Abstract
Objectives:
Bacterial components are used to improve immune responses in patients with respiratory infections. Pharmacological formulations of bacterial components include a mixture of bacterial antigens, some of which are complete inactivated bacteria, that is, named bacterial suspensions; while others are fragments of bacteria, which are presented as bacterial lysates. Although bacterial lysates have been broadly used as immune-stimulators, the biological support for the therapeutic effectiveness of bacterial suspension has not yet been studied. Thus, the aim of our study was to investigate the immunological activity induced by bacterial suspension.
Methods:
This work was an exploratory translational study. Peripheral blood mononuclear cells were obtained from healthy donors and cultured in time–dose dependent assays with a commercial bacterial suspension. Flow cytometry was used for phenotypic analysis and for determining soluble cytokines in culture supernatants.
Results:
We observed that bacterial suspension activates B cells in a dose-dependent manner. Peripheral blood mononuclear cells were able to secrete IL-6 and IL-10 after 24 h of bacterial suspension stimulation. TLR2 expression was observed mainly on CD19+ CD38Lo B cells after 72 h of culture; remarkably, most of the TLR2+ CD19+ cells were also IL-10+.
Conclusion:
Our findings suggest that bacterial suspension induces the activation of B cell subsets as well as the secretion of IL-6 and IL-10. Expression of TLR2 on CD19+ cells could act as an activation loop of IL-10+ B regulatory cells. The clinical implications of these findings are discussed at the end of this article.
Keywords: B cells, Bregs, IL-6, IL-10, bacterial suspension, immunomodulation
Introduction
Bacterial antigens or inactivated bacteria from different species are widely used to prevent recurrent respiratory infections in children and to improve immune responses in elderly patients.1,2 Commercially differing in their pharmaceutical formulations, the complete inactivated bacteria are referred to as bacterial suspensions (BSs), while fragments of bacteria are referred to as bacterial lysates (BLs). BS and BL vary in the number and type of bacteria they contain, and depending on whether they are lysates or suspensions, their biological effects are different. Some BL preparations can induce IL-1b, IL-6, and TNF-a in vitro3 while others activate in vivo T and NK cells4 and produce a protective Th1/Th17 memory immune response,5 induce nasal human beta-defensins,6 or increase the percentage of circulating B cells.7 Although BL are broadly used as immune-stimulators, the biological argument about the therapeutic effectiveness of BS has not been studied yet. Recently, some strains of bacteria were reportedly able to enhance the therapeutic effects of antigen-specific immunotherapy in asthma by the induction of B regulatory cells (Bregs).8 Bregs are a subset of B cells that are functionally defined by IL-10 secretion. These cell subpopulations are involved in the control of inflammation, autoimmunity, and regulation of immune responses.9 This study aimed to investigate the immunomodulatory activities of a commercially available BS on human mononuclear cells.
Material and methods
Study design
This study was an exploratory translational study aimed to explore the immunological activity induced by BS on peripheral blood mononuclear cells (PBMCs) obtained from healthy donors.
Healthy donors
Convenience sampling was used in this study, and five healthy volunteers participated as healthy donors (three men and two women, age range 27–30 years old). Healthy donors were included after a complete medical evaluation. All included subjects had no familial or personal history of atopy, none of them presented with chronic diseases, and all were free of infectious diseases at the time of sampling. Laboratory explorations showed complete blood counts inside normal ranges for age and sex. All participants provided their informed consent for blood sampling after written information was provided. The Medical Investigation (CI-046-2016), Ethics (CEI-2016/10/03), and Biosecurity (CB-046-2016) Committees of the Institute of Ophthalmology “Conde de Valenciana Foundation” in Mexico City approved this study.
Reagents
RPMI-1640 culture medium, Concanavalin A (Con A), and salts were from Sigma Chemical Co. (St. Louis, MO, USA). Lymphoprep (Ficoll 1.077 density) was obtained from Nycomed Pharma (Nyegaard, Oslo, Norway). L-glutamine, 2-mercaptoethanol, and sodium pyruvate were purchased from Gibco BRL. (Rockville, MD, USA). Fetal calf serum was from HyClone Labs. (Logan, UT, USA), Bacterial suspension (Polivacc), a heat-killed whole bacteria suspension, was purchased from IPI ASAC Pharmaceutical Immunology (Alicante, Spain), Brefeldin A was obtained from BD Biosciences (San Jose, CA, USA).
PBMCs
Whole heparinized peripheral blood was diluted 1:2 (vol/vol) in phosphate buffered saline (PBS), pH 7.2. PBMCs were separated on a Ficoll density gradient by centrifugation at 300 g for 30 min at room temperature. After centrifugation, the cells at the interface were collected, washed twice, and counted using a handheld automated cell counter (Millipore Co., Billerica, MA, USA), and viability was assessed by eosin dye exclusion.
Cell cultures
PBMCs were cultured in 24-well flat-bottomed cell culture plates (Costar, Cambridge, MA, USA) at 5 × 106 cells/well in RPMI-1640 medium supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, and 10% heat-inactivated fetal calf serum. Commercial BS were added at different concentrations, starting from a dilution stock of 2.6 × 109 of inactivated bacteria/mL according to the manufacturer. The BS concentrations used were 19.5 × 106, 13 × 106, 7.8 × 106, 3.9 × 106, and 1.5 × 106 bacteria/mL. Protein concentration was determined using a Bio Rad Protein Assay Kit (Bio Rad Laboratories, Philadelphia, PA). The initial stock concentration was at 4.84 µg/mL; then, we calculated protein concentrations per microliter, and the final concentrations were 36.3, 24.2, 14.5, 7.2, and 3.6 ng/mL.
