
Shows p<0.05;
Shows p<0.01.
TUNEL detection of uterine cell apoptosis
Apoptosis of the uterine cells was detected by TUNEL method. Nuclei that were brown or dark brown were judged to be positive. The results showed that apoptotic cells were found in the uterus of each group. There were still some apoptotic cells in the uterus of the normal mice at day 18 of gestation, and the staining was shallow (Figure 7). The number of apoptotic cells increased in PFOA groups, and density of positive cells increased, showing dark brown in a dose-dependent manner.
Figure 7.
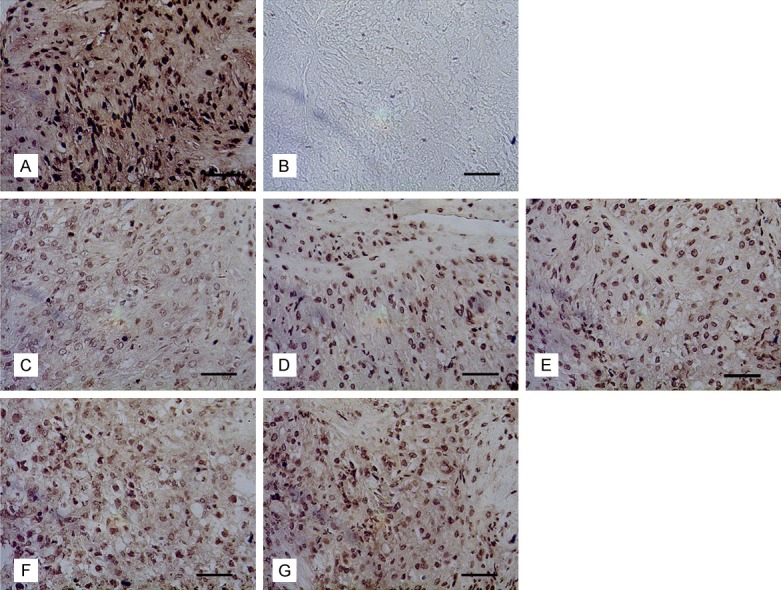
Cell apoptosis in uterus of mice on gestational day 18. Notes: Apoptosis of uterine cells of mice on gestational day 18, Bar = 50 μm. (A) the positive control, (B) the negative control, (C) the control group, (D) the 1 mg/kg PFOA group, (E) the 5 mg/kg PFOA group, (F) the 10 mg/kg PFOA group, (G) the 20 mg/kg PFOA group. Bar = 50 μm.
The number of apoptotic uterine cells in the 1 mg/kg PFOA group was not significantly different compared with that of the control group (P>0.05). After 5 mg/kg PFOA treatment, the number of apoptotic cells in the uterus of the mice at day 18 of gestation was significantly increased compared to the control group (P<0.05). After 10 mg/kg and 20 mg/kg PFOA treatment, the number of apoptotic cells in the uterus was significantly higher than that of the control group (P<0.01), increasing by 18.27% and 19.50%, respectively. See Table 4.
Table 4.
Apoptosis of uterine cells of mice during pregnancy
Notes: Group A was the control group. Mice in groups B, C, D, E were treated with 1 mg/kg, 5 mg/kg, 10 mg/kg, 20 mg/kg PFOA respectively. Compared with the cntrol group.
Shows p<0.05;
Shows p<0.01.
Discussion
Many reports have shown that PFOA has potential reproductive toxicity to animal and human health [24-27]. PFOA (3-20 mg/kg·bw/day) increased maternal liver weight, delayed the growth of offspring, delayed eye opening, and slowed hair growth. High-dose PFOA makes all embryos absorbed [19]. A study of cross-breast feeding shows that the key period for the role of PFOA is in the uterus rather than in lactation [28], and the mechanisms of damage are still rarely reported. Therefore, the current study investigated the mechanism of apoptosis in uteri of pregnant mice by exposure to different concentrations of PFOA. The results showed that exposure to PFOA significantly increased the expressions of the apoptosis factors in uterus, such as Fas, FasL, and casepase-3. In addition, the Bcl-2/Bax ratio was significantly lower than the control group (P<0.01), and the number of apoptotic uterine cells increased in a dose-dependent manner. These results showed that maternal exposure to PFOA accelerated the apoptosis of uterine cells, leading to abortion or fetal growth retardation.
