Abstract
Introduction
Cavernosal nerve (CN) injury is commonly caused by radical prostatectomy surgery, and it might directly lead to erectile dysfunction (ED). Currently, the role of mitogen-activated protein kinase (MAPK) family proteins in phenotypic transformation of corpus cavernosum smooth muscle cell (CCSMC) after CNs injury is poorly understood.
Aim
To investigate the role of p38 MAPK in hypoxia-induced phenotypic transformation of CCSMCs after CN injury.
Methods
In total, 20 Sprague–Dawley rats (male and 8 weeks of age) were randomly divided into 2 groups, including a sham group and CNCI group. In the sham group, rats were sham-operated by identifying 2 CNs without causing direct damage to the CNs. In the CNCI group, rats were subjected to bilateral CN crush injury. CCSMCs were isolated from the normal corpus cavernosum tissues of the Sprague–Dawley rat and then cultured in 21% or 1% O2 concentration context for 48 hours.
Main Outcome Measures
Intracavernous pressure/mean arterial pressure were analyzed to measure erectile response. The impact of hypoxia on penile pathology, as well as the expression of extracellular signal-regulated kinases, the c-Jun NH2-terminal kinase, and p38 MAPK, were analyzed.
Results
Compared with the sham group, the intracavernous pressure/mean arterial pressure rate and α-smooth muscle actin expression of CNCI group were decreased significantly (P = .0001; P = .016, respectively), but vimentin expression was significantly increased (P = .023). Phosphorylated p38 level in CNCI group was decreased significantly (P = .017; sham: 0.17 ± 0.005; CNCI: 0.14 ± 0.02). The CCSMCs in the normoxia group were long fusiform, whereas the morphology of CCSMCs in the hypoxia group became hypertrophic. After hypoxia for 48 hours, the expression of α-smooth muscle actin and phosphorylated p38 MAPK was decreased significantly (P = .01; P = .024, normoxia: 0.66 ± 0.18, hypoxia: 0.26 ± 0.08, respectively), and the expression of hypoxia-inducible factor-1α and collagen I was increased significantly in hypoxia group (P = .04; P = .012, respectively).
Conclusions
Hypoxia induced the phenotypic transformation of CCSMCs after CNCI might be associated with the downregulation of phosphorylated p38 MAPK.
Chen S, Huang X, Kong X, et al. Hypoxia-Induced Phenotypic Transformation of Corpus Cavernosum Smooth Muscle Cells After Cavernous Nerve Crush Injury by Down-Regulating p38 Mitogen-Activated Protein Kinase Expression. Sex Med 2019;7:433–440
Key Words: Erectile Dysfunction, Neurogenic, Phenotypic Transformation, Mitogen-Activated Protein Kinase (MAPK), p38 MAPK, Hypoxia
Introduction
Erectile dysfunction (ED) is one of the most common diseases in men, which usually exhibits the inability of a man achieving or maintaining an erection enough for satisfactory intercourse with his partner. ED was considered as an entirely psychogenic disorder in previous studies until increasing evidence indicated that greater than 80% of patients have an organic etiology, and the organic etiology can be classified as nonendocrine and endocrine.1 Neurogenic ED is the main cause of nonendocrine types, which can be caused by medical diseases (like multiple sclerosis, Parkinson disease, and diabetes), surgical diseases (like spinal cord injury, lumbar disc disease, traumatic brain injury), and radical pelvic surgery (like radical prostatectomy, radical cystectomy, abdominoperineal resection).1
In particular, neurogenic ED is mainly caused by cavernous nerve (CN) injury during radical prostatectomy surgery (RP),2 and it eventually leads a change in penis structure. Corpora cavernosum smooth muscle cells (CCSMCs) account for 42–50% of the corpora cavernosum, which plays an important role in erectile response.3 The penis has a special configuration similar to vascular tissue.4 Similar pathologic features can be found between the vascular system and penis, such as phenotypic transformation.
Phenotypic transformation is defined as the state of smooth muscle cells (SMCs) converted from contractile to synthetic, and it plays a crucial role in vascular disease5 and ED. Phenotypic transformation of CCSMCs occurs in diabetic rats with ED, rats with CN injury, and rats with hyperlipidemia-associated ED.6, 7, 8 However, the function of phenotypic transformation of CCSMCs remains unclear.
Currently, many treatments are suggested for the rehabilitation of erectile function, including daily dosing of phosphodiesterase type 5 inhibitors, intracavernosal injection therapy, or the use of vacuum erection devices.9 However, the treatment of neurogenic ED is still limited. Therefore, phenotypic transformation of CCSMCs may be a new potential target of neurogenic ED.
