Abstract
Background
Low-intensity extracorporeal shock wave therapy (Li-ESWT) has been reported to improve erectile function in patients with moderate-to-severe erectile dysfunction (ED) or even convert phosphodiesterase type 5 inhibitors nonresponders to responders. ED is highly prevalent in hypertensive patients. The effect of Li-ESWT on an animal model of hypertension-associated ED has not been reported.
Aim
To investigate the effect of Li-ESWT on hypertension-associated ED and provide plausible mechanisms of action of Li-ESWT on local mechanisms of penile erection.
Methods
Spontaneously hypertensive rats (SHRs) in the active group (n = 13) received Li-ESWT at energy flux density 0.06 mJ/mm2 (Aries; Dornier MedTech, Wessling, Germany) twice weekly for 6 weeks. The emitter was set to zero for SHRs in the sham group (n = 12). Erectile function was assessed 4 weeks post-treatment by monitoring intracavernosal pressure (ICP) in response to electrical stimulation of cavernous nerve before and after single dose of 0.3 mg/kg intravenous sildenafil. Cavernosal tissue was then evaluated for collagen/smooth muscle content, neuronal nitric oxide synthase (nNOS), and vascular endothelial factor (CD31) expression.
Outcomes
Erectile function was assessed with ICP, erectile tissue remodeling was studied by smooth muscle/collagen ratio, nNOS and CD31 were semiquantitatively evaluated on cavernosal sections.
Results
The improvement of ICP parameters was greater in Li-ESWT–treated rats compared with controls with and without sildenafil. Sildenafil led to 20% increase in area under the intracavernosal pressure curve measured during the entire response/mean arterial pressure at 10 Hz in ESWT_SHR + sildenafil compared with ESWT_SHR. The smooth muscle/collagen ratio increased 2.5-fold in Li-ESWT compared with sham. Expression of CD31 tended to be increased whereas nNOS was unchanged.
Conclusions
Li-ESWT by Aries may represent an effective noninvasive therapeutic alternative and a relevant add-on therapy to phosphodiesterase type 5 inhibitors for ED in hypertensive patients, and it is suggested that it acts via remodeling of the penile tissue and promoting cavernosal vascularization.
Assaly R, Giuliano F, Clement P, et al. Extracorporeal Shock Waves Therapy Delivered by Aries Improves Erectile Dysfunction in Spontaneously Hypertensive Rats Through Penile Tissue Remodeling and Neovascularization. Sex Med 2019;7:441–450
Key Words: Low-Intensity Extracorporeal Shock Wave Therapy, Erectile Dysfunction, Hypertension, Fibrosis
Introduction
Several cross-sectional and longitudinal studies have reported that cardiovascular diseases (CVDs) represent a major risk factor for ED.1 In particular, the prevalence of erectile dysfunction (ED) is greater in men with hypertension (HTN)2, 3 and ranges from 17 to 79% in men with HTN compared with 7 to 24% in normotensive men.4 Penile erection corresponds to filling of the sinusoidal spaces (trabeculae) of the corpora cavernosa with blood caused by concomitant increase in blood inflow and decrease in blood outflow. The role of the nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) pathway in regulating blood engorgement of erectile tissues is well established.5 In response to sexual stimuli, local NO release from endothelial cells and nerve endings result in cGMP formation and smooth muscle relaxation. The enzyme phosphodiesterase type 5 degrades cGMP and therefore phosphodiesterase type 5 inhibitors (PDE5is; eg, sildenafil) were developed as pharmacologic treatments for ED.6
In contrast, men with CVD have impaired NO bioavailability/bioactivity. The potential mechanisms for the pathogenic link for reduced NO bioavailability in HTN include increased generation of reactive oxygen species, secondary to endothelial dysfunction, which scavenge NO, thereby reducing NO bioavailability. Also, decreased NO production in CVD has been reported due to defects in the L-arginine/NO pathway, genetic poly-morphisms in endothelial nitric oxide synthase (eNOS), and reduced availability of cofactors essential to NO formation.7 This may explain why men with CVD are more likely to be nonresponders to PDE5is.8, 9 Moreover, PDE5is are “symptomatic” treatments with no disease-modifying effect on any of the underlying causes of ED. Thus, recently, importance has been given to research for new management strategies of ED with a rehabilitative or curative effect in patients with CVD including HTN.
In the search for a new treatment modality that would provide a disease-modifying effect on ED, Vardi et al10, 11 reported that low-intensity extracorporeal shockwave therapy (Li-ESWT) (i) improved penile hemodynamics and erectile function in patients with ED who respond to PDE5is, or even (ii) converted PDE5i nonresponders to responders.12 Indeed, several randomized clinical trials strongly suggest that Li-ESWT improves erectile function in patients suffering from ED, particularly of vasculogenic etiology,13 and may become a first-line nonpharmacologic treatment for these patients.14
Animal investigations were conducted to explore the mode of action of Li-ESWT in erection. Results obtained in a rat model of type I diabetes showed an increased expression of the endothelial and neuronal NO synthases, eNOS and nNOS, in penile tissue, suggesting NO/cGMP signaling pathway as a target of Li-ESWT in restoring erectile function.15 However, these described effects on protein expression do not seem to have functional relevance, as reported by a recent study that showed in a rat model of type II diabetes, a NO/cGMP pathway-independent proerectile action of Li-ESWT in isolated erectile tissue.16 Furthermore, it has been reported that Li-ESWT enhanced the expression of vascular endothelial growth factor, induced neovascularization, and improved myocardial ischemia.17, 18
Aims
The aims of this study were to explore (i) the therapeutic effect of Li-ESWT alone and combined with sildenafil, in a rat model of HTN-associated ED and (ii) possible mechanisms for the effect of Li-ESWT in this experimental model. The model used is the spontaneously hypertensive rat (SHR). SHRs were previously described to display impaired erectile responses compared with their age-matched counterparts and respond only partially to sildenafil.19, 20 To investigate possible mechanisms, collagen, smooth muscle, microvascularization, and nNOS-positive nerves were explored using histologic/immunohistochemical approaches in erectile tissue of sham vs Li-ESWT–treated SHRs. To our knowledge, this is the first reported study of Li-ESWT in an HTN-associated ED animal model.