Weight is the most direct indicator to detect physical fitness. In this study, we measured maternal weight changes during pregnancy. In the 10 mg/kg and 20 mg/kg PFOA groups, maternal weight had a slow growth tendency from days 1 to 18 of pregnancy. In the 40-mg/kg dosage group, however, weight losses were seen from the fifth day on. These results are consistent with that obtained by Lau and his colleagues [20]. The weight decrease at GD5 coincides with the time of blastocyst implantation in mice. However, other report claims that PFOA treatment increases the number of resorbed embryos and does not significantly affect the number of embryo implantation [29]. Therefore, PFOA-induced maternal abortion may not be via the approach of blastocyst implantation. Exposure to PFOA reduces the number and size of corpus luteum and significantly reduces serum progesterone levels [29]. It is well known that progesterone maintains early fetal growth and development during pregnancy. Thus, we postulate that a low level of progesterone due to PFOA exposure might play a role in accelerating maternal abortion.
Apoptosis of uterine cells is related to the regulation of a variety of genes, whose products play a role in regulating the natural apoptosis of uterine cells by coordinating with each other. Bcl-2 is a key anti-apoptotic protein that maintains the integrity of the mitochondrial outer membrane, whereas Bax promotes apoptosis by increasing mitochondrial membrane permeability and releasing apoptotic factors into the cytoplasm [30]. The high expression of Bcl-2 in the myometrium is conducive to thickening of the uterine smooth muscle and increasing uterine capacity. The weak expression of Bcl-2 in the intima favors the attachment and growth of embryos. Immunohistochemistry results show that Bcl-2 and Bax are mainly expressed in the decidua, the circular and longitudinal muscle in uterus. The expression of Bax in PFOA groups was significantly higher than that in the control group, while the expression of Bcl-2 was significantly decreased. Bcl-2/Bax ratio significantly decreased in a dose-dependent manner. It has been reported that the occurrence of spontaneous abortion is related to the abnormal apoptosis of uterine decidual cells. Compared with normal pregnant mice, the expression of Bcl-2 in spontaneous abortion mice was significantly decreased (P<0.01); the expression of Bax was significantly increased (P<0.01). Presumably PFOA exposure, through the Bcl-2, Bax-mediated mitochondrial pathway, leads to excessive apoptosis of decidual cells, and ultimately abortion.
Fas is a known TNF superfamily receptor that induces apoptotic cell death when it binds to a FAS ligand (FasL), which is mainly expressed in activated T-lymphocytes and natural killer (NK) cells [31]. In addition, Fas and FasL interactions can also mediate graft rejection and immune tolerance [32]. The results of the current study showed that Fas and FasL were mainly expressed in the decidual cells around the blastocyst, and the expressions of Fas and FasL in PFOA-treated groups were higher than that in the control group. Therefore, we speculate another pathway of pregnancy failure may be that PFOA induces apoptosis by upregulating the expression of Fas and FasL in uterine cells and mediates the immune rejection of the embryos.
Caspase-3, a key protease in mammalian apoptosis, is the core of the apoptotic cascade [33]. In the present study, our results demonstrated that all doses of PFOA treatment (1, 5, 10, 20, and 40 mg/kg/day) significantly increased the Caspase-3 expressions in uterus in a dose-dependent manner. This is consistent with Cui et al.’s results [34], in which IC50 and IC80 of PFOA treatment (84.76 and 150.97 µg/ml) significantly increased gene expressions of Caspase-3, P53, Bax, Bcl-2, and NFKB in zebrafish liver cell line. Joswig et al postulate that the apoptosis of uterine epithelium is mainly mediated by caspase-3 [35]. The TUNEL results show that a few uterine apoptotic cells in the control group which are light colored are located in the decidua and uterine smooth muscle. While the density of positive cells in PFOA groups increased, the color deepened in a dose-dependent manner, indicating that PFOA induces a large number of apoptotic cells in uterine tissue. This is consistent with the results of immunohistochemistry in detecting the tendencies of Fas, Fasl, Bcl-2, Bax, and Caspase-3 expression. Our current results support that PFOA exposure induces apoptosis of uterine cells, and as a result, different sized embryos, congestion or embryonic absorption occur.
