Key Points
The incidence of IFIs during VEN-HMA therapy is low, and the used antifungal prophylaxis approach did not influence the risk of IFIs.
The risk of IFIs is higher in nonresponders and those who were treated in the r/r AML setting.
Abstract
The combination of venetoclax with hypomethylating agents (VEN-HMAs) showed promising activity in newly diagnosed and relapsed/refractory (r/r) acute myeloid leukemia (AML). Treatment with VEN-HMAs results in prolonged cytopenia, thereby exposing patients to invasive fungal infections (IFIs). Here, we retrospectively studied a cohort of 119 AML patients treated with VEN-HMAs and analyzed the occurrence of IFIs, as well as our practice of antifungal prophylaxis, with the aim to identify the nature and risk factors for IFIs and their association with the type of antifungal prophylaxis used. The intended antifungal prophylaxis was micafungin in 38% of patients, azoles in 41% of patients, and none in 21% of patients. Older age was associated with no antifungal prophylaxis or micafungin use and lesser use of azoles (P = .043). We recorded 15 (12.6%) patients who developed probable or proven IFIs, with a median onset of 72 days (range, 35-281) after starting therapy. IFIs were more common among nonresponders compared with responders to VEN-HMA therapy (22% vs 6%, P = .0132) and in r/r compared with newly diagnosed AML (19% vs 5%, P = .0498); however, the antifungal prophylaxis used, patient age, hypomethylating agent schedule, history of prior allogeneic transplant, and initial neutropenia duration did not influence the development of IFIs during therapy. We conclude that the overall risk of IFIs during VEN-HMA therapy is low. The risk of IFIs is higher in nonresponders and in those who were treated in the r/r setting; these patients need reevaluation of their antifungal prophylaxis to minimize the risk of IFIs during therapy.
Visual Abstract
Introduction
The combination of venetoclax and hypomethylating agents (VEN-HMAs) has emerged as the standard of care for newly diagnosed elderly and frail patients with acute myeloid leukemia (AML), and it is approved by the US Food and Drug Administration (FDA) for this indication. This combination is active, and the rate of complete remission (CR)/CR with incomplete count recovery (CRi) with VEN-HMAs is markedly higher than with single-agent hypomethylating agents (HMAs) in this setting (70% vs 15%-25%).1-3 VEN-HMAs have also been used in relapsed and refractory (r/r) AML outside of clinical studies, with encouraging results.4-6
Currently, VEN-HMA therapy is administered for an indefinite duration as long as patients remain in CR or derive hematologic benefit from this therapy. VEN-HMA therapy results in profound and prolonged cytopenia, even after achieving CR, thereby continuously exposing patients to infection risk from periods of neutropenia for months or even years. Neutropenia remains the dominant toxicity of VEN-HMAs in patients who achieve CR, and this can be abrogated to some extent by growth factor use and shortening the duration of venetoclax therapy or decreasing the HMA dose during each cycle.
Patients receiving VEN-HMAs are theoretically at highest risk for invasive fungal infections (IFIs) prior to achievement of CR, because the neutropenia is severe and often prolonged (expected to last ≥4 weeks). After achievement of remission, neutropenic periods between cycles of therapy are shorter but tend to become progressively prolonged with additional cycles. It is not clear whether continued antifungal prophylaxis is needed for patients who remain in CR, because they would be expected to have neutrophil recovery between cycles. There is a correlation between the severity and the duration of neutropenia and the risk of IFIs in patients with hematological malignancies.7-9 Limited data are available on the type of infectious complications that are encountered during VEN-HMA therapy, particularly IFIs. There is also no consensus on the choice or duration of antifungal prophylaxis for AML patients treated with VEN-HMAs or in which setting (eg, newly diagnosed vs r/r, age, responders vs nonresponders). The choice of antifungal prophylaxis is also relevant, because venetoclax is a CYP3A4 inhibitor and, therefore, has a significant interaction with the azole class of antifungals, requiring dose adjustment of venetoclax when administered concurrently.10
We reviewed data on a cohort of AML patients treated at our institution with VEN-HMAs and analyzed the occurrence of IFIs and our practice of antifungal prophylaxis during therapy, to identify the types and risk factors for IFIs, as well as the type and efficacy of antifungal prophylaxis.