The BS used in this study contained a total of 14 different inactivated bacteria (BS-14): Streptococcus pneumoniae, Klebsiella pneumoniae, Brahmanella catarrhalis, Staph-ylococcus aureus, Haemophilus influenza, Streptococcus alpha and beta, Enterococcus faecalis (Syn. Streptococcus faecalis), Staphylococcus epidermidis, Bordetella pertussis, Proteus sp., Pseudomonas sp., Escherichia coli, and Corynebacterium pseudodiphtheria. Con A mitogen (2 µg/mL) was used as a positive control for PBMCs stimulation. Brefeldin A (1 µg/106 cells) was added 5 h before culture ended. Cells were harvested every 24 h and were processed to determine CD19, CD38, CD69, TLR2, or IL-10 expression by flow cytometry. Supernatants were collected and stored at −70°C to determine soluble cytokine levels.
Immunofluorescence staining of cell-surface markers
Three or four-color staining was performed on harvested cells by direct immunofluorescence using PE CY5.5- or FITC-labeled anti-CD3 and anti-CD19 monoclonal antibodies (mAbs, e-Biosciences, BioLegend, San Diego, CA, USA) and either APC and/or PE-labeled mAbs against CD38 or CD69, and TLR2 (BD Biosciences, San Jose, CA, USA) for 30 min at 4°C. After incubation, the cells were washed twice with PBS, supplemented with 0.2% Bovine serum albumin and 0.2% sodium Azide (PBA), and immediately analyzed by flow cytometry.
Immunofluorescence staining of intracellular markers
After extracellular staining was performed, the cells were fixed and permeabilized with BD Cytofix/Cytoperm™ solution according to manufacturer’s instructions Then, the cells were incubated with PE-labeled anti-human IL-10 antibody and immediately acquired by flow cytometry.
Flow cytometric analysis
All cells were analyzed for the expression of phenotypic markers on a FACSCalibur cytometer (Becton Dickinson, San Jose, CA) using CellQuest software version 10.0 (BD Biosciences), and 10,000 events were counted. To analyze the staining of cell-surface markers, lymphocytes were first gated by their physical properties (forward and side scatter). To determine B cells, a second gate was drawn based on the immunofluorescence characteristics of the gated cells to determine if the cells were CD3+ or CD3– cells. The CD3– cells were selected, and a CD19 dot plot was created to analyze CD38, CD69, TLR2, or IL-10 staining on CD19+ cells. Data are presented as dot-plots or histograms. Control stains were performed using isotype-matched mAb of unrelated specificity. Background staining was <1% and was subtracted from experimental values.
Determination of soluble cytokines
After stimulation with BS-14, supernatants were collected, and the cytokines, IL-2, IL-4, IL-6, IL-10, TNF, IFN-γ, and IL-17A were measured with cytometric bead arrays, according to the manufacturer’s instructions (Human Th1/Th2/TH17 Kit, BD Biosciences, Franklin Lakes, NJ, USA). Cytokine concentrations were analyzed by flow cytometry in a FACSVerse Cytometer with FCAP Array software, version 3.0 (BD Biosciences).
Statistical analysis
T-tests or Mann–Whitney U tests were used to detect significant differences. The analysis was performed with Prism8 v. 8.2.0 (GraphPad Software, San Diego, CA). Differences were considered statistically significant when the test yielded p values less than 0.05.
Results
BS preferentially activates B cells in a dose-dependent manner
We began by determining the percentage of CD3+ and CD19+ cells that were activated after BS-14 stimulation in time–dose in vitro assays. Under these culture conditions, we determined the percentage of cells expressing CD69 (Figure 1). As shown in Table 1, 4.4-fold higher CD69 expression was observed in CD19+ cells than in CD3+ cells at 24 h with the lowest BS-14 concentration (p = 0.0004). In addition, the percentage of CD19+ CD69+ cells increased in a dose-dependent manner at all evaluated times, but most significantly at 24 h (Table 1).
Figure 1.
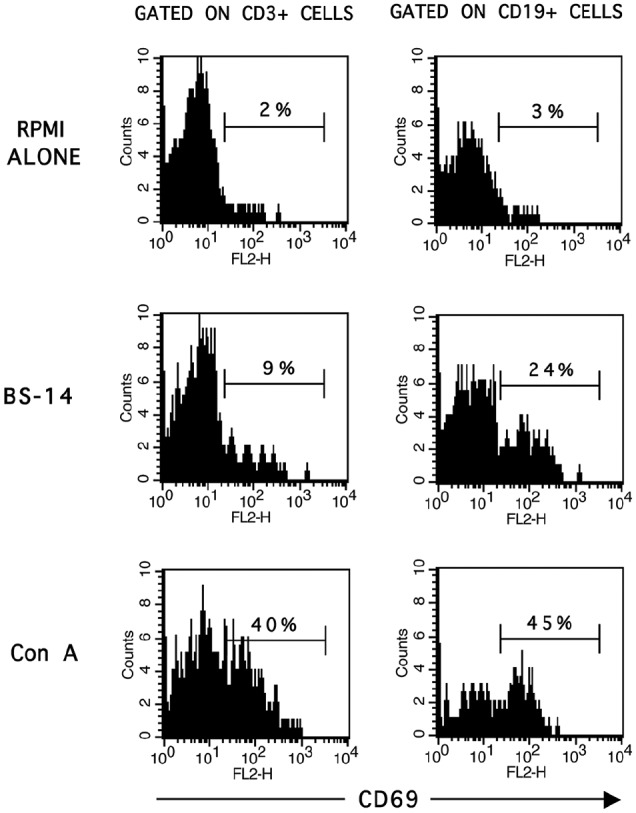
Percentage of CD69 expressing CD3+ and CD19+ cells after BS-14 stimuli. PBMCs were stimulated with 36.3 ng/mL of BS-14, and after 48 h of stimulation, then cells were harvested and stained with fluorescence-conjugated antibodies to CD3, CD19, and CD69 in a triple-immunofluorescence assay as described in the material and methods.
