Abstract
Introduction
Flibanserin, a treatment for hypoactive sexual desire disorder, carries warnings for increased risk of severe hypotension and syncope when used with alcohol. However, these warnings are not informed by studies that used flibanserin’s recommended bedtime dosing because previous alcohol studies assessed flibanserin’s safety during the day.
Aim
The aim of this study was to assess the effects of ethanol in a real-world context in premenopausal women taking flibanserin at bedtime.
Methods
In a randomized, placebo-controlled, double-blind study, 24 healthy premenopausal women (mean age = 34.5 ± 9.9 years; mean body mass index = 25.2 ± 3.4 kg/m2) were dosed with flibanserin or placebo for 3 days to achieve steady-state plasma levels. In a clinical research unit, subjects (n = 22) were provided 2 units of wine (150 mL/unit; 12% ethanol content) or a nonalcoholic beverage with a standardized 3-course evening meal. Flibanserin 100 mg or placebo was administered at bedtime 2.5 hours after the end of the evening meal. On a separate day, subjects were provided the alternative beverage (± alcohol) with the same evening meal and dosed with the same treatment (flibanserin or placebo) at bedtime. After a 5-day washout period, subjects crossed over to the other treatment arm and the protocol was repeated.
Main Outcome Measure
Adverse events (AEs) and vital signs were monitored.
Results
In the absence of ethanol, headaches and hypotension were the only AEs that occurred in ≥2 subjects after flibanserin dosing (placebo corrected rates were 17.4% and 8.7%, respectively). After ethanol consumption, the rate of hypotension after flibanserin dosing was no greater than with flibanserin or placebo after nonalcoholic beverage consumption. There were no instances of orthostatic hypotension or syncope and no serious AEs or AEs leading to study discontinuation.
Conclusion
Flibanserin dosed at bedtime after moderate amounts of alcohol with an evening meal was well-tolerated with no evidence of clinically significant hypotension or syncope.
Millheiser L, Clayton AH, Parish SJ, et al. Safety and Tolerability of Evening Ethanol Consumption and Bedtime Administration of Flibanserin in Healthy Premenopausal Female Subjects. Sex Med 2019;7:418–424.
Key Words: Alcohol Interaction, Flibanserin, Hypoactive Sexual Desire Disorder, Hypotension, Syncope
Introduction
Flibanserin is a multifunctional serotonin (5-HT) agonist and antagonist that is approved in the United States and Canada for the treatment of acquired, generalized, hypoactive sexual desire disorder (HSDD) in premenopausal women.1, 2, 3, 4 HSDD is the most prevalent sexual health problem in pre- and postmenopausal women5 and is most succinctly defined as the persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty; not otherwise accounted for by a general medical or psychiatric condition.6 Preclinical studies suggest that flibanserin activates 5-HT1A receptors and blocks 5-HT2A receptors in the prefrontal cortex that ultimately stimulate dopaminergic and noradrenergic neural circuits to restore sexual desire.7, 8, 9, 10 Consistent with its activity at serotonin receptors, the most common side effects associated with flibanserin are dizziness, somnolence, and nausea, affecting ≥10% of premenopausal women with HSDD in placebo-controlled studies.2
The original prescribing information for flibanserin included a boxed warning regarding risk of severe hypotension and syncope with concomitant alcohol use.2, 3 Although previous alcohol interaction studies have examined the effects of daytime dosing of flibanserin and ethanol intake,11, 12, [13] the effects of alcohol on flibanserin safety in a real-world context when alcoholic beverages are consumed in the evening and in moderation, and flibanserin is taken at bedtime, have not been adequately evaluated. To this end, the objective of the present study was to evaluate the effects of moderate amounts of ethanol consumption with an evening meal on the safety and tolerability of flibanserin taken at bedtime (per prescribing information) in healthy premenopausal women.
Materials and Methods
Study conduct was consistent with ethical principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization. The study protocol was approved by Quorum Review (Seattle, WA) and all participants provided written informed consent.