Patients and methods
Study design and patients
This is a retrospective analysis of AML patients treated with VEN-HMAs at City of Hope Medical Center between February of 2016 and December of 2018. This study included adults who received the combination for newly diagnosed or r/r AML. Patients with IFIs prior to starting VEN-HMA therapy were excluded from the analysis. The study was approved by the City of Hope Institutional Review Board. Objectives of the study were to describe the incidence, type, and risk factors for IFIs during VEN-HMA treatment and antifungal prophylaxis utilization patterns during this therapy, as well as to examine the breakthrough IFIs between antifungal prophylaxes administered and the rate of IFIs in patients not on any prophylaxis prior to the onset of IFIs during treatment.
We recorded probable or proven IFIs (as per European Organization for Research and Treatment of Cancer/Mycoses Study Group definitions) that occurred between the time of initiation of VEN-HMA treatment and when the patient underwent allogeneic hematopoietic cell transplantation (alloHCT), 7 days after starting a different salvage therapy in patients with evidence of leukemia progression, or within 30 days after discontinuing venetoclax in patients who did not receive an alternate antileukemia therapy.11 We recorded the antifungal prophylaxis at the time of initiating therapy (intended antifungal prophylaxis). In a small subset of patients (n = 10), their antifungal prophylaxis could have been modified during the course of treatment, and that was reviewed. The administered antifungal prophylaxis was at the discretion of the treating physician. A subset of newly diagnosed AML patients (n = 15) in this cohort was treated in the phase 1/2 VEN-HMA study (NCT02203773), in which no azoles were allowed; therefore, these patients received micafungin or no antifungal prophylaxis. We recorded initial neutropenia duration for this cohort; it was defined as the number of days between the time that the absolute neutrophil count (ANC) decreased to <500 per microliter after initiating treatment with VEN-HMAs and the time that the ANC recovered to ≥500 per microliter.
Statistical analysis
In univariate analysis, descriptive statistics (including medians, counts, and proportions) were used to summarize patient and treatment characteristics. The univariate correlation between these categorical covariates and IFIs or antifungal prophylaxis was assessed by the Pearson χ2 test or Fisher’s exact test. A multivariable logistic regression model was used to assess the association between IFIs and covariates with univariate P ≤ .1. For each covariate in the multivariable logistic model, the odds ratio (OR) and its 95% confidence intervals (CIs) were listed after being adjusted for other covariates. For other clinical outcomes, such as follow-up time and overall survival (OS), median follow-up time and OS were summarized for response groups vs nonresponse groups and for IFI groups vs non-IFI groups. All analyses were performed using R3.5.1 software.
Results
Patients characteristics
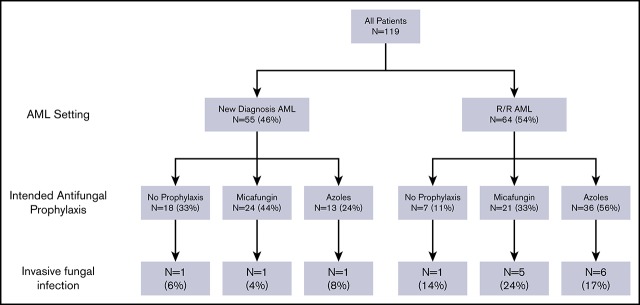
We identified 119 patients with AML who met the inclusion criteria and had no preexisting IFI. Their median age was 69 years (range, 18-86). Fifty-four percent (n = 64) were treated with VEN-HMAs for r/r AML, whereas 46% (n = 55) were treated for newly diagnosed AML. AML was de novo in 53% of patients and secondary in 47% of patients. Only 13% of patients had failed alloHCT before they were treated with VEN-HMAs (Table 1).