Representative histograms of CD3+ and CD19+ gated cells are shown from five different healthy individuals and performed in triplicate.
Table 1.
Frequency of CD3+ CD69+ cells and CD19+ CD69+ cells after BS-14 stimulation.
| % CD3+ CD69+ Mean ± SD |
%CD19+ CD69+ Mean ± SD |
|||||||
|---|---|---|---|---|---|---|---|---|
| [ ] ng/mL | 24 h | 48 h | 72 h | p | 24 h | 48 h | 72 h | p |
| RPMI | 1.6 ± 0.8 | 6.7 ± 2.2 | 6.3 ± 0.8 | – | 5.5 ± 3.8 | 4.4 ± 1 | 5.3 ± 3 | – |
| 3.6 | 4.8 ± 1.5 | 9.7 ± 3.4 | 8.5 ± 2.8 | – | 21.4 ± 2.1*§ | 7.7 ± 4* | 11.8 ± 4.9§ |
*0.006 §0.03 |
| 7.2 | 6.1 ± 2.4 | 10 ± 3 | 9.3 ± 4.8 | – | 23.9 ± 7.7£ | 10 ± 3.4£ | 19 ± 10.2 | £0.04 |
| 14.5 | 6.6 ± 0.8 | 11 ± 2 | 11.6 ± 5.3 | – | 31.3 ± 6.7† | 13.1 ± 5.9† | 21.4 ± 10.4 | †0.02 |
| 24.2 | 8.3 ± 1.5 | 12.8 ± 6.7 | 12.4 ± 5.3 | – | 39.1 ± 3.5** | 22 ± 11** | 27.6 ± 9.3 | **0.03 |
| 36.3 | 7 ± 0.8 | 9.4 ± 1.5 | 13.9 ± 4.3 | – | 47.8 ± 6.7¶,¥ | 21.5 ± 3¶ | 30.3 ±7.9¥ | 0.003 ¥0.04 |
| Con A | 14.5 ± 7.3* | 32.7 ± 11.3 | 41.8 ± 6.3* | *0.04 | 35.4 ± 9.8* | 40.5 ± 10.5 | 59.6 ± 14.7* | *0.03 |
Roswell Park Memorial Institute (RPMI) 1640 Medium.
Mean ± SD. The p columns indicate the degree of significance between the compared cell populations. Symbols indicate comparisons between the time of cultured cells.
BS induced the activation of CD19+ CD38Lo B cells and CD19+ CD38Med B cells
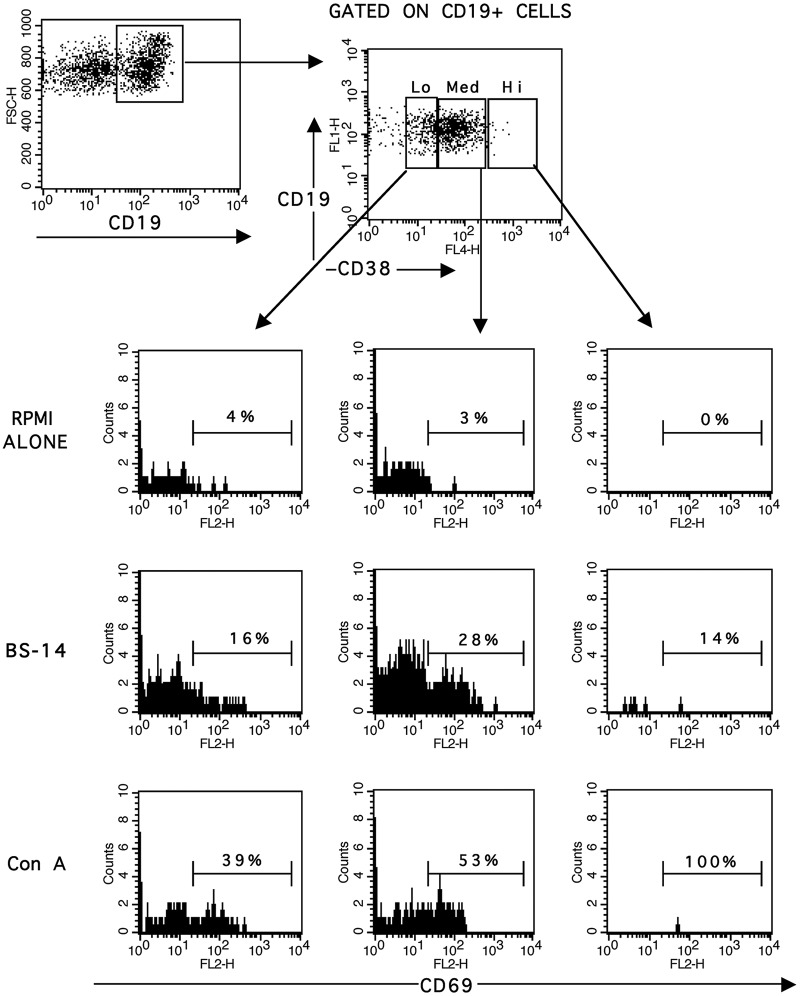
To determine changes in B cell subsets after BS-14 stimulation, we assessed CD38 expression at different times. We did not observe significant differences in the frequency of CD19+ CD38+ cells at any time or at any tested concentration of BS-14. However, when we analyzed the frequency of CD69 on CD19+ CD38+ B cell subsets, we observed a significantly higher percentage of CD19+ CD38Lo CD69+ B cells and CD19+ CD38Med CD69+ B cells at 24 h with the majority of the BS-14 concentrations tested (Table 2), and at 48 and 72 h, only with the highest concentration evaluated. No significant differences were observed in the CD19+ CD38Hi CD69+ cell frequencies (Figure 2).