Study Subjects
All subjects were healthy volunteers providing informed consent and responded to advertisements approved by Quorum Review. Volunteers were offered monetary compensation (US $1,600) for time devoted to study participation and an additional monetary incentive (US $400) for completing the study. Healthy, premenopausal women aged 18 to 55 years with body mass index 18–35 kg/m2 were enrolled. Other major inclusion criteria were a history of regular menstrual cycles during the preceding 6 months, use of a reliable form of contraception during the study, and regular sleep pattern with bedtime occurring between 22:00 and 24:00 hours. All participants were required to have a history of average consumption of ≥2 alcoholic beverages per week but no current or recent (within the last 12 months) history of heavy alcohol intake, defined as an average of >7 standard drinks with ethanol per week (1 standard drink in the United States = 14 g pure ethanol, approximating the alcohol content in 12 ounces of beer with 5% ethanol or 5 ounces of wine with 12% ethanol or 1.5 ounces of distilled spirits with 40% ethanol).14
Exclusion criteria included pregnancy or lactation; hepatic impairment or any other clinically significant medical condition; a history of seizure disorder, orthostatic hypotension, hypotensive events, fainting spells, dizziness, or blackouts; presence of orthostatic hypotension, a resting supine systolic blood pressure <110 mm Hg or diastolic blood pressure <60 mmHg at screening; history of alcohol or other substance dependence (including binge drinking) or treatment for alcohol or other substance abuse within the past 2 years; inability to metabolize alcohol (as determined by unpleasant rapid-onset reactions, such as nasal congestion and skin flushing, after drinking alcohol). Other key exclusion criteria were tobacco use (>10 cigarettes per day or inability to abstain while confined to the clinical research unit), a positive urine test for drugs of abuse (amphetamines, methamphetamines, barbiturates, benzodiazepines, cocaine, cannabinoids, and opiates) or a positive breath test for ethanol at screening, and receipt of any investigational product or participation in any clinical trial within the previous 30 days. Use of concomitant prescription medications, herbal supplements, and over-the-counter products was prohibited, except for hormonal contraceptives and stable-dose (≥30 days) of thyroid medication.
Study Design
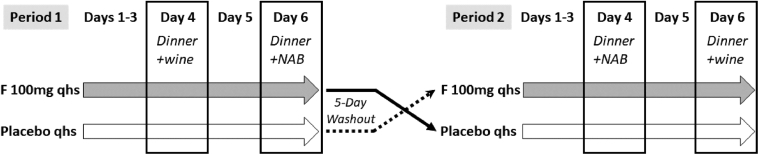
This was a single center, randomized, double-blind, placebo-controlled, 2-period, 4-treatment crossover study conducted between June 2018 and July 2018. A randomized block design was used. Subjects were randomized in a 1:1 ratio to receive either flibanserin 100 mg or placebo (tablets were identical in appearance and size) in treatment period 1, and the alternative therapy in treatment period 2 with a 5-day washout between treatment periods (Figure 1). A pharmacy within the clinical research facility managed the randomization and dispensing of blinded study medication. Pharmacy staff did not otherwise participate in the execution of the study protocol and did not interact with any of the study subjects. Subjects were instructed to take 1 tablet at bedtime with a glass of water for the first 3 days at home in order to achieve steady state.2 Subjects completed a dosing diary to ensure treatment compliance while at home. Subjects reported to the clinical research unit (which includes clinic, pharmacy, dormitory style lodging, and dining facilities) on the morning of day 4. At 18:30–20:00 hours of study days 4 and 6, subjects were provided up to 2 units of wine (1 unit = 5 fluid ounces or 150 mL of 12% ethanol) or up to 2 glasses of matching nonalcoholic beverage during a 3-course evening meal. This amount of wine consumption falls within the definition of moderate ethanol intake, defined as 2 servings of either 12 ounces of beer with 5% ethanol content, 5 ounces of wine with 12% ethanol content or 1.5 ounces of 80-proof spirit. The amount of wine or nonalcoholic beverage consumed was recorded after the evening meal. Flibanserin 100 mg or placebo was administered 5 minutes prior to bedtime (22:30 hour) and subjects were awakened 8 hours after dosing (06:30 hour). Subjects were maintained on flibanserin or placebo at bedtime on day 5. On day 6, subjects were provided the alternative beverage (up to 2 units of wine or matching nonalcoholic beverage) with the same 3-course evening meal provided on day 4, following the same time schedule. Beverage consumption was recorded and either flibanserin or placebo was administered at bedtime, as previously described. On days 4 and 6, vital signs were measured prior to the evening meal, prior to drug administration, and after waking the next morning unless a subject was awakened during the night and experienced signs and symptoms of hypotension. Subjects were discharged from the clinical research unit on day 7 after all postdose procedures had been completed or at the discretion of the research staff.