Mitogen-activated protein kinases (MAPKs) highly participate in embryonic development, as well as proliferation, differentiation, death, and apoptosis of the cell,10, 11 including extracellular signal-regulated kinases (ERK), the c-Jun NH2-terminal kinase (JNK), and p38 MAPK. Activation of JNK and p38 MAPK signaling pathway can promote the apoptosis in the early stage of the corpus cavernosum injury.12, 13 Furthermore, p38 MAPK signaling pathway can induce vascular smooth muscle cell (VSMC) phenotypic transformation.14, 15, 16 However, the effect of the MAPK pathway on phenotypic transformation of CCSMCs after CN injury is still poorly understood. In this study, we aimed to clarify the correlation between MAPK family and phenotypic transformation of CCSMCs after CNCI.
Materials and Methods
Animals
Animal ethics approval for this study was approved by the Committee on the Ethics of Animal Experiments of Zhejiang Chinese Medical University. All rats were kept under the specific-pathogen-free conditions in the animal facility of Zhejiang Chinese Medical University. A total of 20 Sprague–Dawley male rats (male and 8 weeks of age) with a body weight of 330–380 g (SIPPER-BK Laboratory Animals, Shanghai, China) were randomly divided into 2 groups and treated as follows. In the sham group, rats (n = 10) were sham-operated by identifying 2 CNs without causing direct damage to the CNs. In the CNCI group, rats (n = 10) underwent a bilateral cavernous nerve crush injury (CNCI) with the use of a microsurgical vascular clamp to the CN 2–3 mm distal to the major pelvic ganglion for two 60-second periods.17
Measurement of Erectile Function
At the end of 4 weeks after surgery, intracavernous pressure (ICP) and mean arterial pressure were measured to evaluate erectile function as described.18 Then, penile tissues were harvested for further histologic studies after euthanasia.
Cell Culture and Hypoxia
CCSMCs were isolated from the penis tissues of male rats (6 weeks of age) as described19, 20 and cultured by using a tissue explant–adherent method as described.21, 22 CCSMCs, which were from the passage of 3 cells, were identified by the expression of α smooth muscle actin (α-SMA) and desmin. The hypoxic group was cultured in a modular incubator chamber (StemCell, Vancouver, BC, Canada) filled with hypoxia gas (1% O2, 5% CO2, and 94% N2) and set at 37°C. The normoxic group was incubated in the same conditions as the hypoxic group except for the gas (air containing 5% CO2). After 48 hours, the morphologic features of CCSMCs cultured in normoxia or hypoxia were observed in an inverted microscope. Then, CCSMCs were harvested for further studies.
Staining and Immunohistochemistry
Hematoxylin–eosin staining was performed after the tissues were embedded in paraffin and sectioned at 4 mm. The expression of α-SMA, vimentin, ERK, JNK, and p38 in corpus cavernous was examined with immunohistochemical staining as described.7 The primary antibodies are presented in the Supplemental Materials (eTable 1). Images were captured by a LEICA DM 2500 microscope (LEICA, Wetzlar, Germany) under 200× magnification.
Western Blot Analysis
Western blot analyses were performed as described.20 The primary antibodies are presented in Supplemental Materials (eTable 1). Immunoreactivity proteins were visualized with the ChemiDoc touch Imaging System (Bio-Rad, Hercules, CA, USA) and normalized to the β-actin expression level (internal control), then semiquantitative analysis of immunoreactive proteins was performed by Image J software (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence
Parffin-embedded sections of penile tissue were incubated overnight with primary antibodies (eTable 1). After washing in phosphate-buffered saline, the sections were incubated with 2 secondary antibodies (eTable 1) in 1% bovine serum albumin at room temperature for 1 hour. Relevant antibodies were presented in images captured by a LEICA DM 2500 microscope (LEICA) under the 100× magnification.
Quantitative Real-Time Polymerase Chain Reaction
Tissues from each group were collected and lysed according to the TRIzol instructions, and total RNA was extracted. The rat α-SMA, vimentin, ERK, JNK, p38, and β-actin sequences reported in the National Center for Biotechnology Information gene database were designed using Oligo 6.71 primer analysis software, and then were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The primers sequence are presented in Supplemental Materials (eTable 2). The cDNA was synthesized from 1 μg of RNA by applying to the Bio-Rad iScript cDNA synthesis kit (Bio-Rad). Quantitative polymerase chain reaction was performed on the MJ Opticon (Bio-Rad) system using iTaq Universal SYBR Green Supermix (Bio-Rad). The relative expression of mRNA was calculated as: target gene = value of target gene / value of β-actin.