Table 2.
Frequency of CD69 on CD19+ CD38Lo B cells and on CD19+ CD38Med B cells after BS-14 stimulation.
| Time | [ ] ng/mL |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| RPMI | 3.6 | 7.2 | 14.5 | 24.2 | 36.3 | Con A | p | ||
| CD19+ CD38Lo
CD69+ |
24 h | 9.9 ± 5*§ | 15.2 ± 5 | 21.6 ± 6* | 28.2 ± 4* | 36.7 ± 4§ | 44 ± 9§ | 35.8 ± 9 |
*0.04 §<0.004 <0.005 |
| 48 h | 6.4 ± 4* | 13.2 ± 10 | 10.3 ± 7 | 16 ± 5 | 14.2 ± 2 | 22 ± 9* | 39.9 ± 2* | *<0.001 | |
| 72 h | 5.4 ± 3* | 22.1 ± 14 | 37.5 ± 20 | 47.1 ± 21 | 51 ± 21 | 53.7 ± 11* | 58.7 ± 12* | *0.04 | |
| CD19+ CD38Med
CD69+ |
24 h | 7.4 ± 2*§ | 17.8 ± 6 | 24.8 ± 7* | 32.6 ± 6§ | 39.6 ± 2§ | 48.4 ± 9 | 36 ± 11§ |
*0.04 §0.001 <0.001 |
| 48 h | 1.6 ± 1*§ | 5.2 ± 3 | 8.2 ± 3 | 12 ± 7 | 16.4 ± 4 | 21 ± 5* | 38.5 ± 16§ |
*0.01 §0.04 |
|
| 72 h | 4.8 ± 3* | 13.7 ± 13 | 17 ± 13 | 16.5 ± 14 | 23.8 ± 12 | 27.1 ± 11 | 66.3 ± 11* | *0.001 | |
Roswell Park Memorial Institute (RPMI) 1640 Medium.
Mean ± SD. The p columns indicate the degree of significance between the compared cell populations. Symbols indicate comparisons between the concentration of BS-14 in cultured cells.
Figure 2.
Increased CD69 frequency on CD19+ CD38+ cells after BS-14 stimulation. CD19+ cells were gated according to CD38 expression and separated into three cell subsets: CD38Lo, CD38Med, and CD38Hi. Histograms from CD19+ CD38+ cells subpopulation are shown.
The x-axis denotes the frequency of CD69 on the gated cells. PBMCs were stimulated with BS-14 at 36.3 ng/mL, and evaluated after 48 h of stimulation. Con A mitogen was used as a positive stimulation control. Representative FACS plots and histograms from five different healthy individuals are shown and were performed in triplicate.
IL-10 and IL-6 are cytokines induced after BS-14 stimulation
To identify the cytokine profile induced by BS-14 stimulation, we determined the secreted cytokines IL-2, IL-4, IL-6, IL-10, TNF-a, IFN-g, and IL-17A by cytometric bead arrays. A significantly increased concentration of IL-6 was observed at 24 and 48 h after BS-14 stimulation. Interestingly, secretion of IL-10 was significantly increased at 24 h (results are depicted in Table 3). IL-2, IL-4, and IL-17A were not detected at any time following any of the BS-14 treatments. No significant changes in cytokine secretion were observed at 72 h.
Table 3.
Cytokine concentration in the supernatants of cells stimulated with BS-14.
| RPMI | 3.6 | 7.2 | 14.5 | 24.2 | 36.3 | Con A | p | ||
|---|---|---|---|---|---|---|---|---|---|
| IL-6 | 24 h | 6.7 ± 3.9*§ | 26,899 ± 5788* | 29,894 ± 14,989§ | 33,158 ± 10,293 | 36,126 ± 12,139 | 33,588 ± 7538* | 4.2 ± 1.9 |
*0.02 §0.01 ¶0.04 |
| 48 h | 3.9 ± 1.7*§ | 26,601 ± 9756 | 39,280 ± 11,490* | 37,475 ± 10,309* | 28,984 ± 27,201 | 35,331 ± 8704§ | 6166 ± 5999 |
*0.03 §0.02 |
|
| IL-10 | 24 h | ND | 1178 ± 479.3 | 925.5 ± 523.1* | 1049 ± 509.2* | 784.8 ± 363* | 638.1 ± 276.2* | 16.6 ± 12.2* | *0.03 |
| 48 h | ND | 939.2 ± 486.4 | 971.7 ± 607.3 | 702.2 ± 591.4 | 654.6 ± 189.7 | 578.7 ± 297 | 133.4 ± 71.3 | – | |
| TNF-a | 24 h | ND | 248.4 ± 202.2 | 399.2 ± 247.7 | 553.2 ± 338.2 | 386.8 ± 192.6 | 491 ± 292.2 | 40.9 ± 37.2 | – |
| 48 h | ND | 58.90 ± 43 | 68.48 ± 47 | 33.7 ± 9.4 | 193.9 ± 163.8 | 97.35 ± 36 | 341.2 ± 183.1 | – | |
| IFN-g | 24 h | ND | ND | ND | ND | 16.4 ± 4.8 | 21.1± 6.8 | 30.2± 26.6 | – |
| 48 h | ND | ND | 5.5 ± 1.3 | 21 ± 17.6 | 68.7 ± 56.7 | 44.2 ± 23.3 | 216.7 ± 153.6 | – |
Roswell Park Memorial Institute (RPMI) 1640 Medium.