Table 1.
Patient and treatment characteristics
| Characteristic | Data |
|---|---|
| N | 119 |
| Age, median (range), y | 69 (18-86) |
| Sex | |
| Female | 59 (50) |
| Male | 60 (50) |
| AML setting | |
| Newly diagnosed | 55 (46) |
| r/r | 64 (54) |
| AML subtype | |
| De novo | 63 (53) |
| Therapy related | 23 (19) |
| Secondary | 33 (28) |
| Prior alloHCT | |
| Yes | 16 (13) |
| No | 103 (87) |
| 2017 ELN cytogenetics-molecular risk | |
| Favorable | 13 (11) |
| Intermediate | 34 (29) |
| Adverse | 72 (60) |
| HMA | |
| Azacitidine | 16 (13) |
| Decitabine | 103 (87) |
| 5 d | 49 (48) |
| 10 d | 54 (52) |
| VEN-HMA cycles, median (range) | 3 (1-21) |
| Responders | 4 (1-21) |
| Nonresponders | 2 (1-17) |
| Response to VEN-HMAs | |
| Yes | 68 (57) |
| No | 51 (43) |
| Antifungal prophylaxis | |
| None | 25 (21) |
| Micafungin | 45 (38) |
| Azoles | 49 (41) |
| Posaconazole, n | 25 |
| Isavuconazonium, n | 15 |
| Voriconazole, n | 5 |
| Fluconazole, n | 4 |
Unless otherwise noted, data are n (%).
ELN, European LeukemiaNet.
The majority of patients received decitabine as their HMA (87%), and the remainder were treated with 5-azacitidine. For patients who received decitabine, roughly half were treated with a 5-day course or with a 10-day course (48% vs 52%; Table 1). The CR/CRi rate for this cohort was 57% (n = 68), including 36 patients who achieved CR and 32 patients who achieved CRi. AML cytogenetics-molecular risk stratification per 2017 European LeukemiaNet criteria significantly influenced response to VEN-HMAs in our cohort, and patients with adverse genetic risk had an inferior CR/CRi rate compared with patients with favorable/intermediate risk (47% vs 72%; P = .0068).
Patterns of antifungal prophylaxis
The prescribed antifungal prophylaxis at the time of initiating therapy with VEN-HMAs was none in 21% (n = 25), micafungin in 38% (n = 45), and azoles in 41% (n = 49; posaconazole [n = 25], isavuconazole [n = 15], voriconazole [n = 5], and fluconazole [n = 4]). When we analyzed factors that might have influenced the treating physician’s decision regarding antifungal prophylaxis, only older age (>65 years) was associated with the no antifungal prophylaxis approach (26% vs 12%), micafungin use (41% vs 32%), and the lower use of azoles (33% vs 56%) compared with their younger counterparts (P = .043). The AML setting (newly diagnosed vs r/r) was not associated with the choice of antifungal prophylaxis and showed only a trend toward more azole use in the r/r setting (P = .071). The type and duration of HMA therapy were not associated with the selection of antifungal prophylaxis (Table 2).
Table 2.
Patterns of antifungal prophylaxis
| None | Micafungin | Azole | P | |
|---|---|---|---|---|
| AML setting | .0714 | |||
| Newly diagnosed (n = 55) | 18 (33) | 24 (44) | 13 (24) | |
| r/r (n = 64) | 7 (11) | 25 (39) | 32 (50) | |
| Patient age, y | .0426 | |||
| ≤65 (n = 41) | 5 (12) | 13 (32) | 23 (56) | |
| >65 (n = 78) | 20 (26) | 32 (41) | 26 (33) | |
| HMA type and schedule | .5194 | |||
| Decitabine | ||||
| 5 d (n = 49) | 11 (22) | 21 (43) | 17 (35) | |
| 10 d (n = 54) | 9 (17) | 20 (37) | 25 (46) | |
| Azacitidine (n = 16) | 5 (31) | 4 (25) | 7 (44) |
Data are n (%).