Statistics
Differences between 2 groups were analyzed by the independent t test. All variables were reported as mean ± standard deviation. A 2-sided P-value <.05 was considered statistically significant. SPSS 23.0 software (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Results
CN Injury Significantly Decreased Erectile Response
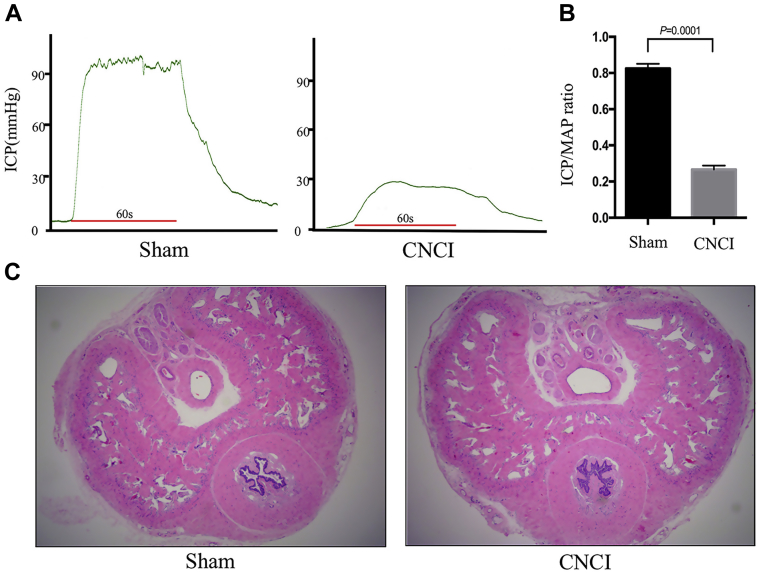
At the end of the fourth week, the CNCI group saw a significant decline in ICP compared with that of the sham group (Figure 1A). In addition, the ICP/mean arterial pressure rate of CNCI group was significantly lower than the sham group (P = .0001; sham: 0.83 ± 0.03; CNCI: 0.27 ± 0.02) (Figure 1B). Hematoxylin–eosin staining showed that the penile morphology of CNCI group was similar to the sham group when viewed on a microscope (Figure 1C).
Figure 1.
Effect of bilateral cavernous nerve injury on erectile response and corpora cavernosa morphologic alteration. Panel A shows the values of ICP in both groups. Panel B shows the ICP/MAP ratio in both groups. Panel C shows representative hematoxylin–eosin staining photomicrographs in both groups (magnification ×40). Statistical analysis by independent t test. CNCI = cavernous nerve crush injury; ICP = intracavernous pressure; MAP = mean arterial pressure.
Marker Protein Expression of Phenotypic Transformation After CN Injury
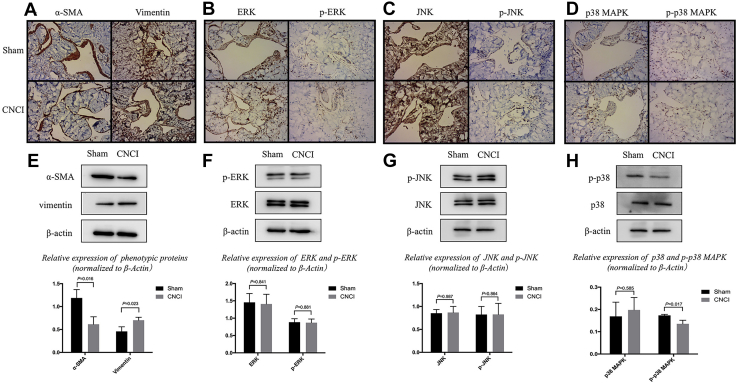
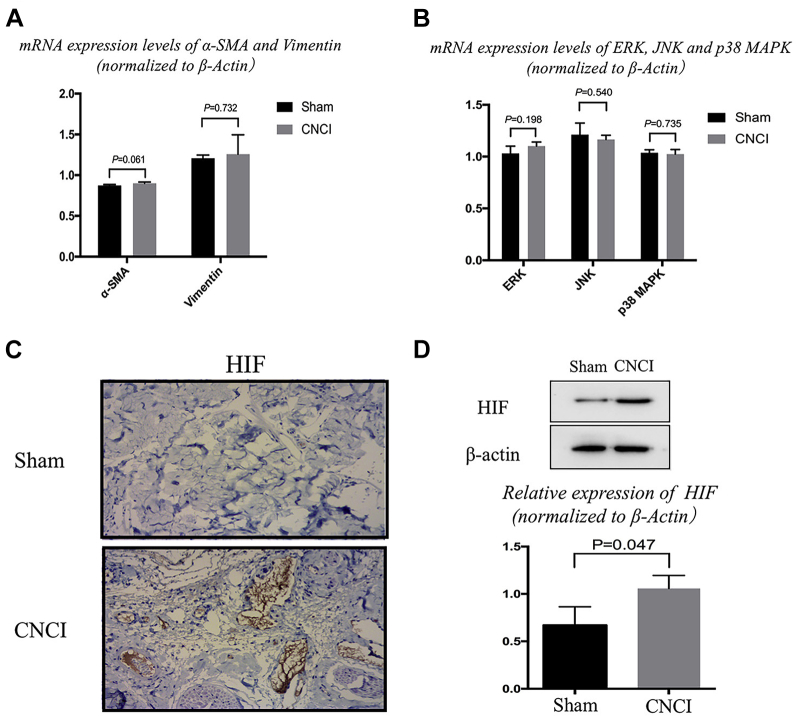
Immunohistochemical staining results revealed that the expression of α-SMA and vimentin in the CNCI group was decreased and increased, respectively, as compared with that in the sham group (Figure 2A). Subsequently, the results of western blot analysis also indicated that the expression of α-SMA and vimentin was significantly downregulated (P = .016; sham: 1.19 ± 0.19; CNCI: 0.61 ± 0.16) and upregulated (P = .023; sham: 0.46 ± 0.1; CNCI: 0.7 ± 0.06), respectively (Figure 2E). However, the mRNA levels of α-SMA and vimentin did not reach statistical significance (Figure 3A).
Figure 2.