ND: not detected, or below the limits of detection. The results are in pg/mL. Kit detection limits were as follows: IL-2, 2.6 pg/mL; IL-4, 4.9 pg/mL; IL-6, 2.4 pg/mL; IL-10, 4.5 pg/mL; IL-17A, 18.9 pg/mL; IFN, 3.7 pg/mL; and TNF-a, 3.8 pg/mL. Symbols indicate comparisons between the concentration of BS-14 in cultured cells.
TLR2 is mainly expressed on CD19+ CD38Lo/Med B cells after 72 h of BS-14 stimulation
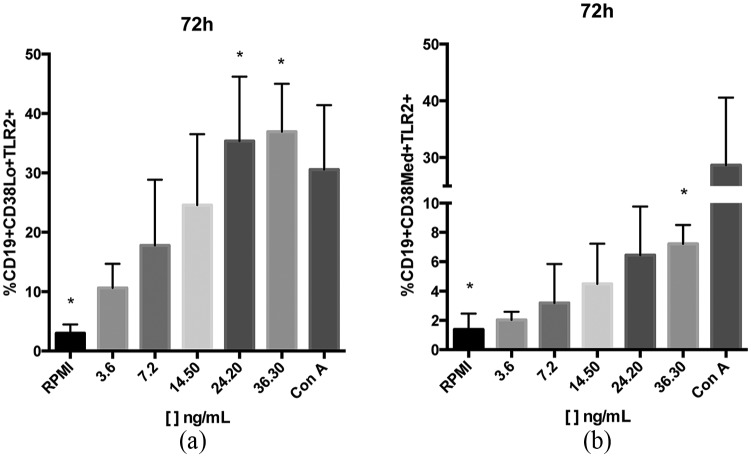
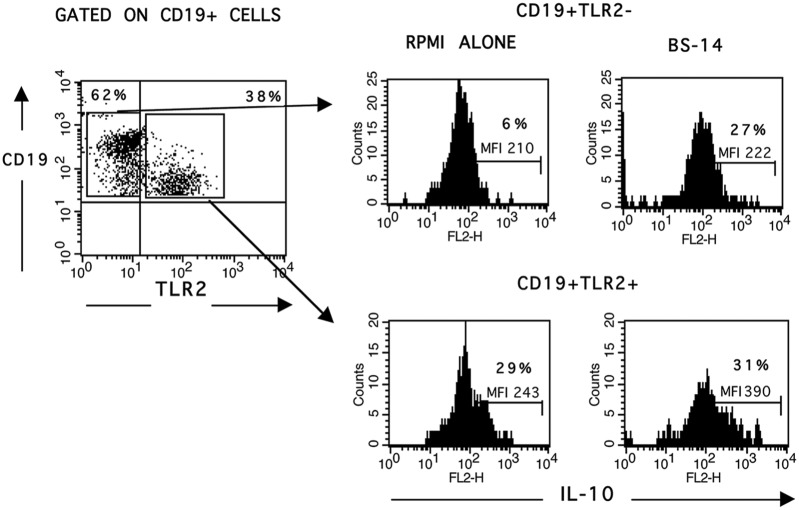
We evaluated the frequency of TLR2 expression on B cell subpopulations at 24, 48, and 72 h after BS-14 stimuli. We observed that TLR2 expression increased by 11.9-fold with 24.2 ng/mL and significantly increased by 12.4-fold with 36.3 ng/mL on CD19+ CD38Lo B cells at 72 h. In addition, it increased by 5.2-fold on CD19+ CD38Med B cells at 72 h with only 36.3 ng/mL (Figure 3 and Table 4). Interestingly, when we analyzed for IL-10 expression in TLR2– and TLR2+ B cells, we observed that the TLR2+ B cells were also IL-10+, and after stimulation, the mean fluorescence intensity (MFI) was increased even though the percentage did not significantly change (Figure 4).
Figure 3.
Increased TLR2 frequency on CD19+ CD38+ cells after 72 h of BS-14 stimulation. Percentage of TLR2+ cells in (a) CD19+ CD38Lo B cells, and in (b) CD19+ CD38Med B cells.
Significant differences are indicated (*). n = 5 different healthy individuals were evaluated, and all assays were performed in triplicate.
Table 4.