Ten (8%) patients in this cohort had their intended antifungal prophylaxis changed during the course of treatment, including 8 patients who were responders. Five patients who initially received micafungin had prophylaxis discontinued (no prophylaxis) after achieving CR/CRi, 4 patients who were initially receiving micafungin were switched to azole, and 1 patient who was not on prophylaxis was placed on micafungin. None of these patients developed an IFI upon modification of antifungal prophylaxis.
The incidence and characteristics of IFIs
The median follow-up for responders and nonresponders on VEN-HMA therapy was 210 days and 85 days, respectively. We identified 15 (12.6%) patients (total of 16 episodes, because 1 patient had 2 episodes of IFI) with documented IFIs (13 were breakthrough IFIs on micafungin/azole prophylaxis) during VEN-HMA treatment, including 7 proven cases and 8 probable cases. Aspergillus was the most common cause of IFIs (n = 7; 47%), followed by Mucor (n = 5; 33%), with 4 of 5 cases of mucormycosis developing on azole prophylaxis; the lung was the most commonly involved organ (n = 11; 73%). The median time from initiating VEN-HMAs to the onset of IFIs was 72 days (range, 35-281); it was 107 days (range, 35-281) in responders and 66 days (range, 38-115) in nonresponders. Among responders who achieved CR/CRi and developed IFIs, the onset of IFI occurred before ANC recovery (≥1000 per microliter) in 3 patients (75%). Table 3 depicts patient and IFI characteristics, as well as details regarding the type of IFI.
Table 3.
Characteristics of IFIs during VEN-HMA therapy
| UPN | Age, y/sex | AML setting | AML type | Antifungal prophylaxis | Response | Prior alloHCT | HMA regimen (d) | Onset of IFI, d | Diagnosis of IFI | Fungal infection | Site |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77/M | Untreated | De novo | None | CR | No | Azacitidine (7) | 281 | Probable | Aspergillus niger | Lung |
| 2 | 68/F | r/r | Therapy related | Micafungin | CR | No | Decitabine (5) | 35 | Proven | Mucor | Disseminated; lung, erector spinae muscles, liver and spleen. |
| 3 | 75/M | r/r | De novo | Fluconazole | CRi | No | Decitabine (5) | 104 | Proven | Rhizopus spp. | Sinusitis and brain infarct |
| 4 | 70/M | r/r | De novo | Micafungin | CRi | No | Azacitidine (7) | 109 | Probable and proven | Aspergillus Mucor - 11/2018 developed on CRESEMBA then treated with AmBisome | Lung liver/spleen, had splenectomy. |
| 5 | 55/F | r/r | Secondary | Posaconazole | Refractory | Yes | Decitabine (5) | 56 | Proven | Scedosporium prolificans | Blood |
| 6 | 62/M | r/r | De novo | Micafungin | Refractory | No | Decitabine (10) | 92 | Probable | Aspergillus | Lung |
| 7 | 66/M | Untreated | De novo | Micafungin (patient was being treated with ABELCET empirically when BALF from 8/9/2017 was positive for hyphae) | Refractory | No | Decitabine (5) | 61 | Probable | Unspecified mold infection | Lung |
| 8 | 57/M | r/r | Secondary | Micafungin | Refractory | No | Decitabine (10) | 38 | Probable | Aspergillus | Lung |
| 9 | 45/M | Untreated | Therapy related | Isavuconazonium | Refractory | No | Decitabine (10) | 71 | Proven | Rhizopus spp. | Sinus/orbit |
| 10 | 32/F | r/r | De novo | Posaconazole | Refractory | Yes | Decitabine (10) | 73 | Probable | Aspergillus | Lung |
| 11 | 74/F | r/r | De novo | None | Refractory | No | Azacitidine (7) | 60 | Proven | Scedosporium apiospermum | Skin/deep tissue |
| 12 | 71/F | r/r | Secondary | Isavuconazonium | Refractory | No | Azacitidine (7) | 115 | Probable | Mucor | Lung |
| 13 | 56/F | r/r | De novo | Posaconazole | Refractory | Yes | Azacitidine (7) | 59 | Probable | Aspergillus | Lung |
| 14 | 68/M | r/r | Secondary | Isavuconazonium | Refractory | No | Decitabine (5) | 89 | Proven | Aspergillus fumigatus | Lung |
| 15 | 55/F | r/r | Therapy related | Micafungin | Refractory | No | Decitabine (10) | 43 | Probable | Penicillium spp. | Lung |
BALF, bronchoalveolar lavage fluid; F, female; M, male; UPN, unique patient number.