Expression of proteins of phenotypic transformation and MAPK by immunohistochemistry and Western blot. Panel A shows the results of marker proteins of phenotypic transformation performed by immunohistochemical staining in both groups. Compared with the sham group, weak expression of α-SMA and strong expression of vimentin were detected in cytoplasm in the CNCI group. Panels B-D shows the results of MAPK presented by immunohistochemical staining in both groups. The site of expression of all was the nucleus. Only the expression of p-p38 MAPK in the sham group was stronger than that in CNCI group. In Panel E, compared with the sham group, the expression of α-SMA was decreased significantly and vimentin increased significantly in the CNCI group (mean ± standard deviation). In Panels F-H, compared with the sham group, the expression of p-p38 was significantly decreased in the CNCI group, and for the others there was no statistical significance between both groups. (mean ± standard deviation). The data were normalized by β-actin expression. α-SMA = α-smooth muscle actin; CNCI = cavernous nerve crush injury; ERK = extracellular signal-regulated kinases; JNK = the c-Jun NH2-terminal kinase; MAPK = mitogen-activated protein kinase; p-ERK = phosphorylated ERK, p-JNK = phosphorylated JNK, p-p38 MAPK = phosphorylated p38 mitogen-activated protein kinase (magnification ×200). Statistically significant (independent t test): α-SMA (P = .016); vimentin (P = .023); p-p38 MAPK (P = .017).
Figure 3.
Expression of HIF-1α in cavernous tissue. In Panels A and B, there were no significant differences in α-SMA, vimentin, ERK, JNK, and p38 between both groups. In Panel C, the result of HIF-1α was presented by immunohistochemical staining in both groups. Strong expression of HIF-1α was detected in CNCI group. In Panel D, the results of western blot show that HIF-1α increased significantly in CNCI groups compared with sham group (mean ± standard deviation) (magnification ×200). Statistically significant (independent t test): HIF-1α (P = .047). α-SMA = α-smooth muscle actin; CNCI = cavernous nerve crush injury; ERK, extracellular signal-regulated kinase; HIF-1α = hypoxia-inducible factor-1α; JNK = c-Jun NH2-terminal kinase.