Material and Methods
Adult male SHRs (11 weeks of age; Elevage Janvier, Le Genest-Saint-Isle, France) were housed 1 week before the beginning of the experiments at the animal facility with free access to standard chow and water and maintained on inversed 12-hour dark/light cycle (light off 10:00 am/10:00 pm). All procedures were approved by the local ethical committee (CEE47) and performed in accordance with the legislation on the use of laboratory animals (National Institutes of Health publication No. 85-23, revised 1996) and Animal Care Regulations in force in France as of 1988 (authorization from competent French Ministry of Agriculture - Agreement No. B78-423-1, July 2017). After the 1-week acclimation period, SHR rats were randomly divided into 2 experimental groups either receiving Li-ESWT protocol (ESWT_SHR, n = 13) or not (sham_SHR, n = 12).
Li-ESWT Protocol
The Li-ESWT protocol implemented here was adapted from our previous study16 with few modifications. Treatment protocol consisted of 2 treatment sessions per week for 6 weeks. Shockwaves were delivered to isoflurane-anesthetized rats using an electromagnetic shockwave emitter attached to a compact unit (Aries; Dornier MedTech, Wessling, Germany). To facilitate coverage and transmission of the shockwaves, the penis of SHR was manually stretched and submerged in a water-filled cage (37°C), in proximity to the calibrated Li-ESWT applicator. During each session, 2000 shocks were delivered via the applicator to the penis at 2 focal 10-mm separated zones of the mid shaft and the base of the penis at an energy flux density of 0.06 mJ/mm2 and a frequency of 6Hz. Sham_SHR were treated exactly the same way as the ESWT_SHR but no shockwaves were delivered. At the end of the 6-week Li-ESWT protocol, a 4-week washout period was implemented, and then erectile function of ESWT_SHR (n = 13) and Sham_SHR (n = 12) rats was assessed using the electrical stimulation of cavernous nerve (ES CN) procedure.
Erectile Function Evaluation
Erectile function was assessed in 19- to 20-week-old anesthetized SHRs using the ES CN procedure as previously described.21, 22 In summary, the procedure consisted of measuring changes in the pressure at the corpus cavernosum (CC) in response to ES CN. Rats were anesthetized with xylazine (10 mg/kg) and ketamine (90 mg/kg) and their temperature maintained at 37°C using a homeothermic pad. Rats were tracheotomized to prevent aspiration of saliva and the carotid artery was catheterized with polyethylene tubing filled with heparinized saline (50 IU/mL) to record blood pressure via a pressure transducer (750; Elcomatic. Glasgow, United Kingdom). The CN was exposed at the lateral aspect of the prostate, with the aid of a dissecting microscope and mounted on a bipolar platinum electrode connected to an electrical stimulator (AMS 2100; Phymep, Paris, France). A 25-gauge needle connected to a catheter filled with heparinized saline (50 IU/mL) was inserted into 1 CC to monitor intracavernosal pressure (ICP).
After a 5-minute baseline recording period, electrical stimulations (6 V, 1-millisecond pulse, 45-second duration) of varying frequency (0, 2, 3, 4, 5, 7.5, and 10 Hz) were applied to CN every 3 minutes in a randomized order in view of establishing a frequency–response curve for ICP. At the end of the first series of electrical stimulation, an intravenous injection of sildenafil 0.3 mg/kg was performed, a dose shown in this model to improve erectile response without eliciting blood pressure–lowering effect.23 Then, a second series of electrical stimulation (same parameters as the first series) was applied to CN 4 minutes after intravenous sildenafil, to establish a second frequency–response curve.
Erectile responses to ES CN were expressed as a ratio of ICP (mm Hg) / mean arterial pressure (MAP; mm Hg) × 100, ICP being the difference between the ICP in the flaccid state, ie, before stimulation and ICP during the plateau phase of the erectile response, and MAP, the mean arterial pressure during the plateau phase and a ratio of AUC45/MAP, with AUC45 being the area under the ICP curve measured during the 45 seconds of electrical stimulation. In addition, the penile detumescence was reported by computing the ratio of AUCtot/MAP, with AUCtot being the area under the ICP curve measured during the entire response.
At the end of the ES CN experiment, the rats were euthanized and the penis of 6 of 13 sham_SHR and 8 of 12 ESWT_SHR were harvested and cut into 2 parts with the proximal division being fixed in 10% neutral buffered formalin solution for 48 hours and the distal division being cryopreserved for histologic and immunohistological analyses, respectively.
Histologic Analysis
The fixed proximal portion of the penis of 6 of 13 sham_SHR and 8 of 12 ESWT_SHR was paraffin embedded and 5-μm sections were performed using a microtome (RM 2245; Leica Biosystems, Nanterre, France). The sections were positioned on SuperFrost plus glass slides (Thermo Fisher Scientific, Waltham, MA, USA), dried, and stained for evaluation of smooth muscle/collagen ratio with Masson’s trichrome method using an automated multistainer (ST5020 &CV5030 Coverslipper; Leica Biosystems). The acquisition of slides images was then performed using Aperio AT2 - Scanner for On-Screen Diagnosis (Leica Biosystems). The percent of smooth muscle and the percent of collagen were computed in the total area of CC from each section by a blinded experimenter using the positive pixel count algorithm (Leica Biosystems). For each animal, 4 transverse sections of penis were stained and analyzed and a mean smooth muscle/collagen ratio was determined.