Figure 1.
Study design. F = flibanserin; NAB = nonalcoholic beverage; qhs = bedtime dosing.
Assessments
Medical assessments were performed by clinical research staff who were the equivalent of physician assistants or registered nurses and were supervised by a medical doctor. Safety assessments included adverse event (AE) monitoring, measurements of vital signs, and physical examination findings. AEs were coded according to the Medical Dictionary for Regulatory Activities version 21 and classified according to chronological criteria. The primary end point for this study was the proportion of subjects who experienced syncope or orthostatic hypotension associated AEs of special interest requiring medical intervention, such as Trendelenburg position or smelling salts to prevent significant increase in severity in AEs. Secondary end points included the proportion of subjects with orthostatic hypotension, defined as a drop in systolic blood pressure of ≥20 mmHg from the supine to the standing position and/or a drop in diastolic blood pressure of ≥10 mmHg from supine to standing position15; the proportion of subjects experiencing symptomatic orthostatic hypotension (onset of dizziness, light-headedness, or faintness after change of position) that resulted in no standing blood pressure measurement; the proportion of subjects who experienced hypotension, defined as systolic blood pressure <90 mmHg and/or diastolic pressure <60 mmHg; and rates of syncope, orthostatic hypotension, dizziness, and somnolence, defined as events of interest.
Results
A total of 59 volunteers were screened, 27 were assigned as potential candidates, and 24 subjects were enrolled with an age range of 18–51 years (mean ± SD = 34.5 ± 9.9 years) and mean body mass index of 25.2 ± 3.4 kg/m2 (Table 1). Volunteers were excluded (n = 32) for out of range vitals or laboratories, medical history, substance abuse habits, gynecologic history, or other reasons that included inability to adhere to protocol requirements. Self-reported alcohol consumption at screening for those enrolled into the study ranged from 2–6 drinks per week (mean ± SD = 3.1 ± 1.2 drinks/week). Most subjects (n = 22) completed both treatment periods and 2 subjects discontinued after enrollment. One of the subjects who discontinued was randomized to flibanserin during treatment period 1 and provided study medication but did not report for check-in at the clinical research unit on day 4. This subject was not assessed for AEs and was excluded from the safety analysis. A second subject who was randomized to placebo during treatment period 1 withdrew consent after reporting to the clinical research unit on day 4. Although a treatment diary was not submitted, medication vials were returned empty and it was assumed that all tablets were taken as directed. This subject reported no AEs and was included in the safety analysis for nonalcohol exposure days.
Table 1.
Demographic and baseline characteristics of study cohort
| Characteristics | Mean ± SD | Range |
|---|---|---|
| Age, years | 34.5 ± 9.9 | 18.0–51.0 |
| Weight, kg | 64.7 ± 9.2 | 45.8–82.6 |
| Height, cm, mean, SD | 160.0 ± 4.6 | 153.5–171.3 |
| Body mass index, kg/m2 | 25.2 ± 3.4 | 19.0–32.0 |
| Current alcohol use, # drinks per week | 3.1 ± 1.2 | 2.0–6.0 |
| Characteristics | n (%) | |
| Total subjects enrolled | 24 (100) | |
| Race | ||
| White | 22 (91.7) | |
| Black/African American | 2 (8.3) | |
| Hispanic/Latino ethnicity | 12 (50.0) | |
| Tobacco use | ||
| Former | 12 (50.0) | |
| Never | 12 (50.0) | |
| Contraception method | ||
| Abstinence | 5 (20.8) | |
| Condom with spermicide | 13 (54.2) | |
| IUD with hormones | 1 (4.2) | |
| Systemic hormonal | 2 (8.3) | |
| Tubal occlusion | 3 (12.5) |
IUD = intrauterine device.