Predictors for IFIs
IFIs were more common in patients who did not achieve CR/CRi with VEN-HMAs compared with patients who did (22% vs 6%; P = .0132). Among responders, 6% (n = 2) of patients who achieved CR and 6% (n = 2) of those who achieved CRi developed IFIs. Additionally, treatment of AML with VEN-HMAs in the r/r setting was associated with higher rates of IFIs compared with treatment in the newly diagnosed setting (19% vs 5%; P = .0498). The intended antifungal agent (P = .826), prior alloHCT (P = .427), patient age (P = .287), duration of initial neutropenia (>30 days or ≤30 days; P = .728), and the type and schedule of HMA (P = .054) did not influence the incidence of IFIs during VEN-HMA therapy (Table 4). In multivariable analysis, which included factors with P ≤ .1, therapy with VEN-HMA in the r/r setting was independently associated with an increased incidence of IFIs (OR, 4.19; 95% CI, 1.13-21.12; P = .049), whereas response to therapy (achieving CR or CRi) reduced the incidence of IFIs (OR, 0.28; 95% CI, 0.07-0.93; P = .047; Table 5). Surprisingly, 5-azacitidine as the HMA was associated with a higher risk for IFIs compared with decitabine (OR, 5.92; 95% CI, 1.25-30.19; P = .026). The reasons for this difference remain unclear and could be skewed as a result of the small number of patients in the 5-azacitidine group (n = 16). The median OS was not different between patients who did and did not develop IFIs during VEN-HMA therapy (225 days vs 325 days; P = 1.00).
Table 4.
Predictors for IFI during VEN-HMA therapy
| IFI | P | ||
|---|---|---|---|
| Yes | No | ||
| AML setting | .0498 | ||
| Newly diagnosed (n = 55) | 3 (5) | 52 (95) | |
| r/r (n = 64) | 12 (19) | 52 (81) | |
| Patient age, y | .287 | ||
| ≤65 (n = 41) | 7 (17) | 34 (83) | |
| >65 (n = 78) | 8 (10) | 70 (90) | |
| Antifungal prophylaxis | .8262 | ||
| None (n = 25) | 2 (8) | 23 (92) | |
| Micafungin (n =45) | 6 (13) | 39 (87) | |
| Azoles (n = 49) | 7 (14) | 42 (86) | |
| HMA type and schedule | .0536 | ||
| Decitabine | |||
| 5 d (n = 49) | 5 (10) | 44 (90) | |
| 10 d (n = 54) | 5 (9) | 49 (91) | |
| Azacitidine (n = 16) | 5 (31) | 11 (69) | |
| Response to therapy | .0132 | ||
| Yes (n = 68) | 4 (6) | 64 (94) | |
| No (n = 51) | 11 (22) | 40 (78) | |
| Prior alloHCT | .4268 | ||
| Yes (n = 16) | 3 (19) | 13 (81) | |
| No (n = 102) | 12 (12) | 90 (88) | |
| Neutropenia duration, d | .7283 | ||
| ≤30 (n = 24) | 2 (8) | 22 (92) | |
| >30 (n = 76) | 11 (14) | 65 (86) | |
Data are n (%).
Table 5.