Immunohistological Analysis: CD31 and nNOS
Antibody against CD31 (1:500, Mouse Monoclonal CD31/PECAM-1 Antibody; Bio-Techne, Lille Cedex, France) was used as a marker of penile microvascularization. Antibody against nNOS (1:1000, anti-nNOS neuronal antibody; BD Biosciences, Franklin Lakes, NJ, USA) was used as a marker of nonadrenergic noncholinergic neurons. The frozen division of the penis of 6 of 13 sham_SHR and 8 of 12 ESWT_SHR was sectioned (10 μm in thickness) using a cryostat and sections were positioned on SuperFrost plus glass slides, dried for 30 minutes at room temperature, and stored at –20°C until immuno-detection processing.
Tissue sections were incubated with the primary antibody. The sections were then washed and incubated with the corresponding secondary antibody according to the manufacturer’s instructions. After rinsing, color reaction was detected using Vector Impact DAB (Vector Laboratories, Burlingame, CA, USA). The slides were then counterstained using hematoxylin, dehydrated, and mounted. Two negative control sections were obtained by replacing either the secondary or the primary antibody by the corresponding buffer. For each animal, 4 sections per marker were processed for semiquantitative analysis of CD31 and nNOS expression. For CD31, DAB-stained dots were manually counted on 4 sections per animal and 4 fields per section at ×200 magnification and the number of dots was then reported per section and per animal. For nNOS, DAB-stained dots were manually counted on both sides of the CC at ×400 magnification on 4 tissue sections per animal and the number of dots was then reported per section and per animal. The expression of nNOS also was evaluated on dorsal nerve branches and around dorsal arteries on 4 sections per animal and 2 fields per section at ×400 magnification by scoring the intensity of labeling as follows: 0; absence, 1; mild; 2; moderate; 3, intense. The scores were then averaged per section and per animal. These semiquantitative analyses were performed by an experimenter blinded to the experimental groups.
Statistical Analysis
All results are presented as mean ± standard error of the mean. For ES CN data, comparisons of frequency–response curves were performed using 2-way analysis of variance (anova) test with treatment (ESWT or sham with or without intravenous sildenafil) and frequency of stimulation as parameters. For histologic study, the Student t test was used to compare percent of collagen and percent smooth muscle as well as smooth muscle/collagen ratio and number of immunopositive dots. The nonparametric Mann–Whitney U test was used to compare scores of immunolabeling intensity. Statistical analysis was performed with GraphPad Prism 6.05 software (GraphPad, San Diego, CA, USA). P values <.05 were considered significant.
Drugs and Chemicals
Sildenafil citrate was purchased from Carbosynth Ltd. (Newbury, United Kingdom). All other drugs and chemicals were purchased from Sigma-Aldrich (Lyon, France).
Results
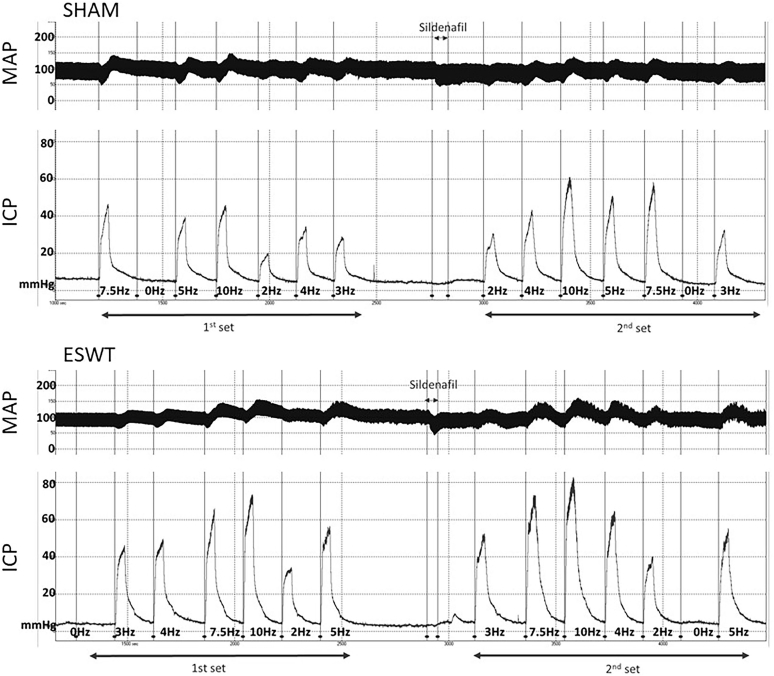
SHRs were treated with Li-ESWT or sham treatment (12 sessions). All SHRs tolerated the Li-ESWT well. Some penile reddening and petechia were observed immediately after treatment but subsided within 24 hours. At 4 weeks after the final treatment, each rat was assessed by ES CN first without, then with intravenous 0.3 mg/kg sildenafil (Figure 1, representative tracings). Hence, the ES CN data consist of 4 groups: sham_SHR; sham_SHR + sildenafil; ESWT_SHR; and ESWT_SHR + sildenafil.
Figure 1.
Representative tracings of ICP and MAP. ESWT = extracorporeal shock wave therapy; ICP = intracavernosal pressure; MAP = mean arterial pressure.