Frequency of TLR2 on CD19+ CD38Lo B cells and on CD19+ CD38Med B cells after BS-14 stimulation.
| Time | [ ] ng/mL |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| RPMI | 3.6 | 7.2 | 14.5 | 24.2 | 36.3 | Con A | p | ||
| CD19+ CD38Lo
TLR2+ |
24 h | 1.6 ± 1.6 | 3.2 ± 1.8 | 5.3 ± 2.8 | 5 ± 2.4 | 9.2 ± 3.3 | 3 ± 1 | 1.5 ± 1.1 | – |
| 48 h | 5.8 ± 3.8 | 1.4 ± 0.4 | 7.7 ± 5.1 | 6.2 ± 3 | 6.9 ± 2.7 | 5.5 ± 1.5 | 10.4 ± 6.7 | – | |
| 72 h | 2.9 ± 1.5* | 10.6 ± 4 | 17.8 ± 11 | 24.6 ± 11.9 | 35.4 ± 10.8* | 36.9 ± 8* | 30.6 ± 10.8 | *0.02 | |
| CD19+ CD38Med
TLR2+ |
24 h | 1.7 ± 1.5 | 1.6 ± 1.6 | 1.9 ± 0.9 | 1.8 ± 1.6 | 4.9 ± 1 | 2.2 ± 0.9 | 0 ± 0 | – |
| 48 h | 2.3 ± 1.5 | 0.2 ± 0.1 | 1.1 ± 1 | 1.2 ± 1.1 | 2.7 ± 2.3 | 1.1 ± 0.8 | 1.7 ± 1.2 | – | |
| 72 h | 1.4 ± 1.1* | 2 ± 0.9 | 3.2 ± 2.7 | 4.5 ± 2.7 | 6.4 ± 3.3 | 7.2 ± 1.3* | 28.7 ± 11.9 | *0.01 | |
Roswell Park Memorial Institute (RPMI) 1640 Medium.
Mean ± SD. Symbols indicate comparisons between the concentration of BS-14 in cultured cells.
Figure 4.
TLR2+ cells are the main source of IL-10. After 72 h of stimulation with BS-14 at 36.3 ng/mL, TLR2 expression was assessed on CD19+ cells by flow cytometry. Intracellular IL-10 was evaluated in TLR2– and TLR2+ B cells. The histograms show CD19+ TLR2-IL-10+ (upper right) and CD19+ TLR2+ IL-10+ (low right), demonstrating that TLR2+ B cells are the primary source of IL-10.
Representative FACS plots and histograms from five different healthy individuals, which were performed in triplicate.
Discussion
BLs have long been used to prevent recurrent respiratory tract infections in children and to improve immune responses in elderly patients.1,2 Large numbers of BLs are commercially available, but the amount of bacteria and the type of bacterial mixture in BL induces a broad range of effects on the immune system.3–7 Here, we studied a commercial formulation of 14 inactivated bacteria (BS-14) commonly used as immune-stimulant. The mechanism by which BS-14 impacts the immune response had not been previously evaluated, and this is the first work that explored it.
First, we analyzed the frequency of CD69 on CD3+ and CD19+ cells after BS-14 stimulation in PBMCs. It is well known that CD69 is an activation antigen that is induced early after activation.10,11 Our results showed that B cells were mainly activated by BS-14, and most of these activated B cells had CD38Lo or CD38Med expression. CD38 is an ectoenzyme that is expressed in early B cells and in mature B cells.12 The staining of CD38 allows for distinction between precursor cells (CD38+), transitional cells (CD38++), and plasmablast cells (CD38+++),13 suggesting that activated B cells by BS-14 were mainly precursor and transitional B cells. Similar results were reported by Lanzilli et al.,4 who evaluated a formulation of BL that were orally administered in tablets and, after 100 days of treatment, they reported changes in the frequency of circulating T, NK, and B cells. Interestingly, the observed B cells were phenotypically early precursors (CD19+ CD27– IgM+/–) and early memory B cells (CD19+ CD27+ IgM++).
In addition, in our work after BS-14 stimulation, the cytokines detected in the supernatant were mainly IL-6 and IL-10. IL-6 is a pro-inflammatory cytokine that enhances Ig secretion by activated B cells and causes differentiation into plasma cells14 while IL-10 is an anti-inflammatory cytokine with a central role in regulating immune responses to pathogens.15 Interestingly, IL-6 and IL-10 can be induced after TLR2 ligation16–18 and both cytokines are involved in the activation of a subset of memory B cells.19,20 In this context, whether the activation of transitional B cells after BS stimulation corresponds to a switched memory phenotype (CD19+ CD27+ IgD– IgM–)13 is not known, and further investigation is needed.
On the contrary, in this work, we observed that TLR2+ B cells were the most important source of IL-10. TLR2 is an innate receptor that recognizes specific components conserved among microorganisms.21 After BS-14 stimulation, TLR2 was mainly expressed on precursor B cells at 72 h. Some of the bacteria contained in BS-14 can induce TLR2, such as Klebsiella pneumonia and Pseudomonas sp.16,22–24 Remarkably, it has been reported that some TLR2 ligands induce IL-10, thereby leading to activation of regulatory B cells and the attenuation of T effector functions, which contribute to immune regulation.25 It is possible that BS-14 stimulation functionally expands a subgroup of Bregs characterized by TLR2 expression and IL-10 production.