Days with No Alcohol Consumption
A total of 16 AEs were reported by 10 subjects (43.5%) taking flibanserin and 10 AEs were reported by 6 subjects (26.1%) taking placebo on days with no ethanol intake. There were no deaths and no serious AEs. As shown in Table 2, the only events that occurred in 2 or more subjects taking flibanserin were headache (6 events in 5 subjects) and hypotension (3 events in 3 subjects). Headache and hypotension occurred more frequently in subjects taking flibanserin than in those taking placebo. All AEs experienced by subjects taking flibanserin were mild with the exception of 1 episode of moderate headache. For subjects taking placebo, all AEs were mild with the exception of 1 episode of moderate insomnia. There were no severe AEs and there were no AEs that led to study discontinuation.
Table 2.
Number of subjects with AEs on days with no ethanol dosing
| AE | Flibanserin N = 23 n (%) | Placebo N = 23 n (%) |
|---|---|---|
| Subjects with any AEs | 10 (43.5) | 6 (26.1) |
| Headache | 5 (21.7) | 1 (4.3) |
| Hypotension | 3 (13.0) | 1 (4.3) |
| Insomnia | 1 (4.3) | 1 (4.3) |
| Nausea | 1 (4.3) | 0 (0.0) |
| Mood swings | 1 (4.3) | 0 (0.0) |
| Nasal congestion | 1 (4.3) | 0 (0.0) |
| Pharyngeal paraesthesia | 1 (4.3) | 0 (0.0) |
| Pollakiuria | 1 (4.3) | 0 (0.0) |
| Sneezing | 1 (4.3) | 0 (0.0) |
| Anxiety | 0 (0.0) | 1 (4.3) |
| Cough | 0 (0.0) | 1 (4.3) |
| Dizziness | 0 (0.0) | 1 (4.3) |
| Fatigue | 0 (0.0) | 1 (4.3) |
| Limb injury | 0 (0.0) | 1 (4.3) |
| Myalgia | 0 (0.0) | 1 (4.3) |
| Somnolence | 0 (0.0) | 1 (4.3) |
AE = adverse event.
Days with Alcohol Consumption
Alcoholic and nonalcoholic beverage consumption with evening meals was identical in all treatment arms and occurred over a mean duration of 22 minutes. Overall, duration of beverage consumption was somewhat shorter when a nonalcoholic beverage was provided (median duration = 28–32 minutes) compared to an alcoholic beverage (median duration = 44–48 minutes). All subjects consumed the maximum volume of the 2 glasses of beverage provided and alcohol dosing is estimated to be 0.4 g/kg in subjects who consumed wine with their evening meal.
No subjects experienced an instance of syncope or AEs associated with orthostatic hypotension that required medical intervention (primary end point). Further, no subjects experienced orthostatic hypotension or any other AE of interest (dizziness, somnolence, and syncope), and no subjects had missing standing blood pressure due to dizziness, light-headedness, or faintness upon change of position. Predictably, subjects only reported “feeling drunk” after having consumed ethanol with their evening meal and an equal number of subjects (n = 2) experienced this AE whether they were taking flibanserin or placebo (Table 3).
Table 3.
Number of subjects with AEs on days with ethanol dosing
| AE | Ethanol n (%) |
No ethanol n (%) |
||
|---|---|---|---|---|
| Flibanserin N = 22 | Placebo N = 22 | Flibanserin N = 22 | Placebo N = 22 | |
| Subjects with any AE | 4 (18.2) | 4 (18.2) | 6 (27.3) | 3 (13.6) |
| Hypotension | 3 (13.9) | 2 (9.1) | 6 (27.3) | 3 (13.6) |
| Feeling drunk | 2 (9.1) | 2 (9.1) | 0 (0.0) | 0 (0.0) |
| Headache | 0 (0.0) | 1 (4.5) | 0 (0.0) | 0 (0.0) |
The table shows only events that occurred after alcohol intake on days 4 and 6, until taking vital signs after rising the following morning.
AE = adverse event.
AEs were classified as hypotension at the discretion of the investigator, based upon descriptive signs and symptoms (eg, lightheadedness or feeling faint) reported by subjects and do not necessarily correlate with hypotension assessed by blood pressure measurement. After meals with nonalcoholic drinks in the evening, more subjects reported hypotension as an AE after flibanserin treatment (n = 6) than after placebo treatment (n = 3; Table 3). Similarly, when blood pressure was measured during assessment of vital signs, hypotension (defined as <90 mmHg for systolic or <60 mmHg for diastolic blood pressure) was detected in 8 subjects after flibanserin treatment and in 5 subjects after placebo treatment (Table 4). This suggests that some subjects were either asymptomatic or less aware of their drop in blood pressure.