Effect of Li-ESWT Protocol on Erectile Function
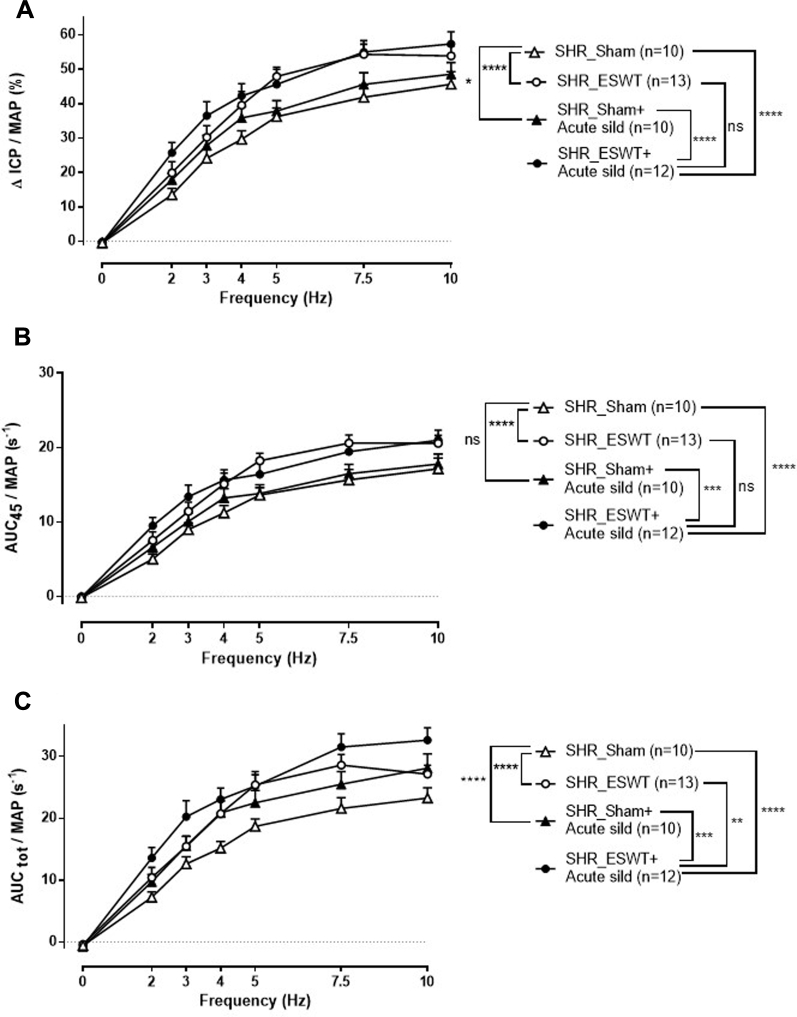
In sham_SHR, intravenous sildenafil led to increased erectile responses to ES CN (Figure 2). In ESWT_SHR, erectile responses were improved, as shown by the significant increase in ICP/MAP and AUC45/MAP when compared with sham_SHR (2-way anova, P < .0001; Figure 2A and B). The penile detumescence was also improved in ESWT_SHR. as shown by the significant increase in AUCtot/MAP when compared with sham_SHR (2-way anova, P < 0.0001; Figure 2C). In addition, erectile function of ESWT_SHR was significantly increased compared with erectile function of sham_SHR + sildenafil (2-way anova, P < .01 for ICP/MAP; P < .001 for AUC45/MAP; Figure 2).
Figure 2.
Effect of Li-ESWT protocol delivered by Aries on erectile function in SHRs. Erectile responses to ES CN of varying frequency were obtained in anesthetized rats who received (SHR_ESWT) or not (sham) the Li-ESWT protocol. The first series of ES CN was performed in absence of sildenafil and the second one after intravenous delivery of 0.3 mg/kg sildenafil (sild). Erectile responses are expressed as ICP/MAP (Panel A), AUC45/MAP (Panel B), and erectile detumescence as AUCtot/MAP (Panel C). Data are mean ± standard error of the mean. Statistics: 2-way analysis of variance; **P < .01, ***P < .001, ****P <.0001 overall significance. AUC45 = area under the intracavernosal pressure curve measured during the 45 seconds of electrical stimulation; AUCtot = area under the intracavernosal pressure curve measured during the entire response; ES CN = electrical stimulation of cavernous nerve; ICP = intracavernosal pressure; Li-ESWT = low-intensity extracorporeal shock wave therapy; MAP = mean arterial pressure; SHR = spontaneously hypertensive rat.
Effect of Li-ESWT Protocol Combined With Sildenafil on Erectile Function
The effect of intravenous sildenafil on erectile responses was potentiated by Li-ESWT, as shown by the significant increase (2-way anova, P < .001) in ICP/MAP and AUC45/MAP in ESWT_SHR + sildenafil as compared with sham_SHR + sildenafil (Figure 2A and B). Erection duration of ESWT_SHR + sildenafil was also significantly increased compared with ESWT alone (+20% increase; 2-way anova, P < .01 for AUCtot/MAP) (Figure 2C).
Effect of ESWT Protocol on Smooth Muscle/Collagen Ratio in CC
At microscopic level, CC is characterized by sinusoidal spaces (trabeculae) separated by collagen (blue staining in Figure 3), the main component of connective tissue, and smooth muscle cells (red staining in Figure 3). Total cavernosal area was not significantly different between ESWT_SHR and sham_SHR. However, a 3-fold increase in the smooth muscle/collagen ratio was found in ESWT_SHR (Figure 3B) as compared with sham_SHR (Figure 3A), a result explained by an increase in smooth muscle and decrease in collagen in ESWT_SHR (Table 1).
Figure 3.
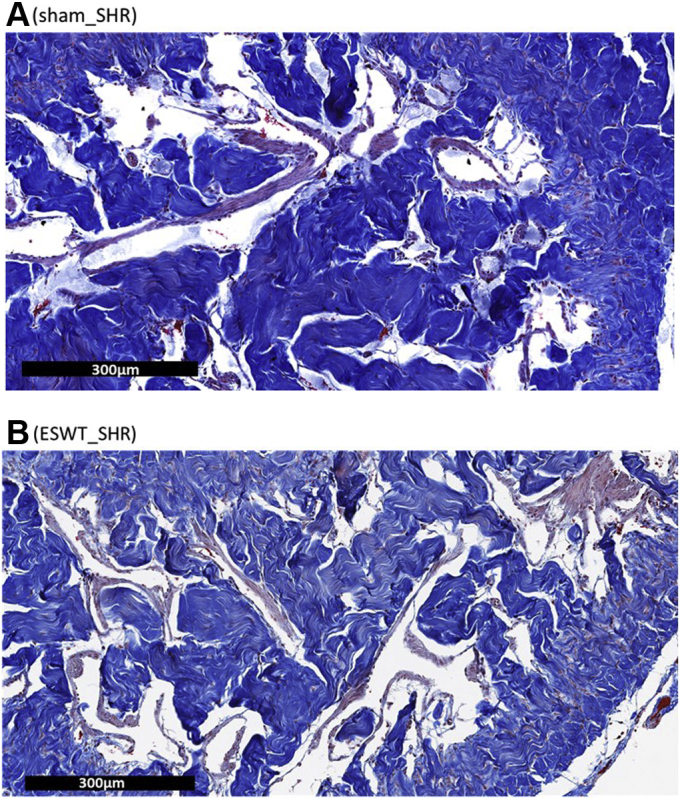
Representative images of SHR corpus cavernosum sections stained with Masson’s trichrome. Sections of penis were obtained in sham rats (Panel A) or rats who received the Li-ESWT protocol delivered by Aries (Panel B). Collagen fibers stained blue and smooth muscle fibers stained red. Magnification ×400. Li-ESWT = low-intensity extracorporeal shock wave therapy; SHR = spontaneously hypertensive rat.