Significantly Decreased Phosphorylated p38 Expression After CN injury
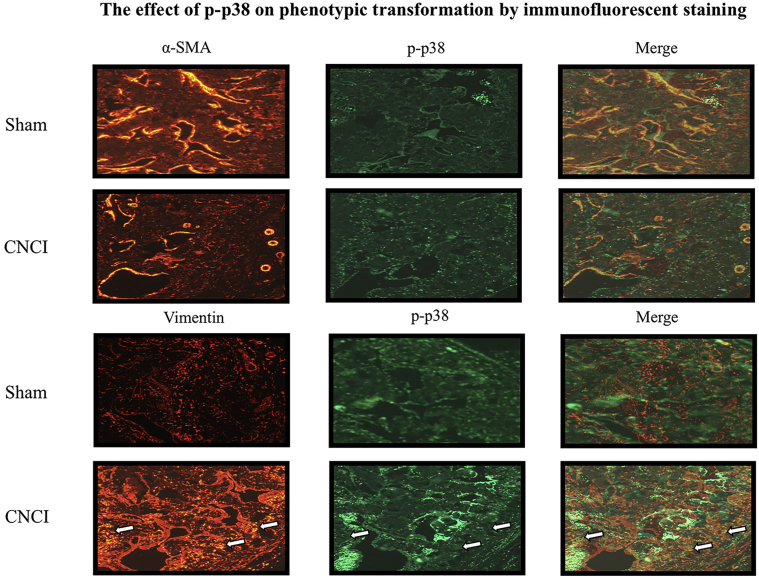
Immunohistochemical staining and western bolt were applied to investigate the expression of the MAPK family, containing p38 MAPK, phosphorylated p38 MAPK (p-p38 MAPK), JNK, phosphorylated-JNK, ERK, and phosphorylated-ERK. Only the expression of p-p38 MAPK was decreased dramatically in the CNCI group (P = .017; sham: 0.17 ± 0.005; CNCI: 0.14 ± 0.02) (Figure 2B-D, Figure 2F-H). However, the mRNA levels of p38 did not reach statistical significance (Figure 3B). Immunofluorescence staining showed that the expression of phosphorylated p38 was decreased in similar locations of the corpus cavernosum, whereas the expression of vimentin was increased (Figure 4, white arrows).
Figure 4.
The effect of p-p38 on phenotypic transformation by immunofluorescent staining. Representative expressions of α-SMA, vimentin, and p-p38 MAPK are presented. White arrows represent smooth muscle cells in the corpus cavernosum (magnification ×100). α-SMA = α-smooth muscle actin; CNCI = cavernous nerve crush injury; p-p38 MAPK = phosphorylated p38 mitogen-activated protein kinase.
Hypoxia-Inducible Factor 1-α (HIF-1α) Expression In Vivo
The expression of HIF-1α at protein level was significantly increased in the CNCI group compared with that of the sham group (P = .047; sham: 0.67 ± 0.19; CNCI: 1.06 ± 0.14) (Figure 3C and D).
CCSMC Identification and the Morphologic Change of CCSMCs After 48 Hours
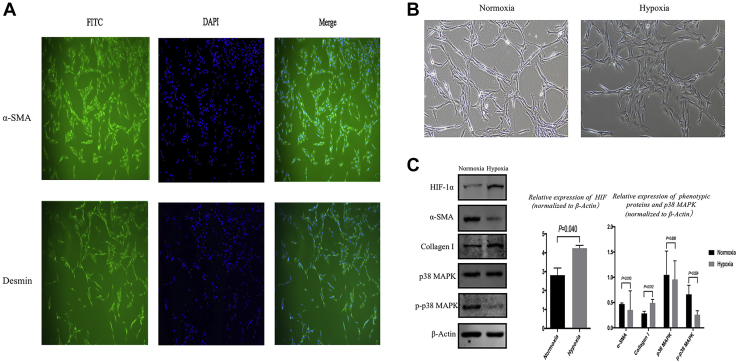
SMCs were isolated according to the positive expression of α-SMA and desmin by immunofluorescence staining (Figure 5A) as in a previous study.23 The primary CCSMCs appeared to be spindle shaped initially and then tended to become hypertrophic after hypoxia for 48 hours (Figure 5B).
Figure 5.
Phenotypic transformation and p-p38 in CCSMCs. Panel A shows the results of CCSMC identification presented by immunofluorescent staining in the 2 groups. Panel B shows the morphologic change of CCSMCs. In Panel C, compared with the normoxia group, α-SMA and p-p38 were decreased significantly in the hypoxia group, and collagen I and HIF-1α were increased significantly in the hypoxia group (mean ± standard deviation) (magnification ×100). Statistically significant (independent t test): α-SMA (P = .01); collagen I (P = .012); p-p38 MAPK (P = .024); HIF-1α (P = .04). α-SMA = α-smooth muscle actin; CCSMC = corpora cavernosum smooth muscle cell; HIF-1α = hypoxia-inducible factor-1α; MAPK = mitogen-activated protein kinase; p-p38 MAPK = phosphorylated p38 mitogen-activated protein kinase.
Phenotypic Transformation and Phosphorylated p38 In Vitro
The expression of HIF-1α and collagen I at protein level was increased significantly (P = .04; normoxia: 2.81 ± 0.38; hypoxia: 4.24 ± 0.15; P = .012, normoxia: 0.29 ± 0.04, hypoxia: 0.49 ± 0.07, respectively), but the expression of α-SMA and phosphorylated p38 MAPK at protein level was obviously decreased (P = .01, normoxia: 0.47 ± 0.02; hypoxia: 0.35 ± 0.38; P = .024, normoxia: 0.66 ± 0.18, hypoxia: 0.26 ± 0.08, respectively). However, the expression of p38 MAPK was not statistically significant (P = .808; normoxia: 1.05 ± 0.47; hypoxia: 0.96 ± 0.37) (Figure 5C).