It is undeniable that other TLRs could be participating in the activation of IL-10+ B cells, as both gram-positive and gram-negative bacteria were present in the BS. In this context, it has been reported that peptidoglycan, and lipoteichoic acid from S. aureus, induces large quantities of IL-1026,27 and proliferation of B1 cells.18 Moreover, prolonged stimulation by Lipopolysaccharide (LPS) induces clonal expansion of Bregs,28 and TLR4 ligation on Bregs could suppress CD4+ T cell proliferation.29 Interestingly, LPS stimulation promotes maturation of B10 pro-cells from the human blood into IL-10+ B competent cells, which parallels mouse regulatory B10 cells.30
Thus, the clinical implications of our findings are relevant, as it is well known31–34 that the activation and expansion of Bregs are fundamental to control immune responses, and the mechanisms of suppression are IL-10 dependent. Moreover, it has been suggested that dysfunction or low frequencies of circulating Bregs are related to allergy and autoimmune diseases.35–37 Hence, BS-14 could be easily applied as an adjuvant therapy in patients with chronic inflammatory diseases to therapeutically downregulate immune response through Breg induction, and the evaluation of circulating Bregs could be proposed as efficacy biomarker.
Limitations
This research was performed with a small healthy donor sample size, as it was necessary to first explore the ability of BS-14 to induce functional changes in PBMCs. This unknown function of BS-14 in PBMCs was the reason that we performed our first exploration using Con A as a positive control. After we analyzed the results, we observed that BS-14 mainly activated B cells, rather than T cells. This finding was interesting because it is known that BL activates mainly T and NK cells, without activation of B cells.4,5,38 In this work, we explored the impact of BS-14 on PBMCs comparing with Con A, a polyclonal mitogen used to study activation of T cells.39 Therefore, we know that it is essential to perform new assays using a pokeweed mitogen (PKW) as a positive control, as PKW is a polyclonal mitogen used to evaluate B cell function since activation observed on B cells by Con A is a consequence to cellular cooperation.39 By performing these new assays on PBMCs or on isolated B cells, we will be able to know the real impact of BS on B cell function. Nevertheless, the use of Con A as a positive control on cultured cells did not change our findings related to the effect of BS-14 on B cells. It is undeniable that pharmacological formulation of bacteria in BL versus BS may activate the immune response differentially. Our study gives the first insights that bacterial formulations commonly used in clinical practice have different action mechanisms, and this can be explained if we understand the mechanism of activation of immune cells depending on the antigen. BLs are fragments of bacteria antigens and are quickly captured by antigen-presenting cells, then stimulating T cells; while the TLRs or even BCR could be participating in recognition of BS, activating mainly B cells. The last limitation in our study was that the manufacturer did not provide the relative proportions of the different strains contained in BS-14, and as all experiments were performed in an ex vivo model, more studies are needed to evaluate both the role of each bacterial strain on immune cells and the activation or expansion of IL-10 producing B cells in BS-14 treated patients.
Conclusion
Our findings demonstrate that BS-14 induces activation of precursor and transitional B cells, and secretion of IL-6 and IL-10, favoring differentiation of B memory cells. In addition, the expression of TLR2 on B cells could be involved in an activation loop of IL-10+ B cells (Bregs) (Figure 5). Our results provide evidence that at the cellular level, BS-14 could be used therapeutically to diminish pathogenic immune responses. To address this question, clinical studies are needed to elucidate the real impact of BS-14 and other bacterial formulations on the modulation of protective versus pathogenic immune responses.
Figure 5.
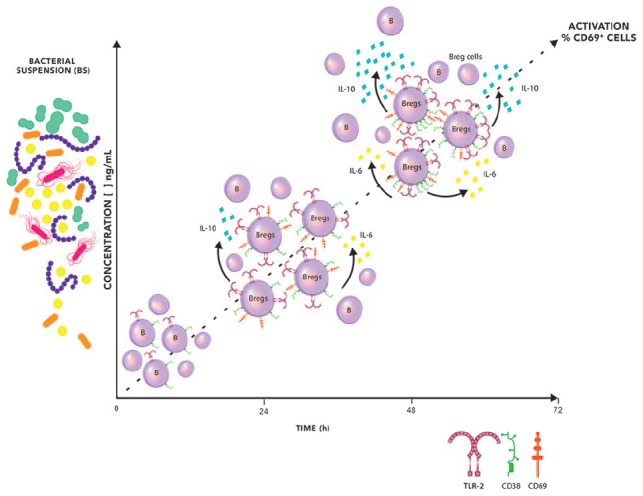
The hypothetic sequence of events after stimulation of mononuclear cells with BS. BS-14 induces activation of B cells in a dose and time-dependent manner. B cell activation developed inside a microenvironment enriched with IL-6 and IL-10, which increased the expression of TLR2 on the B cells.
A possible role for TLR2 on CD19+ cells is as a contributor in an activation loop of CD19+ CD38+ IL-10+ Breg subsets.
Acknowledgments
Thanks are due to Maria Consuelo Andaluz, for her critical analysis of this work; Verónica Martínez, for her technical assistance; and Jesús Esteban Mendoza, for the art in Figure 5.
Footnotes
Authors’ Note: A.S. was a doctoral student from Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM) and received fellowship 347009 from CONACYT. J.N. is a doctoral student from Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM) and received fellowship 330168 from CONACYT; H.V.-S. is a doctoral student from Programa de Doctorado en Ciencias Médicas, Odontológicas y de la Salud (Farmacología Clínica), Universidad Nacional Autónoma de México (UNAM) and received fellowship 294674 from CONACYT.
Author Contributions: A.S. designed and performed experiments; J.E.N. performed the experiments; H.V.-S. analyzed the data and wrote the paper; and M.C.J.-M. designed experiments, analyzed data, and wrote the paper.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Department of Biochemistry, Faculty of Medicine, UNAM and Fundación Conde de Valenciana.