Table 1.
Quantitative measurements from Masson’s trichrome staining
| Sham SHR (n = 6) | ESWT SHR (n = 8) | |
|---|---|---|
| Collagen, % | 51 ± 4.1 | 38 ± 5.6 |
| Smooth muscle, % | 29 ± 3.5 | 43 ± 6.2 |
| Ratio smooth muscle/collagen | 0.6 ± 0.1 | 1.9 ± 0.6 |
| Total cavernosal area, mm2 | 3.38 ± 0.12 | 3.87 ± 1.5 |
Effect of Li-ESWT protocol delivered by Aries on cavernosal histology in SHR. Masson’s trichrome staining was performed on penis section of rats who received (SHR_ESWT) or not (sham) the Li-ESWT protocol. The amount of collagen and smooth muscle fibers is expressed as percentage of the total area of cavernosal tissue. Data are mean ± standard error of the mean.
Li-ESWT = low-intensity extracorporeal shock wave therapy; SHR = spontaneously hypertensive rat.
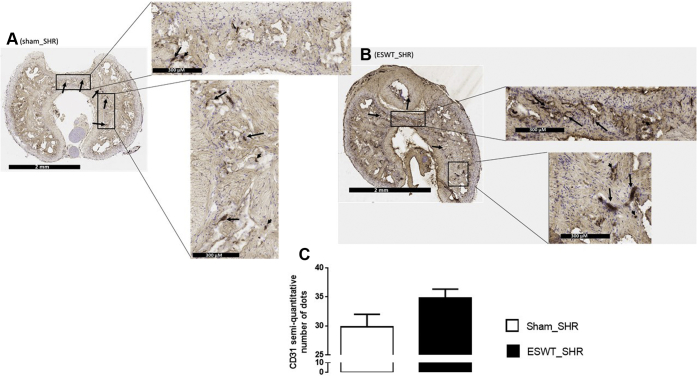
Effect of ESWT Protocol on CD31 Expression in CC
In both ESWT_SHR and sham_SHR, CD31 immunopositive cells were identified mostly on the inner wall surface of the vasculature or inside the vessel walls of the CC (black arrows, Figure 4). A cytoplasmic reactivity was also occasionally seen (black arrowhead, Figure 4). Semiquantitative analysis revealed a trend for an increase in CD31 expression in ESWT_SHR compared with sham_SHR (Student t test, P = .08; Figure 4C).
Figure 4.
Representative images of SHR corpus cavernosum sections immunostained for CD31 and semiquantitative evaluation of CD31. Sections of penis were obtained in sham rats (Panel A) or rats who received the Li-ESWT protocol delivered by Aries (Panel B). Immunopositive cells stained brown (arrows). Magnification: ×40 (left panel), ×400 (right panel). The mean number of dots is presented in Panel C. Li-ESWT = low-intensity extracorporeal shock wave therapy; SHR = spontaneously hypertensive rat.
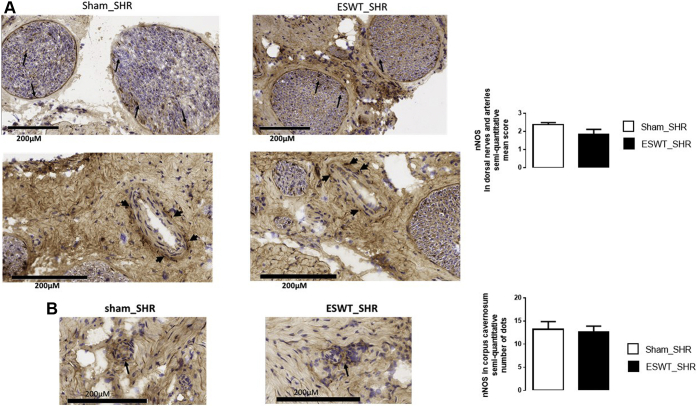
Effect of ESWT Protocol on nNOS Expression in CC
In both ESWT_SHR and sham_SHR, nNOS immunostaining was detected in the adventitia of dorsal arteries (black arrowhead, Figure 5A) and in fibers of the dorsal nerve (black arrows, Figure 5A). Moreover, a discrete diffuse pattern of immunostaining was observed within the CC likely associated with cavernosal nerve endings (black arrows, Figure 5B). Whatever the location considered, nNOS expression was similar between ESWT_SHR and sham_SHR.
Figure 5.
Representative images of SHR corpus cavernosum sections immunostained for nNOS. Sections of penis were obtained in sham rats (left panel) or rats who received the Li-ESWT protocol delivered by Aries (right panel). Immunopositive cells stain brown (arrows) were found in dorsal arteries and dorsal nerves (Panel A) and corpus cavernosum (Panel B). Magnification: ×400. Li-ESWT = low-intensity extracorporeal shock wave therapy; nNOS = neuronal nitric oxide synthase; SHR = spontaneously hypertensive rat.