Table 4.
Number of subjects with hypotension on days with ethanol dosing
| Ethanol n (%) |
No ethanol n (%) |
|||
|---|---|---|---|---|
| Flibanserin N = 22 | Placebo N = 22 | Flibanserin N = 22 | Placebo N = 22 | |
| Subjects with hypotension (systolic or diastolic BP) | 5 (22.7) | 5 (22.7) | 8 (36.4) | 5 (22.7) |
| Subjects with systolic BP <90 mmHg | 1 (4.5) | 1 (4.5) | 2 (9.1) | 0 (0.0) |
| Subjects with diastolic BP <60 mmHg | 5 (22.7) | 5 (22.7) | 8 (36.4) | 5 (22.7) |
BP = blood pressure.
The table shows only events that occurred after alcohol intake on days 4 and 6, until taking vital signs after rising the following morning.
After consumption of ethanol with evening meals, the number of subjects reporting hypotension as an AE was similar in subjects treated with either flibanserin (n = 3) or placebo (n = 2). Again, blood pressure measurements detected greater numbers of subjects with hypotension but there was no difference between flibanserin and placebo treatment (n = 5; Table 4).
Orthostatic increases in heart rate (≥20 beats/minute) were also more prevalent in subjects treated with flibanserin after evening meals with nonalcoholic drinks (n = 17; 77.3%) compared to subjects treated with placebo (n = 13; 59.1%) but this was not exacerbated with ethanol intake during the evening meal (n = 14; 63.6% for flibanserin vs n = 12; 54.5% for placebo).
Discussion
This study assessed the safety of flibanserin with ethanol when consumed in a real-world context. Study participants consumed a total of 300 mL of wine (12% ethanol content) with an evening meal and achieved an estimated ethanol dose of 0.4 g/kg. Flibanserin dosing occurred at bedtime, 2–3 hours after the evening meal, as is most likely to occur in patients who are prescribed flibanserin. It should also be noted that the effects of ethanol in this study were assessed after achieving steady-state blood levels of flibanserin.
Importantly, there were no AEs associated with orthostatic hypotension or syncope during the course of this study. These data suggest that when flibanserin is dosed at bedtime at least 2 hours after ethanol consumption, the risk for clinically significant hypotension-related AEs is mitigated. Our findings are consistent with a separate postapproval alcohol interaction study in premenopausal women in which ethanol (0.4 g/kg), consumed 2, 4, or 6 hours prior to daytime flibanserin dosing, did not increase rates of hypotension, orthostatic hypotension, or syncope compared to placebo.13 The number of subjects who experienced somnolence or dizziness was greater after flibanserin dosing compared with placebo in that study but there was no consistent trend for either AE between any of the ethanol timing conditions.13
In an earlier alcohol interaction study conducted prior to the approval of flibanserin in the United States, investigators found an increased incidence of dizziness, fatigue, sedation, hypotension, and syncope-related events in healthy subjects who were administered ethanol and flibanserin at the same time.11 However, this earlier study was performed mostly in men (23 of 25 total subjects), and ethanol (0.4 g/kg or 0.8 g/kg) and flibanserin were dosed in the morning after an overnight fast and a light breakfast. Ethanol was consumed in concentrated form (95%) within 10 minutes. Interestingly, another postapproval study, performed in 96 premenopausal women, used a similar protocol (ie, ethanol consumed in concentrated form within 10 minutes) to study the effects of concomitant ethanol (0.2–0.6 g/kg) and a single dose of flibanserin 100 mg administered in the morning.12 Although the number of subjects experiencing dizziness or the inability to stand for measurement of orthostatic vital signs increased when flibanserin was taken with ethanol, there were no reports of syncope or orthostatic hypotension that required medical intervention.12
Studies using this “worst case” context are severely limited in generalizability with regard to the typical premenopausal patient with HSDD, as indicated by sociodemographic data. In a study involving 1,088 premenopausal women diagnosed with acquired, generalized HSDD at 33 clinical sites throughout the United States, the mean age of the study cohort was 37.1 ± 8.9 years, 81.8% were married or living with a partner, and 50.9% had ≤1 alcoholic drink per day in a typical week, whereas 37.8% either did not consume alcohol at the time of the study or had never consumed alcohol.16 Data from phase III clinical trials for flibanserin additionally delineated that study participants were in stable, long-term relationships with a mean duration of >10 years.17, 18, 19 In contrast, in Western cultures, extreme intake of alcoholic beverages or binge drinking has the highest prevalence during late adolescence and early adulthood and decreases with increasing age.20, 21 In addition to the differences in sociodemographic parameters associated with lower levels of alcohol consumption in the typical patient with HSDD, it is unlikely that women with alcohol use disorders would receive a diagnosis of HSDD given the criteria that symptoms cannot be due to another disorder, including substance abuse.22
Another important consideration in evaluating the safety of flibanserin is that data from phase I studies suggest that acute daytime dosing increases the risk of hypotension and syncope.22 The risk of hypotension-related AEs is largely mitigated by bedtime dosing, as evidenced by the phase III trials where hypotension and syncope were 0.39% in the flibanserin-treated group vs 0.21% in the placebo-treated group,22 AE rates that are defined as “uncommon” or “infrequent” (0.1% to <1%) by the World Health Organization.23 Similarly, in the present study, adherence to the bedtime dosing regimen for flibanserin likely minimized AE rates.