Conclusion
PFOA exposure in the gestation period can cause embryo loss and retarded fetal growth, suggesting that the toxicity of PFOA is continuous. The expression of Fas, FasL, Bax, and Caspase-3 in the uterus was elevated by PFOA, and the expression of Bcl-2 was down-regulated. The ratio of Bcl-2/Bax was decreased, resulting in varying degrees of apoptosis of uterine cells that significantly increased in a dose-dependent manner. We come to the conclusion that PFOA-induced excessive apoptosis of uterine cells may be an important cause for the loss of mouse embryos and the retarded growth of the fetus.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 31502111).
Disclosure of conflict of interest
None.
References
- 1.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 2.Olivero-Verbel J, Tao L, Johnson-Restrepo B, Guette-Fernández J, Baldiris-Avila R, O’byrne-Hoyos I, Kannan K. Perfluorooctanesulfonate and related fluorochemicals in biological samples from the north coast of Colombia. Environ Pollut. 2006;142:367–372. doi: 10.1016/j.envpol.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, Lignell S, Lindström G. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996-2004, in Sweden. Environ Health Perspect. 2007;115:226–230. doi: 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen T, Zhang L, Yue JQ, Lv ZQ, Xia W, Wan YJ, Li YY, Xu SQ. Prenatal PFOS exposure induces oxidative stress and apoptosis in the lung of rat off-spring. Reprod Toxicol. 2012;33:538–545. doi: 10.1016/j.reprotox.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45:7954–7961. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Zhang Y, Tan D, Geng Y, Wang L, Peng Y, He Z, Xu Y. Perfluorinated compounds in greenhouse and open agricultural producing areas of three provinces of china: levels, sources and risk assessment. Int J Environ Res Public Health. 2016;13:1224–1237. doi: 10.3390/ijerph13121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wania F. A global mass balance analysis of the source of perfluorocarboxylic acids in the Arctic Ocean. Environ Sci Technol. 2007;41:4529–35. doi: 10.1021/es070124c. [DOI] [PubMed] [Google Scholar]
- 8.Maestri L, Negri S, Ferrari M. Determination of perfluorooctanoic acid and perfluorooctanesulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry. Rapid Commun Mass Sp. 2006;20:2728–2734. doi: 10.1002/rcm.2661. [DOI] [PubMed] [Google Scholar]
- 9.Lau C. Perfluoroalkyl acids: recent research highlights. Reprod Toxicol. 2012;33:405–409. doi: 10.1016/j.reprotox.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Fagbayigbo BO, Opeolu BO, Fatoki OS, Olatunji OS. Validation and determination of nine PFCS in surface water and sediment samples using UPLC-QTOF-MS. Environ Monit Assess. 2018;190:346–352. doi: 10.1007/s10661-018-6715-2. [DOI] [PubMed] [Google Scholar]
- 11.Murakami M, Adachi N, Saha M, Morita C, Takada H. Levels, temporal trends, and tissue distribution of perfluorinated surfactants in freshwater fish from Asian countries. Arch Environ Contam Toxicol. 2011;61:631–641. doi: 10.1007/s00244-011-9660-4. [DOI] [PubMed] [Google Scholar]
- 12.Karrman A, Domingo JL, Llebaria X, Nadal M, Bigas E, van Bavel B, Lindström G. Biomonitoring perfluorinated compounds in Catalonia, Spain: concentrations and trends in human liver and milk samples. Environ Sci Pollut Res. 2010;17:750–758. doi: 10.1007/s11356-009-0178-5. [DOI] [PubMed] [Google Scholar]
- 13.Ahrens L, Siebert U, Ebinghaus R. Total body burden and tissue distribution of polyfluorinated compounds in harbor seals (Phoca vitulina) from the German Bight. Mar Pollut Bull. 2009;58:520–525. doi: 10.1016/j.marpolbul.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy GL Jr, Butenhoff JL, Olsen GW, O’Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 15.White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol. 2011;127:16–26. doi: 10.1016/j.jsbmb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng F, Sheng N, Zhang H, Yan S, Zhang J, Wang J. Perfluorooctanoic acid exposure disturbs glucose metabolism in mouse liver. Toxicol Appl Pharm. 2017;335:41–48. doi: 10.1016/j.taap.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Peng S, Yan L, Zhang J, Shen H. Hepatotoxicity of perfluorooctanoic acid in human hepatocytes using metabonomics. Se Pu. 2012;30:123–127. doi: 10.3724/sp.j.1123.2011.12059. [DOI] [PubMed] [Google Scholar]