Discussion
In this study, the effect of ESWT was assessed in SHRs, a well-established and clinically predictive model of ED associated with HTN. It was previously shown that SHRs display impaired erectile responses compared with their age-matched counterparts WKY rats.24 This result was further confirmed in studies using the SHR model.25, 26 Therefore, we considered that a WKY control group was not necessary in this study to comply with ethical requirements in force in the European Union as of 2013, ie, reduction of animal number, even though this constitutes a limitation of this study, with WKY being necessary for comparison of ICP and histology results. Although a WKY group was not included, comparison of SHR responses in this study with historical data from age-matched WKY shows that erectile responses of SHR at 10 Hz were decreased by 50% compared with age-matched normotensive WKY.
The protocol of Li-ESWT delivered by the Aries applied to SHRs was found effective, which extends the beneficial role of Li-ESWT on ED in experimental diabetes15, 16 to experimental HTN. In the model of HTN-associated ED, intravenous sildenafil treatment improved erectile response to ES CN in SHRs, confirming previous observations.25 The pro-erectile effect of sildenafil in SHRs was characterized by a more marked increase in AUCtot/MAP of the erectile response that takes into account the detumescence phase of erection, ie, return to flaccid state once ES CN is over. The other parameters measuring intensity of penile tumescence (ICP/MAP) or its maintenance during stimulation (AUC45/MAP) were not significantly increased, indicating mild response to sildenafil in SHRs. Erectile function relies on the recruitment of the NO/cGMP pathway.5, 6 Within erectile tissue, NO, by activating the enzyme catalyzing cGMP synthesis (soluble guanylate cyclase), causes increase in intracellular cGMP levels that lead to vasodilation of penile arteries and relaxation of cavernosal smooth muscle cells. The resulting blood engorgement of erectile tissue is responsible for penile erection. Degradation of cGMP levels is regulated by cGMP-specific PDEs, among which type 5 PDE is the most predominant in the human cavernosal tissue.27 The PDE5is, first-line treatment for ED, act in promoting cGMP accumulation more specifically in erectile tissue and thus require a functional, at least partially, NO/cGMP pathway. Indeed, men suffering from conditions associated with reduced NO bioavailability (eg, diabetes, HTN, atherosclerosis, etc) benefit less than the general population from PDE5is.7, 28 It also has been reported in SHRs that levels of expression of eNOS and nNOS were reduced in cavernosal tissue29 as well as an impaired relaxation mediated by neurogenic and endothelial NO of the cavernosal strips.24, 30 Thus, impairment of NO/cGMP pathway in SHRs may explain reduced efficacy of sildenafil in SHRs as observed in the present experiments.
Then, it was hypothesized that Li-ESWT improved erectile function in SHRs through modulation of NO/cGMP pathway. It has indeed been shown that Li-ESWT enhances NO synthesis in cells31 and increases levels of expression of eNOS and nNOS in cavernosal tissue of rats with streptozotocin-induced diabetes.15, 32, 33 Conversely, functional studies did not confirm the reported effect on protein expression. Indeed, the pro-erectile action of Li-ESWT was found independent of NO/cGMP pathway in Goto–Kakizaki rats, a model of type II diabetes.16 The latest findings are corroborated by the present immunohistochemical investigation in penile tissues of SHRs revealing no difference in the expression of nNOS-positive nerves neither in the CC nor around the dorsal arteries or within the dorsal nerves in ESWT SHRs, suggesting a NO/cGMP-independent pathway for the pro-erectile effect of Li-ESWT in SHRs.
Accumulation of collagen in SHR corpora cavernosa has already been reported.24, 34 Clinically, increase in connective tissue content including collagen to the detriment of smooth muscle cells is a common histological feature in erectile tissue of patients with ED.35, 36 Collagen deposit is hypothesized to cause stiffness of the cavernosal tissue, to reduce the corporal smooth muscle compliance and elasticity, and to modify the intercellular communication through cell-to-cell contact, leading to diminution of blood engorgement of the penis. A 3-fold increase in the smooth muscle/collagen ratio in CC of SHRs subject to Li-ESWT protocol has been found. Thus, we postulate that the facilitator effect on erectile response of Li-ESWT in SHR could be attributed, at least partly, to the remodeling effect of Li-ESWT delivered by Aries on the corporal architecture of SHRs. Indeed, Li-ESWT has been reported to induce collagen matrix changes in tendinous structures in ponies,37 to inhibit the expression of transforming growth factor beta 1 in post-burn hypertrophic scars contributing to the antifibrotic effects of ESWT38 and to recruit endogenous MSCs with up-regulation of alpha-smooth muscle actin.33 In addition, it is to be verified in future studies whether a modification of the Li-ESWT protocol, ie, greater frequency and/or prolonging shock waves stimulation, may lead to more pronounced effects on cavernosal tissue remodeling.
It is well known that improving vascularization to erectile tissue is crucial in restoring erectile function. The investigations in animal models of ischemia18, 39 or diabetes40 support the potential effect of Li-ESWT in promoting neovascularization possibly by upregulating expression of angiogenic-related growth factors.32, 33 Furthermore, it was recently reported that Li-ESWT enhanced angiogenesis in cavernosal tissue of streptozotocin-induced diabetes in rats.41 The results of the present immunohistochemical exploration of the expression of the vascular endothelial factor CD31 are in line with the aforementioned observations. Indeed, application of Li-ESWT protocol with Aries increased CD31 expression in CC of SHRs even though this conclusion has to be confirmed since the increase did not reach statistical significance.
Improving erectile function through the NO-cGMP independent pathway might be beneficial for the treatment of patients with vasculogenic ED and insufficient responders to PDE5is, notably patients with diagnosed CVD.8, 9 Consequently, palliating the impairment of NO bioavailability in these disease states by acting via an NO-cGMP independent pathway might be beneficial for the treatment of patients suffering from ED and not experiencing satisfactory results from PDE5is. This study showed additive effects when Li-ESWT was combined with sildenafil. Indeed, Li-ESWT delivered by Aries significantly potentiated erectile responses of SHRs treated with sildenafil alone whereas in presence of sildenafil, a significant increase in AUCtot/MAP was recorded as compared with Li-ESWT alone. Thus, these results suggest that Li-ESWT delivered by Aries may improve erectile function in patients who respond to PDE5is and convert PDE5i insufficient responders to responders.