Although this study was designed with a real-world context, it does have several limitations. These include small sample size, recruitment of healthy premenopausal women who were not assessed for HSDD, and strict adherence to dosing flibanserin within 5 minutes of bedtime, preventing the assessment of orthostatic vital signs in the hours immediately after flibanserin administration. However, it is reassuring that rates of dizziness, hypotension, orthostatic hypotension, or syncope were not increased over those of placebo when flibanserin was dosed during the day at least 2 hours after ethanol consumption in a previous study.13 In August 2019, the safety information for flibanserin in the US was updated to remove the alcohol contraindication and to clarify that the risk of hypotension and syncope can be mitigated by separating flibanserin bedtime dosing and prior alcohol consumption by at least 2 hours.24
Conclusion
In summary, these data demonstrate the lack of a clinically significant alcohol interaction with flibanserin when moderate amounts of ethanol are consumed with an evening meal 2–3 hours prior to bedtime dosing of flibanserin 100 mg in healthy premenopausal women without the diagnosis of HSDD. Our findings are supportive of the changes to the label for flibanserin that now permit ingestion of alcohol up to 2 hours prior to flibanserin bedtime dosing. The importance of bedtime dosing should be emphasized and postmarketing pharmacovigilance data should be helpful in assessing safety signals with increasing real-world use in patients with HSDD.
Statement of Authorship
Category 1
-
(a)Conception and Design
- Leah Millheiser
-
(b)Acquisition of Data
- Leah Millheiser
-
(c)Analysis and Interpretation of Data
- Leah Millheiser; Noel N. Kim
Category 2
-
(a)Drafting the Article
- Leah Millheiser; Anita H. Clayton; Sharon J. Parish; Sheryl A. Kingsberg; Noel N. Kim; James A. Simon
-
(b)Revising It for Intellectual Content
- Leah Millheiser; Anita H. Clayton; Sharon J. Parish; Sheryl A. Kingsberg; Noel N. Kim; James A. Simon
Category 3
-
(a)Final Approval of the Completed Article
- Leah Millheiser; Anita H. Clayton; Sharon J. Parish; Sheryl A. Kingsberg; Noel N. Kim; James A. Simon
Footnotes
Conflict of Interest: Dr Millheiser reports serving as a consultant to or on the advisory boards of Valeant Pharmaceuticals North America LLC, Duchesnay USA, AMAG Pharmaceuticals, Willow, ExploraMed Development, LLC, Aytu BioScience, Inc., and TherapeuticsMD, Inc.; being a stockholder in Viveve Medical, Inc., Aytu BioScience, Inc., and Willow; and was the Chief Medical Officer of Sprout Pharmaceuticals, Inc. at the time of manuscript development. Dr Clayton reports receiving research and grant support from Endoceutics Inc., Janssen Pharmaceuticals, Inc., Sage Therapeutics, and Takeda Pharmaceuticals U.S.A., Inc.; serving as a consultant to or on the advisory board for Acadia, Alkermes PLC, AMAG Pharmaceuticals, Inc., Fabre-Kramer Pharmaceuticals, Inc., Fabre-Kramer Pharmaceuticals, Inc., Lundbeck, Otsuka, Palatin Technologies, Inc., S1 Biopharma, Inc., Sprout Pharmaceuticals, and Takeda; being a stock shareholder of Euthymics Bioscience and S1 Biopharma, Inc.; and having royalties and copyrights at Ballantine Books/Random House, the Changes in Sexual Functioning Questionnaire, and Guilford Publications. Dr Parish reports serving as a consultant to Dare Bioscience, JDS Therapeutics, Strategic Science Technologies, and