Discussion
Phenotypic transformation is highly involved in the pathologic development of VSMCs.24 Many vascular diseases, such as atherosclerosis and restenosis, are closely associated with phenotypic changes from the contractile to synthetic phenotype for VSMCs.25, 26 Furthermore, α-SMA, SM myosin heavy chain, vimentin, and collagen I have been found as phenotypic markers for VSMCs.27, 28, 29 Also, corpus cavernosum is considered as a specific vascular tissue with phenotypic transformation.4 He et al30 have suggested that erectile function significantly improves in diabetic rats with ED by transforming CCSMCs from synthetic to the contractile phenotype. In addition, the phenotypic transformation of CCSMCs also occurs in rats with CN injury.7, 21
The occurrence of ED after RP is closely related to neurogenic factors, especially CN injury. CNCI is widely applied in animal experiments to imitate the injury that often occurs after RP. Previous studies have reported that the 1-, and 4-, and 12-week intervals represent the acute, subacute, and chronic phases following CN injury, respectively.31, 32 Yang et al7 have shown that phenotypic transformation occurs in CCSMCs at 12 weeks after CN injury. In our study, downregulated α-SMA and upregulated vimentin indicated the phenotypic transformation of CCSMCs can occur at the fourth week after CN injury. Hence, we speculated that phenotypic transformation of CCSMCs after CN injury is the main cause of pathologic development.
MAPKs can regulate various basic life activities of cells through multifunctional signals, including embryonic development, cell proliferation, cell differentiation, and cell death–related pathways.10 In mammals, ERK, JNK, and p38 are the main MAPK proteins.11 Furthermore, vascular studies show that phenotypic transformation of VSMCs is highly associated with p38 MAPK and ERK.14, 15, 16 In our study, expression of p-p38 MAPK decreased significantly in CNCI with upregulated vimentin and downregulated α-SMA. It was likely that p38 MAPK also participated in the occurrence of phenotypic transformation of CCSMC after CNCI.
We also found that HIF-1α was increased significantly at the fourth week after CNCI, and it indicated that hypoxia was already present in the corpus cavernosum at the fourth week after CNCI. In vitro, the expression of p-p38 MAPK also decreased significantly in isolated CCSMCs with the downregulated α-SMA and the upregulated collagen I after acute hypoxia for 48 hours. Therefore, we speculated that hypoxia could induce the phenotypic transformation of CCSMCs after CNCI and that it might be closely associated with the downregulated p-p38 MAPK.
However, based on the current studies,11 we found that p38 MAPK usually is activated by environmental stresses and inflammatory cytokines. For example, phosphorylated p38 is upregulated by various pathogenic factors such as angiotensin II and streptozotocin.33, 34, 35 However, our results were inconsistent with these studies. Downregulation of the p38 activity might promote the production of synthetic SM cells. Therefore, the p38 MAPK signaling pathway might play a critical role in ED after CNCI. However, the potential effect of p38 still needs to be further studied.
There were some limitations in our study. First, we did not use a blocking or activating method to determine the causal relationship between dysregulated p38 and phenotypic transformation of cavernosal SM in vivo and vitro. However, we identified that p38 rather than ERK or JNK might involve phenotypic transformation of cavernosal tissue following partial CN damages. Second, our experiments were performed at only one time point (4 weeks) after CN injury. However, the selection of time point based on some previous studies.7, 9
Conclusions
In summary, hypoxia can induce the phenotypic transformation of CCSMCs after CNCI, and the occurrence of phenotypic transformation is likely associated with the downregulated p-p38 MAPK. Moreover, p38 MAPK might be a potential target for the treatment of ED by reversing the phenotypic transformation of CCSMC. Finally, the causal relationship between p38 MAPK signaling pathway and phenotypic transformation still needs to be further clarified.
Statement of Authorship
Category 1
-
(a)Conception and Design
- Sixiang Chen; Xiaojun Huang; Bodong Lv
-
(b)Acquisition of Data
- Xianghui Kong; Zhaohui Sun
-
(c)Analysis and Interpretation of Data
- Xianghui Kong; Zhaohui Sun; Miaoyong Ye; Ke Ma
Category 2
-
(a)Drafting the Article
- Sixiang Chen; Xiaojun Huang
-
(b)Revising It for Intellectual Content
- Sixiang Chen; Fan Zhao; Wenjie Huang; Tingting Tao
Category 3
-
(a)Final Approval of the Completed Article
- Sixiang Chen; Xiaojun Huang
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: This work was supported by National Natural Science Foundation of China (No. 81874400) and Zhejiang Provincial Natural Science Foundation of China (No. LY17H040013).