Multivariable analysis of variables in Table 4 (P < .1)
| Estimate | SE | z value | Pr (>|z|) | OR | 95% CI (for OR) | |
|---|---|---|---|---|---|---|
| Intercept | −2.78 | 0.81 | −3.41 | 0.0006 | 0.06 | 0.01-0.26 |
| r/r (vs newly diagnosed) | 1.43 | 0.73 | 1.97 | 0.0485 | 4.19 | 1.13-21.12 |
| Responder (vs nonresponder) | −1.28 | 0.65 | −1.98 | 0.0473 | 0.28 | 0.07-0.93 |
| Decitabine (5 vs 10 d) | 0.25 | 0.69 | 0.36 | 0.7194 | 1.28 | 0.32-5.16 |
| HMA (azacitidine vs 10 d) | 1.78 | 0.8 | 2.23 | 0.0255 | 5.92 | 1.25-30.19 |
Pr, probability; SE, standard error.
Discussion
In this study, we have analyzed a relatively large cohort of AML patients treated with VEN-HMAs, and we show an overall low incidence of probable or proven IFIs. As expected, the incidence appears to be higher in patients treated for r/r AML compared with newly diagnosed cases. The incidence of IFIs in newly diagnosed AML patients in our cohort (5%) is in the range of the 8% rate observed by DiNardo et al in their phase 1B dose-escalation and expansion study of VEN-HMAs1 and the 19% incidence in r/r patients, and that is comparable to what was observed in the same setting in a previous study.5 It is important to point out that this relatively low rate of IFIs in the phase 1B study was observed, despite the fact that the study prohibited azole prophylaxis because of the known drug-drug interaction between venetoclax and azoles.10 Therefore, 46% of patients in that study received echinocandin prophylaxis.1 The low incidence of IFIs during VEN-HMA therapy, despite profound neutropenia, is likely a reflection of the efficacy of this treatment; responding patients are protected from IFIs by virtue of neutrophil count recovery between cycles of therapy. Data on the incidence of IFIs during single-agent HMA treatment in AML are lacking; however, overall, it appears to be low (∼6%).12
Antifungal prophylaxis that provides antimold activity can be considered standard of care during induction therapy for AML.13-15 However, the choice of agent varies, and azoles have been widely used in this setting; posaconazole is FDA approved for this indication.15 In the landmark randomized study that established posaconazole as FDA-approved prophylaxis during neutropenia in patients with AML or myelodysplastic syndrome undergoing induction chemotherapy, posaconazole reduced the rate of IFIs compared with fluconazole or itraconazole (2% vs 8%).15 The efficacy of echinocandins in this setting has been evaluated in small studies that suggest probable benefit of micafungin in reducing IFIs.16 The complicating issue of VEN-HMAs and the concurrent use of azoles is CYP3A4 enzyme inhibition and, thereby, the increased levels of venetoclax and resultant bone marrow suppression.10 Pharmacokinetic data exist for the concurrent use of venetoclax with the strong CYP3A4 inhibitor posaconazole.17 Although there are guidelines for reducing the dose of venetoclax when used concurrently with azoles,18 there is a risk for underdosing venetoclax if the patient is not compliant or is unable to take the azole. Moreover, most trials of venetoclax prohibited azole use; therefore, physicians were left with the choice of using no prophylaxis or an echinocandin. Although intermittent or low-dose liposomal amphotericin B can be used as prophylaxis, it is hampered by its side effect profile, which includes nephrotoxicity, electrolyte abnormalities, and infusion reactions.19
As expected, we identified that the practice of antifungal prophylaxis during VEN-HMA therapy is diverse, even within our institution, which has 1 of the largest experiences with this new combination therapy. Azoles were used in 41% of our cohort. This could be related, in part, to the subset of elderly patients in this cohort who were enrolled in an early clinical trial of VEN-HMAs in which azole prophylaxis was prohibited. Additionally, it appears that there is a tendency to administer azole prophylaxis in r/r AML, which is in line with the greater concern for IFIs in this setting because of the prolonged and recurrent neutropenia experienced by these patients.