Conclusions
This study showed that Li-ESWT with Aries may represent an effective noninvasive therapeutic alternative for ED in hypertensive patients, alone or in combination with PDE5is to salvage PDE5is insufficient responders. Moreover, this study suggests that potential mechanisms of action of Li-ESWT in HTN-associated ED may include cavernosal tissue remodeling in favor of smooth muscle cells, and increased penile microvascularization. However, statistical significance was not reached for these histologic and immunohistological evaluations and, thus, additional studies are needed to confirm these conclusions.
Statement of Authorship
Category 1
-
(a)Conception and Design
- Rana Assaly; François Giuliano; Pearline Teo; Jacques Bernabe; Delphine Behr-Roussel
-
(b)Acquisition of Data
- Rana Assaly; Miguel Laurin; Maryline Favier; Jacques Bernabe; Delphine Behr-Roussel
-
(c)Analysis and Interpretation of Data
- Rana Assaly; Miguel Laurin; Maryline Favier; Delphine Behr-Roussel
Category 2
-
(a)Drafting the Article
- Rana Assaly; Pierre Clement; Pearline Teo; François Giuliano; Delphine Behr-Roussel
-
(b)Revising It for Intellectual Content
- Laurent Alexandre; François Giuliano
Category 3
-
(a)Final Approval of the Completed Article
- Rana Assaly; François Giuliano; Pierre Clement; Miguel Laurin; Maryline Favier; Pearline Teo; Laurent Alexandre; Jacques Bernabe; Delphine Behr-Roussel
Acknowledgments
Trichrome Masson’s staining was performed by Cochin HistIM plateform, Paris, France.
Footnotes
Conflict of Interest: Pearline Teo is employee of Dornier MedTech. The other authors report no conflict of interest.
Funding: None.
References
- 1.Gandaglia G., Sun M., Popa I. Cardiovascular mortality in patients with metastatic prostate cancer exposed to androgen deprivation therapy: a population-based study. Clin Genitourin Cancer. 2015;13:e123–e130. doi: 10.1016/j.clgc.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Feldman H.A., Goldstein I., Hatzichristou D.G. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano F.A., Leriche A., Jaudinot E.O. Prevalence of erectile dysfunction among 7689 patients with diabetes or hypertension, or both. Urology. 2004;64:1196–1201. doi: 10.1016/j.urology.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 4.Hackett G., Kirby M., Wylie K. British Society for Sexual Medicine guidelines on the management of erectile dysfunction in men—2017. J Sex Med. 2018;15:430–457. doi: 10.1016/j.jsxm.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Lue T.F. Erectile dysfunction. N Engl J Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 6.Hurt K.J., Musicki B., Palese M.A. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermann M., Flammer A., Luscher T.F. Nitric oxide in hypertension. J Clin Hypertens (Greenwich) 2006;8:17–29. doi: 10.1111/j.1524-6175.2006.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saenz de Tejada I., Angulo J., Cellek S. Physiology of erectile function. J Sex Med. 2004;1:254–265. doi: 10.1111/j.1743-6109.04038.x. [DOI] [PubMed] [Google Scholar]
- 9.Kendirci M., Tanriverdi O., Trost L. Management of sildenafil treatment failures. Curr Opin Urol. 2006;16:449–459. doi: 10.1097/01.mou.0000250286.60237.a6. [DOI] [PubMed] [Google Scholar]
- 10.Vardi Y., Appel B., Kilchevsky A. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol. 2012;187:1769–1775. doi: 10.1016/j.juro.2011.12.117. [DOI] [PubMed] [Google Scholar]
- 11.Vardi Y., Appel B., Jacob G. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol. 2010;58:243–248. doi: 10.1016/j.eururo.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Gruenwald I., Appel B., Vardi Y. Low-intensity extracorporeal shock wave therapy--a novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy. J Sex Med. 2012;9:259–264. doi: 10.1111/j.1743-6109.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- 13.Lu Z., Lin G., Reed-Maldonado A. Low-intensity Extracorporeal shock wave treatment improves erectile function: a systematic review and meta-analysis. Eur Urol. 2017;71:223–233. doi: 10.1016/j.eururo.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 14.Sokolakis I., Dimitriadis F., Teo P. The basic science behind low-intensity extracorporeal shockwave therapy for erectile dysfunction: a systematic scoping review of pre-clinical studies. J Sex Med. 2019;16:168–194. doi: 10.1016/j.jsxm.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Qiu X., Lin G., Xin Z. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10:738–746. doi: 10.1111/jsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assaly-Kaddoum R., Giuliano F., Laurin M. Low intensity extracorporeal shock wave therapy improves erectile function in a model of type II diabetes independently of NO/cGMP pathway. J Urol. 2016;196:950–956. doi: 10.1016/j.juro.2016.03.147. [DOI] [PubMed] [Google Scholar]
- 17.Kuo Y.R., Wang C.T., Wang F.S. Extracorporeal shock-wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ-induced diabetes. Wound Repair Regen. 2009;17:522–530. doi: 10.1111/j.1524-475X.2009.00504.x. [DOI] [PubMed] [Google Scholar]
- 18.Nishida T., Shimokawa H., Oi K. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation. 2004;110:3055–3061. doi: 10.1161/01.CIR.0000148849.51177.97. [DOI] [PubMed] [Google Scholar]
- 19.Behr-Roussel D., Gorny D., Mevel K. Erectile dysfunction: an early marker for hypertension? A longitudinal study in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R276–R283. doi: 10.1152/ajpregu.00040.2004. [DOI] [PubMed] [Google Scholar]