Supplementary data related to this article can be found at https://doi.org/10.1016/j.esxm.2019.08.005.
Supplementary Data
References
- 1.Yafi F.A., Jenkins L., Albersen M. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez-Salamanca J.I., La Fuente J.M., Martínez-Salamanca E. α1A-adrenergic receptor antagonism improves erectile and cavernosal responses in rats with cavernous nerve injury and enhances neurogenic responses in human corpus cavernosum from patients with erectile dysfunction secondary to radical prostatectomy. J Sex Med. 2016;13:1844–1857. doi: 10.1016/j.jsxm.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Nehra A., Goldstein I., Pabby A. Mechanisms of venous leakage: a prospective clinicopathological correlation of corporeal function and structure. J Urol. 1996;156:1320–1329. doi: 10.1016/s0022-5347(01)65578-2. [DOI] [PubMed] [Google Scholar]
- 4.Kovanecz I., Nolazco G., Ferrini M.G. Early onset of fibrosis within the arterial media in a rat model of type 2 diabetes mellitus with erectile dysfunction. BJU Int. 2009;103:1396–1404. doi: 10.1111/j.1464-410X.2008.08251.x. [DOI] [PubMed] [Google Scholar]
- 5.Rangrez A.Y., Massy Z.A., Metzinger-Le Meuth V. mIR-143a and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet. 2011;4:197–205. doi: 10.1161/CIRCGENETICS.110.958702. [DOI] [PubMed] [Google Scholar]
- 6.Wei A.Y., He S.H., Zhao J.F. Characterization of corpus cavernosum smooth muscle cell phenotype in diabetic rats with erectile dysfuction. J Int J Impot Res. 2012;24:196–201. doi: 10.1038/ijir.2012.16. [DOI] [PubMed] [Google Scholar]
- 7.Yang F., Zhao J.F., Shou Q.Y. Phenotypic modulation of corpus cavernosum smooth muscle cells in a rat model of cavernous neurectomy. PLoS One. 2014;15 doi: 10.1371/journal.pone.0105186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu X., Fandel T.M., Lin G. Cavernous smooth muscle hyperplasia in a rat model of hyperlipidemia-associated erectile dysfunction. BJU Int. 2011;108:1866–1872. doi: 10.1111/j.1464-410X.2011.10162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song W.H., Son H., Kim S.W. Role of Jun amino-terminal kinase (JNK) in apoptosis of cavernosal tissue during acute phase after cavernosal nerve injury. Asian J Androl. 2018;20:50–55. doi: 10.4103/aja.aja_10_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raman M., Chen W., Cobb M.H. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 11.Morrison D.K. MAP kinase pathways. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a011254. pii: a011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lysiak J.J., Yang S.K., Klausner A.P. Tadalafil increases Akt and extracellular signal-regulated kinase 1/2 activation, and prevents apoptotic cell death in the penis following denervation. J Urol. 2008;179:779–785. doi: 10.1016/j.juro.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Park J., Chai J.S., Kim S.W. Inhibition of Jun N-terminal kinase improves erectile function by alleviation of cavernosal apoptosis in a rat model of cavernous nerve injury. Urology. 2018;113:253.e9–253.e16. doi: 10.1016/j.urology.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X., Chen J., Wang S. Serum amyloid A Induces a vascular smooth muscle cell phenotype switch through the p38 MAPK signaling pathway. Biomed Res Int. 2017;2017:4941379. doi: 10.1155/2017/4941379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi K., Takahashi M., Nishida W. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res. 2001;89:251–258. doi: 10.1161/hh1501.094265. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z., Niu J., Zhang Z. The role of lysophosphatidic acid receptors in phenotypic modulation of vascular smooth muscle cells. Mol Biol Rep. 2010;37:2675–2686. doi: 10.1007/s11033-009-9798-6. [DOI] [PubMed] [Google Scholar]
- 17.Cho M.C., Park K., Kim S.W. Restoration of erectile function by suppression of corporal apoptosis, fibrosis and corporal veno-occlusive dysfunction with Rho-kinase inhibitors in a rat model of cavernous nerve injury. J Urol. 2015;193:1716–1723. doi: 10.1016/j.juro.2014.10.099. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita S., Fujii S., Kamiyama Y. Impact of tissue sealing sheet on erectile dysfunction in a rat model of nerve-sparing radical prostatectomy. J Sex Med. 2016;13:1448–1454. doi: 10.1016/j.jsxm.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Pilatz A., Schultheiss D., Gabouev A.I. Isolation of primary endothelial and stromal cell cultures of the corpus cavernosum penis for basic research and tissue engineering. Eur Urol. 2005;47:710–718. doi: 10.1016/j.eururo.2005.01.008. discussion 718–719. [DOI] [PubMed] [Google Scholar]