Despite the variation in antifungal prophylaxis use, including a substantial number of patients who did not receive prophylaxis, the choice of antifungal treatment did not appear to affect the risk of IFI in our study. The IFI risk was higher in patients who fail to achieve CR/CRi with VEN-HMAs. This would be expected because of the cumulative duration of neutropenia experienced by such patients. In fact, breakthrough IFIs on antifungal prophylaxis occurred in the context of prolonged neutropenia or prior alloHCT. Given the fact that CRs occur in about two thirds of newly diagnosed patients on VEN-HMA therapy, these responders appear to be a low-risk group who may need antifungal prophylaxis only during induction, provided a CR is achieved. Neutrophil recovery upon achieving remission and between cycles of therapy would be expected to provide adequate protection from IFIs. In the case of relapsed disease or patients who have relapsed after alloHCT, consideration should be given to the prior history of IFIs while choosing prophylaxis. The majority of IFIs in our cohort (11/15) were pulmonary, and half were Aspergillus spp.; therefore, a surveillance strategy using periodic computed tomography and serum Aspergillus galactomannan assays could also be tested in patients who are not receiving prophylaxis. Given the paucity of data, physician judgment is required in each individual case to estimate the risk of IFIs and to decide which prophylaxis therapy is required during VEN-HMA therapy; however, antimold prophylaxis appears to be warranted in patients treated for r/r AML, given the higher incidence of IFIs during therapy. Based on the limited data available, we have developed a guideline to address this issue (Table 6) at our institution. It is reassuring that the CR rate for VEN-HMAs was comparable among patients who did or did not receive strong CYP3A4 inhibitors (with dose reduction of venetoclax).20
Table 6.
Proposed guidelines for antifungal prophylaxis during VEN-HMA therapy
| For patients with newly diagnosed AML treated with VEN-HMAs, no clear benefit from administering antifungal prophylaxis was observed for all patients |
| For patients who are expected to have lower response to VEN-HMAs, such as those with adverse-risk genetics, antifungal prophylaxis should be strongly considered |
| If the decision is made to administer antifungal prophylaxis, no class of antifungal had an advantage over another |
| Micafungin is an acceptable choice that can allow administration of venetoclax without dose modification |
| Limited data support administering azoles with appropriate venetoclax dose reduction without impacting AML response |
| For de novo AML patients who achieve CR with neutrophil recovery, the benefit of continuing antifungal prophylaxis during postremission cycles remains debatable |
| Although our data did not show a clear benefit of antifungal prophylaxis for patients with r/r AML treated with VEN-HMAs, who are at a higher risk for IFIs, based on their higher risk we recommend antifungal prophylaxis, particularly for the following subsets: |
| Patients with lower likelihood of response due to adverse risk genetics |
| Early post-alloHCT relapse |
| Secondary prophylaxis for patients with history of IFIs |
Many unanswered questions remain regarding the choice and duration of antifungal prophylaxis during VEN-HMA therapy, and no data exist currently. This issue will be more relevant in the near future with the increasing use of venetoclax-based combinations in AML in the frontline and r/r settings. Our study is limited by its sample size, its retrospective nature, and biases inherent in such an analysis. Notwithstanding these factors, our study provides real-world data on the incidence of IFIs during VEN-HMA therapy and the practice of antifungal prophylaxis that could lead to a systematic study of this important issue.
Authorship
Contribution: I.A., S.D., and V.P. designed the study and wrote the manuscript; J.Z. performed statistical analyses; and all authors collected, assembled, analyzed, and interpreted data and approved the final draft of the manuscript.