TherapeuticsMD; being on the scientific advisory boards for AMAG Pharmaceuticals, Inc.; and receiving writing support, with no compensation, from AMAG, Sprout Pharmaceuticals, and TherapeuticsMD. Dr Kingsberg reports serving as a consultant to, on the advisory board for, or as a clinical investigator for AMAG Pharmaceuticals, Inc., Dare, Duchesnay USA, Emotional Brain, Endoceutics, Inc., GTx, Inc., Ivix, Lupin Pharmaceuticals, Inc., Materna, Palatin Technologies, Inc., Pfizer Inc., Sermonix Pharmaceuticals, Strategic Scientific Solutions, Sprout Pharmaceuticals, TherapeuticsMD, Inc., and Valeant Pharmaceuticals North America LLC. Dr Kim reports being a consultant for Sprout Pharmaceuticals, Inc. and Strategic Science and Technologies LLC; and receiving research funding from Valeant Pharmaceuticals, Inc. and Sprout Pharmaceuticals, Inc. Dr Simon reports (within the past year) receiving grant/research support from AbbVie Inc., Agile Therapeutics, Allergan PLC, Bayer HealthCare LLC, Dornier MedTech, Endoceutics, Inc., GTx, Inc, Ipsen, Myovant Sciences, New England Research Institutes, Inc., ObsEva SA, Palatin Technologies, Inc., Symbio Research, Inc., TherapeuticsMD, Inc., and Tissue Genesis; serving as a consultant to or on the advisory board for AbbVie Inc., Allergan PLC, AMAG Pharmaceuticals, Inc., Amgen Inc., Ascend Therapeutics US, LLC, Bayer HealthCare Pharmaceuticals, Inc., CEEK Enterprises, LLC, Covance Inc., Millendo Therapeutics, Inc., Mitsubishi Tanabe Pharma S.A., Sebela Pharmaceuticals, Inc., Sanofi S.A., Sebela Pharmaceuticals, Inc., Shionogi Inc., Symbiotec Pharma Lab Pvt. Ltd., TherapeuticsMD, Inc., and Valeant Pharmaceuticals North America LLC; serving on the speakers’ bureau for AMAG Pharmaceuticals, Inc., Duchesnay USA, Novo Nordisk, Shionogi Inc., and Valeant Pharmaceuticals North America LLC; and being a stock shareholder of Sermonix Pharmaceuticals LLC.
References
- 1.Clayton A.H., Goldstein I., Kim N.N. The International Society for the Study of Women's Sexual Health Process of Care for Management of Hypoactive Sexual Desire Disorder in Women. Mayo Clin Proc. 2018;93:467–487. doi: 10.1016/j.mayocp.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Addyi (flibanserin) tablets, for oral use [package insert in United States] Sprout Pharmaceuticals, Inc.; Raleigh, NC: 2018. [Google Scholar]
- 3.Addyi (flibanserin) tablets, for oral use [product monograph in Canada] Sprout Pharmaceuticals, Inc.; Raleigh, NC: 2018. [Google Scholar]
- 4.Health Canada Summary Basis of Decision for Addyi (May 2018) https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00392 Available at:
- 5.Shifren J.L., Monz B.U., Russo P.A. Sexual problems and distress in United States women: Prevalence and correlates. Obstet Gynecol. 2008;112:970–978. doi: 10.1097/AOG.0b013e3181898cdb. [DOI] [PubMed] [Google Scholar]
- 6.Parish S.J., Goldstein A.T., Goldstein S.W. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions-Part II. J Sex Med. 2016;13:1888–1906. doi: 10.1016/j.jsxm.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Borsini F., Evans K., Jason K. Pharmacology of flibanserin. CNS Drug Rev. 2002;8:117–142. doi: 10.1111/j.1527-3458.2002.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marazziti D., Palego L., Giromella A. Region-dependent effects of flibanserin and buspirone on adenylyl cyclase activity in the human brain. Int J Neuropsychopharmacol. 2002;5:131–140. doi: 10.1017/S1461145702002869. [DOI] [PubMed] [Google Scholar]