- 20.Oudot A., Oger S., Behr-Roussel D. A new experimental rat model of erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: the testosterone-supplemented spontaneously hypertensive rat. BJU Int. 2012;110:1352–1358. doi: 10.1111/j.1464-410X.2012.11085.x. [DOI] [PubMed] [Google Scholar]
- 21.Bernabe J., Rampin O., Giuliano F. Intracavernous pressure changes during reflexive penile erections in the rat. Physiol Behav. 1995;57:837–841. doi: 10.1016/0031-9384(94)00309-s. [DOI] [PubMed] [Google Scholar]
- 22.Giuliano F., Rampin O., Bernabe J. Neural control of penile erection in the rat. J Auton Nerv Syst. 1995;55:36–44. doi: 10.1016/0165-1838(95)00025-s. [DOI] [PubMed] [Google Scholar]
- 23.Oger-Roussel S., Behr-Roussel D., Caisey S. Bladder and erectile dysfunctions in the type 2 diabetic Goto-Kakizaki rat. Am J Physiol Regul Integr Comp Physiol. 2014;306:R108–R117. doi: 10.1152/ajpregu.00033.2013. [DOI] [PubMed] [Google Scholar]
- 24.Behr-Roussel D., Chamiot-Clerc P., Bernabe J. Erectile dysfunction in spontaneously hypertensive rats: pathophysiological mechanisms. Am J Physiol Regul Integr Comp Physiol. 2003;284:R682–R688. doi: 10.1152/ajpregu.00349.2002. [DOI] [PubMed] [Google Scholar]
- 25.Fibbi B., Morelli A., Marini M. Atorvastatin but not elocalcitol increases sildenafil responsiveness in spontaneously hypertensive rats by regulating the RhoA/ROCK pathway. J Androl. 2008;29:70–84. doi: 10.2164/jandrol.107.003152. [DOI] [PubMed] [Google Scholar]
- 26.Wilkes N., White S., Stein P. Phosphodiesterase-5 inhibition synergizes rho-kinase antagonism and enhances erectile response in male hypertensive rats. Int J Impot Res. 2004;16:187–194. doi: 10.1038/sj.ijir.3901149. [DOI] [PubMed] [Google Scholar]
- 27.Waldkirch E., Uckert S., Sigl K. Expression and distribution of cyclic GMP-dependent protein kinase-1 isoforms in human penile erectile tissue. J Sex Med. 2008;5:536–543. doi: 10.1111/j.1743-6109.2007.00735.x. [DOI] [PubMed] [Google Scholar]
- 28.Albersen M., Shindel A.W., Mwamukonda K.B. The future is today: emerging drugs for the treatment of erectile dysfunction. Expert Opin Emerg Drugs. 2010;15:467–480. doi: 10.1517/14728214.2010.480973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y., Jiang J., He Y. Icariin combined with breviscapine improves the erectile function of spontaneously hypertensive rats. J Sex Med. 2014;11:2143–2152. doi: 10.1111/jsm.12614. [DOI] [PubMed] [Google Scholar]
- 30.Ushiyama M., Morita T., Kuramochi T. Erectile dysfunction in hypertensive rats results from impairment of the relaxation evoked by neurogenic carbon monoxide and nitric oxide. Hypertens Res. 2004;27:253–261. doi: 10.1291/hypres.27.253. [DOI] [PubMed] [Google Scholar]
- 31.Mariotto S., de Prati A.C., Cavalieri E. Extracorporeal shock wave therapy in inflammatory diseases: molecular mechanism that triggers anti-inflammatory action. Curr Med Chem. 2009;16:2366–2372. doi: 10.2174/092986709788682119. [DOI] [PubMed] [Google Scholar]
- 32.Lei H., Xin H., Guan R. Low-intensity pulsed ultrasound improves erectile function in streptozotocin-induced type I diabetic rats. Urology. 2015;86:1241–1248. doi: 10.1016/j.urology.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Zhou F., Li G.Y. Evaluation of the effect of different doses of low energy shock wave therapy on the erectile function of streptozotocin (STZ)-induced diabetic rats. Int J Mol Sci. 2013;14:10661–10673. doi: 10.3390/ijms140510661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang R., Chen J.H., Jin J. Ultrastructural comparison of penile cavernous tissue between hypertensive and normotensive rats. Int J Impot Res. 2005;17:417–423. doi: 10.1038/sj.ijir.3901329. [DOI] [PubMed] [Google Scholar]
- 35.El-Sakka A.I., Yassin A.A. Amelioration of penile fibrosis: myth or reality. J Androl. 2010;31:324–335. doi: 10.2164/jandrol.109.008730. [DOI] [PubMed] [Google Scholar]
- 36.Wespes E., Goes P.M., Schiffmann S. Computerized analysis of smooth muscle fibers in potent and impotent patients. J Urol. 1991;146:1015–1017. doi: 10.1016/s0022-5347(17)37990-9. [DOI] [PubMed] [Google Scholar]
- 37.Bosch G., de Mos M., van Binsbergen R. The effect of focused extracorporeal shock wave therapy on collagen matrix and gene expression in normal tendons and ligaments. Equine Vet J. 2009;41:335–341. doi: 10.2746/042516409x370766. [DOI] [PubMed] [Google Scholar]
- 38.Cui H.S., Hong A.R., Kim J.B. Extracorporeal shock wave therapy alters the expression of fibrosis-related molecules in fibroblast derived from human hypertrophic scar. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aicher A., Heeschen C., Sasaki K. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114:2823–2830. doi: 10.1161/CIRCULATIONAHA.106.628623. [DOI] [PubMed] [Google Scholar]
- 40.Zins S.R., Amare M.F., Tadaki D.K. Comparative analysis of angiogenic gene expression in normal and impaired wound healing in diabetic mice: effects of extracorporeal shock wave therapy. Angiogenesis. 2010;13:293–304. doi: 10.1007/s10456-010-9186-9. [DOI] [PubMed] [Google Scholar]
- 41.Shan H.T., Zhang H.B., Chen W.T. Combination of low-energy shock-wave therapy and bone marrow mesenchymal stem cell transplantation to improve the erectile function of diabetic rats. Asian J Androl. 2017;19:26–33. doi: 10.4103/1008-682X.184271. [DOI] [PMC free article] [PubMed] [Google Scholar]