- 20.Chung H., Jung S.H., Ryu J.K. Isolation and characterization of smooth muscle cells from rat corpus cavernosum tissue for the study of erectile dysfunction. Korean J Urol. 2012;53:556–563. doi: 10.4111/kju.2012.53.8.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X., Zhao J.F., Zhao F. The protective effect of salidroside on hypoxia-induced corpus cavernosum smooth muscle cell phenotypic transformation. Evid Based Complement Alternat Med. 2017;2017:3530281. doi: 10.1155/2017/3530281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv B., Zhao J., Yang F. Phenotypic transition of corpus cavernosum smooth muscle cells subjected to hypoxia. Cell Tissue Res. 2014;357:823–833. doi: 10.1007/s00441-014-1902-0. [DOI] [PubMed] [Google Scholar]
- 23.Dahiya R., Sikka S., Hellstrom W.J. Phenotypic and cytogenetic characterization of a human corpus cavernosum cell line (DS-1) Biochem Mol Biol Int. 1993;30:559–569. [PubMed] [Google Scholar]
- 24.Beamish J.A., He P., Kottke-Marchant K. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:467–491. doi: 10.1089/ten.teb.2009.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Numaga-Tomita T., Shimauchi T., Oda S. TRPC6 regulates phenotypic switching of vascular smooth muscle cells through plasma membrane potential-dependent coupling with PTEN. FASEB J. 2019;33:9785–9796. doi: 10.1096/fj.201802811R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma S., Motevalli S.M., Chen J. Precise theranostic nanomedicines for inhibiting vulnerable atherosclerotic plaque progression through regulation of vascular smooth muscle cell phenotype switching. Theranostics. 2018;8:3693–3706. doi: 10.7150/thno.24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L., Gao L., Nickel T. Lactate promotes synthetic phenotype in vascular smooth muscle cells. Circ Res. 2017;121:1251–1262. doi: 10.1161/CIRCRESAHA.117.311819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orr A.W., Lee M.Y., Lemmon J.A. Molecular mechanisms of collagen isotype-specific modulation of smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2009;29:225–231. doi: 10.1161/ATVBAHA.108.178749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong W., Li B., Yang P. CD137-CD137L interaction modulates neointima formation and the phenotype transformation of vascular smooth muscle cells via NFATc1 signaling. Mol Cell Biochem. 2018;439:65–74. doi: 10.1007/s11010-017-3136-4. [DOI] [PubMed] [Google Scholar]
- 30.He S., Zhang T., Liu Y., Liu L. Myocardin restores erectile function in diabetic rats: phenotypic modulation of corpus cavernosum smooth muscle cells. Andrologia. 2015;47:303–309. doi: 10.1111/and.12261. [DOI] [PubMed] [Google Scholar]
- 31.Musicki B., Champion H.C., Becker R.E. Erection capability is potentiated by long-term sildenafil treatment: role of blood flow-induced endothelial nitric-oxide synthase phosphorylation. Mol Pharmacol. 2005;68:226–232. doi: 10.1124/mol.104.010678. [DOI] [PubMed] [Google Scholar]
- 32.Kim T.B., Cho M.C., Paick J.S. Is it possible to recover erectile function spontaneously after cavernous nerve injury? Time-dependent structural and functional changes in corpus cavernosum following cavernous nerve injury in rats. World J Mens Health. 2012;30:31–39. [Google Scholar]
- 33.Shatanawi A., Romero M.J., Iddings J.A. Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho kinase/p38 mitogen-activated protein kinase/arginase pathway. Int J Physiol Cell Physiol. 2011;300:C1181–C1192. doi: 10.1152/ajpcell.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nangle M.R., Cotter M.A., Cameron N.E. Correction of nitrergic neurovascular dysfunction in diabetic mouse corpus cavernosum by p38 mitogen-activated protein kinase inhibition. Int J Impot Res. 2006;18:258–263. doi: 10.1038/sj.ijir.3901414. [DOI] [PubMed] [Google Scholar]
- 35.Abdel Aziz M.T., Rezq A.M., Atta H.M. Molecular signaling of a novel curcumin derivative versus tadalafil in erectile dysfunction. Andrologia. 2015;47:616–625. doi: 10.1111/and.12309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.