- 18.Tsuda S. Differential toxicity between perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) Toxicol Sci. 2016;41:SP27–SP36. doi: 10.2131/jts.41.SP27. [DOI] [PubMed] [Google Scholar]
- 19.Bach CC, Bech BH, Brix N, Nohr EA, Bonde JP, Henriksen TB. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol. 2015;45:53–67. doi: 10.3109/10408444.2014.952400. [DOI] [PubMed] [Google Scholar]
- 20.Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 2006;90:510–518. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- 21.Yahia D, EI-Nasser MA, Abedel-Latif M, Tsukuba C, Yoshida M, Sato I, Tsuda S. Effects of perfluorooctanoic acid (PFOA) exposure to pregnant mice on reproduction. J Toxicol Sci. 2010;35:527–533. doi: 10.2131/jts.35.527. [DOI] [PubMed] [Google Scholar]
- 22.Dixon D, Reed CE, Moore AB, Gibbs-Flournoy EA, Hines EP, Wallace EA, Stanko JP, Lu Y, Jefferson WN, Newbold RR, Fenton SE. Histopathologic changes in the uterus, cervix and vagina of immature CD-1 mice exposed to low doses of perfluorooctanoic acid (PFOA) in a uterotrophic assay. Reprod Toxicol. 2012;33:506–512. doi: 10.1016/j.reprotox.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao PL, Ehresman DJ, Rae JM, Chang SC, Frame SR, Butenhoff JL, Kennedy GL, Peters JM. Comparative in vivo and in vitro analysis of possible estrogenic effects of perfluorooctanoic acid. Toxicology. 2014;326:62–73. doi: 10.1016/j.tox.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. The navigation guide-evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014;122:1028–1039. doi: 10.1289/ehp.1307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, Halldorsson TI, Becher G, Haug LS, Toft G. Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Hum Reprod. 2013;28:3337–3348. doi: 10.1093/humrep/det382. [DOI] [PubMed] [Google Scholar]
- 26.Lee JW, Lee JW, Kim K, Shin YJ, Kim J, Kim S, Kim H, Kim P, Park K. PFOA-induced metabolism disturbance and multi-generational reproductive toxicity in oryzias latipes. J Hazard Mater. 2017;340:231–240. doi: 10.1016/j.jhazmat.2017.06.058. [DOI] [PubMed] [Google Scholar]
- 27.Midic U, Vincent KA, Vandevoort CA, Latham KE. Effects of long-term endocrine disrupting compound exposure on macaca mulatta embryonic stem cells. Reprod Toxicol. 2016;65:382–393. doi: 10.1016/j.reprotox.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf CJ, Fenton SE, Schmid JE, Calafat AM, Kuklenyik Z, Bryant XA, Thibodeaux J, Das KP, White SS, Lau CS, Abbott BD. Developmental toxicity of perfluorooctanoic acid in the CD-1 mouse after cross-foster and restricted gestational exposures. Toxicol Sci. 2007;95:462–473. doi: 10.1093/toxsci/kfl159. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Zhou L, Xu J, Zhang L, Li M, Xie X, Xie Y, Luo D, Zhang D, Yu X, Yang B, Kuang H. Maternal exposure to perfluorooctanoic acid inhibits luteal function via oxidative stress and apoptosis in pregnant mice. Reprod Toxicol. 2017;69:159–166. doi: 10.1016/j.reprotox.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 31.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 32.Kim KM, Adachi T, Nielsen PJ, Terashima M, Lamers MC, Köhler G, Reth M. Two new proteins preferentially associated with membrane immunoglobulin D. Embo J. 1994;13:3793–3800. doi: 10.1002/j.1460-2075.1994.tb06690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budihardjo I, Oliver H, Lutter M. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 34.Cui Y, Liu W, Xie W. Investigation of the effects of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) on apoptosis and cell cycle in a zebrafish (Danio rerio) liver cell line. Inter J Env Res Pub Heal. 2015;12:15673–15682. doi: 10.3390/ijerph121215012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joswig A, Gabriel HD, Kibschull M. Apoptosis in uterine epithelium and decidua in response to implantation: evidence for two different pathways. Reprod Biol Endocrinol. 2003;1:44–52. doi: 10.1186/1477-7827-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]