Conflict-of-interest disclosure: I.A. serves on advisory boards for AbbVie and Agios Pharmaceuticals, is a member of the speakers bureau for Jazz Pharmaceuticals, and is a consultant for Autolus Therapeutics. S.D. has acted as a consultant for and serves on advisory boards for Merck, Clinigen, and Janssen, is a member of the speakers bureau for Merck, and has received research support from Merck, Shire, and Chimerix. A.S. has served as a consultant for Kadmon Corporation and has received research funding from Celgene. R.N. serves on advisory boards for Merck and Celgene and has a research collaboration with Jazz Pharmaceuticals. A.S.S. is a member of the speakers bureau for Amgen, Celgene, and Stemline Therapeutics. G.M. is a member of the speakers bureau for AbbVie. V.P. has served on advisory boards for AbbVie and Jazz Pharmaceuticals and is member of the speakers bureau for Jazz Pharmaceuticals, Amgen, Novartis, and AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Ibrahim Aldoss, Gehr Family Center for Leukemia Research, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope Medical Center, 1500 East Duarte Rd, Duarte, CA 91010; e-mail: ialdoss@coh.org.
References
- 1.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lübbert M, Rüter BH, Claus R, et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica. 2012;97(3):393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldoss I, Yang D, Aribi A, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica. 2018;103(9):e404-e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiNardo CD, Rausch CR, Benton C, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93(3):401-407. [DOI] [PubMed] [Google Scholar]
- 6.Aldoss I, Yang D, Pillai R, et al. Association of leukemia genetics with response to venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Am J Hematol. 2019;94(10):E253-E255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prentice HG, Kibbler CC, Prentice AG. Towards a targeted, risk-based, antifungal strategy in neutropenic patients. Br J Haematol. 2000;110(2):273-284. [DOI] [PubMed] [Google Scholar]
- 8.Portugal RD, Garnica M, Nucci M. Index to predict invasive mold infection in high-risk neutropenic patients based on the area over the neutrophil curve. J Clin Oncol. 2009;27(23):3849-3854. [DOI] [PubMed] [Google Scholar]
- 9.Gerson SL, Talbot GH, Hurwitz S, Strom BL, Lusk EJ, Cassileth PA. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100(3):345-351. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal SK, DiNardo CD, Potluri J, et al. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther. 2017;39(2):359-367. [DOI] [PubMed] [Google Scholar]
- 11.De Pauw B, Walsh TJ, Donnelly JP, et al. ; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group . Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pomares H, Arnan M, Sánchez-Ortega I, Sureda A, Duarte RF. Invasive fungal infections in AML/MDS patients treated with azacitidine: a risk worth considering antifungal prophylaxis? Mycoses. 2016;59(8):516-519. [DOI] [PubMed] [Google Scholar]
- 13.Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25(34):5471-5489. [DOI] [PubMed] [Google Scholar]
- 14.Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol. 2018;36(30):3043-3054. [DOI] [PubMed] [Google Scholar]
- 15.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348-359. [DOI] [PubMed] [Google Scholar]
- 16.Epstein DJ, Seo SK, Huang YT, et al. Micafungin versus posaconazole prophylaxis in acute leukemia or myelodysplastic syndrome: a randomized study. J Infect. 2018;77(3):227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wexler D, Courtney R, Richards W, Banfield C, Lim J, Laughlin M. Effect of posaconazole on cytochrome P450 enzymes: a randomized, open-label, two-way crossover study. Eur J Pharm Sci. 2004;21(5):645-653. [DOI] [PubMed] [Google Scholar]
- 18.Mei M, Aldoss I, Marcucci G, Pullarkat V. Hypomethylating agents in combination with venetoclax for acute myeloid leukemia: update on clinical trial data and practical considerations for use. Am J Hematol. 2019;94(3):358-362. [DOI] [PubMed] [Google Scholar]
- 19.Mandhaniya S, Swaroop C, Thulkar S, et al. Oral voriconazole versus intravenous low dose amphotericin B for primary antifungal prophylaxis in pediatric acute leukemia induction: a prospective, randomized, clinical study. J Pediatr Hematol Oncol. 2011;33(8):e333-e341. [DOI] [PubMed] [Google Scholar]
- 20.Pollyea DA, Pratz KW, Jonas BA, et al. Venetoclax in combination with hypomethylating agents induces rapid, deep, and durable responses in patients with AML ineligible for intensive therapy. Blood. 2018;132(suppl 1):285. [Google Scholar]