- 9.Stahl S.M., Sommer B., Allers K.A. Multifunctional pharmacology of flibanserin: Possible mechanism of therapeutic action in hypoactive sexual desire disorder. J Sex Med. 2011;8:15–27. doi: 10.1111/j.1743-6109.2010.02032.x. [DOI] [PubMed] [Google Scholar]
- 10.Croft H.A. Understanding the role of serotonin in female hypoactive sexual desire disorder and treatment options. J Sex Med. 2017;14:1575–1584. doi: 10.1016/j.jsxm.2017.10.068. [DOI] [PubMed] [Google Scholar]
- 11.Stevens D.M., Weems J.M., Brown L. The pharmacodynamic effects of combined administration of flibanserin and alcohol. J Clin Pharm Ther. 2017;42:598–606. doi: 10.1111/jcpt.12563. [DOI] [PubMed] [Google Scholar]
- 12.Sicard E., Clayton A., Simon J. Effects of alcohol administered with flibanserin in healthy premenopausal women: A randomized, double-blind, single-dose, seven-way crossover study. 19th Annual Fall Scientific Meeting of SMSNA. November 8−11, 2018 Paper presented at. Miami, FL. [Google Scholar]
- 13.Simon JA, Clayton AH, Kingsberg SA, et al. Effects of timing of flibanserin administration relative to alcohol intake in healthy premenopausal women: A randomized, double-blind, crossover study. J Sex Med. 10.1016/j.jsxm.2019.08.006. E-pub ahead of print. [DOI] [PubMed]
- 14.National Institute on Alcohol Abuse and Alcoholism What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink Available at:
- 15.Kaufman H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125–126. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 16.Rosen R.C., Maserejian N.N., Connor M.K. Characteristics of premenopausal and postmenopausal women with acquired, generalized hypoactive sexual desire disorder: The Hypoactive Sexual Desire Disorder Registry for women. Menopause. 2012;19:396–405. doi: 10.1097/gme.0b013e318230e286. [DOI] [PubMed] [Google Scholar]
- 17.Derogatis L.R., Komer L., Katz M. Treatment of hypoactive sexual desire disorder in premenopausal women: Efficacy of flibanserin in the VIOLET study. J Sex Med. 2012;9:1074–1085. doi: 10.1111/j.1743-6109.2011.02626.x. [DOI] [PubMed] [Google Scholar]
- 18.Thorp J., Simon J., Dattani D. Treatment of hypoactive sexual desire disorder in premenopausal women: Efficacy of flibanserin in the DAISY study. J Sex Med. 2012;9:793–804. doi: 10.1111/j.1743-6109.2011.02595.x. [DOI] [PubMed] [Google Scholar]
- 19.Katz M., DeRogatis L.R., Ackerman R. Efficacy of flibanserin in women with hypoactive sexual desire disorder: Results from the BEGONIA trial. J Sex Med. 2013;10:1807–1815. doi: 10.1111/jsm.12189. [DOI] [PubMed] [Google Scholar]
- 20.Wilsnack R.W., Wilsnack S.C., Gmel G. Gender differences in binge drinking. Alcohol Res. 2018;39:57–76. doi: 10.35946/arcr.v39.1.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keyes K.M., Miech R. Age, period, and cohort effects in heavy episodic drinking in the US from 1985 to 2009. Drug Alcohol Depend. 2013;132:140–148. doi: 10.1016/j.drugalcdep.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dooley E.M., Miller M.K., Clayton A.H. Flibanserin: From bench to bedside. Sex Med Rev. 2017;5:461–469. doi: 10.1016/j.sxmr.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization Definitions https://www.who.int/medicines/areas/quality_safety/safety_efficacy/trainingcourses/definitions.pdf Available at:
- 24.Addyi (flibanserin) tablets, for oral use [updated package insert in United States]. Raleigh, NC: Sprout Pharmaceuticals, Inc; 2019.