ORCID iD: Maria C Jiménez-Martínez  https://orcid.org/0000-0003-3982-9097
https://orcid.org/0000-0003-3982-9097
References
- 1. Cazzola M, Anapurapu S, Page CP. Polyvalent mechanical bacterial lysate for the prevention of recurrent respiratory infections: a meta-analysis. Pulm Pharmacol Ther 2012; 25(1): 62–68. [DOI] [PubMed] [Google Scholar]
- 2. Ricci R, Palmero C, Bazurro G, et al. The administration of a polyvalent mechanical bacterial lysate in elderly patients with COPD results in serological signs of an efficient immune response associated with a reduced number of acute episodes. Pulm Pharmacol Ther 2014; 27(1): 109–113. [DOI] [PubMed] [Google Scholar]
- 3. Luan H, Zhang Q, Wang L, et al. OM85-BV induced the productions of IL-1β, IL-6, and TNF-α via TLR4- and TLR2-mediated ERK1/2/NF-κB pathway in RAW264.7 cells. J Interferon Cytokine Res 2014; 34(7): 526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lanzilli G, Traggiai E, Braido F, et al. Administration of a polyvalent mechanical bacterial lysate to elderly patients with COPD: effects on circulating T, B and NK cells. Immunol Lett 2013; 149(1–2): 62–67. [DOI] [PubMed] [Google Scholar]
- 5. Rial A, Ferrara F, Suarez N, et al. Intranasal administration of a polyvalent bacterial lysate induces self-restricted inflammation in the lungs and a Th1/Th17 memory signature. Microbes Infect 2016; 18(12): 747–757. [DOI] [PubMed] [Google Scholar]
- 6. Guaní-Guerra E, Negrete-García MC, Montes-Vizuet R, et al. Human β-defensin-2 induction in nasal mucosa after administration of bacterial lysates. Arch Med Res. 2011; 42(3): 189–194. [DOI] [PubMed] [Google Scholar]
- 7. Rosaschino F, Cattaneo L. Strategies for optimizing compliance of paediatric patients for seasonal antibacterial vaccination with sublingually administered Polyvalent Mechanical Bacterial Lysates (PMBL). Acta Biomed 2004; 75(3): 171–178. [PubMed] [Google Scholar]
- 8. Liao HY, Tao L, Zhao J, et al. Clostridium butyricum in combination with specific immunotherapy converts antigen-specific B cells to regulatory B cells in asthmatic patients. Sci Rep 2016; 6: 20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol 2015; 194(4): 1395–1401. [DOI] [PubMed] [Google Scholar]
- 10. Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells 1994; 12(5): 456–465. [DOI] [PubMed] [Google Scholar]
- 11. Van Belle K, Herman J, Boon L, et al. Comparative in vitro immune stimulation analysis of primary human B cells and B cell lines. J Immunol Res 2016; 2016: 5281823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Funaro A, Morra M, Calosso L, et al. Role of the human CD38 molecule in B cell activation and proliferation. Tissue Antigens 1997; 49(1): 7–15. [DOI] [PubMed] [Google Scholar]
- 13. Wehr C, Kivioja T, Schmitt C, et al. The EURO class trial: defining subgroups in common variable immunodeficiency. Blood 2008; 111(1): 77–85. [DOI] [PubMed] [Google Scholar]
- 14. Muraguchi A, Hirano T, Tang B, et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med 1988; 167(2): 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev 2008; 226: 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hilmi D, Parcina M, Stollewerk D, et al. Heterogeneity of host TLR2 stimulation by Staphylocoocus aureus isolates. PLoS ONE 2014; 9(5): e96416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomlinson G, Chimalapati S, Pollard T, et al. TLR-mediated inflammatory responses to treptococcus pneumoniae are highly dependent on surface expression of bacterial lipoproteins. J Immunol 2014; 193(7): 3736–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leech JM, Lacey KA, Mulcahy ME, et al. IL-10 plays opposing roles during Staphylococcus aureus systemic and localized infections. J Immunol 2017; 198(6): 2352–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bancos S, Phipps RP. Memory B cells from older people express normal levels of cyclooxygenase-2 and produce higher levels of IL-6 and IL-10 upon in vitro activation. Cell Immunol 2010; 266(1): 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin W, Cerny D, Chua E, et al. Human regulatory B cells combine phenotypic and genetic hallmarks with a distinct differentiation fate. J Immunol 2014; 193(5): 2258–2266. [DOI] [PubMed] [Google Scholar]
- 21. Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 2003; 21: 335–376. [DOI] [PubMed] [Google Scholar]
- 22. Regueiro V, Moranta D, Campos MA, et al. Klebsiella pneumoniae increases the levels of toll-like receptors 2 and 4 in human airway epithelial cells. Infect Immun 2009; 77(2): 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shin HS, Lee JH, Paek SH, et al. Pseudomonas aeruginosa-dependent upregulation of TLR2 influences host responses to a secondary Staphylococcus aureus infection. Pathog Dis 2013; 69(2): 149–156. [DOI] [PubMed] [Google Scholar]
- 24. Paolillo R, Romano Carratelli C, Sorrentino S, et al. Expression of IL-23, VEGF and TLR2/TLR4 on mononuclear cells after exposure to Pseudomonas aeruginosa. Int J Immunopathol Pharmacol 2011; 24(4): 961–973. [DOI] [PubMed] [Google Scholar]
- 25. Kuan YC, Wu YJ, Hung CL, et al. Trametes versicolor protein YZP activates regulatory B lymphocytes—gene identification through de novo assembly and function analysis in a murine acute colitis model. PLoS ONE 2013; 8(9): e72422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frodermann V, Chau TA, Sayedyahossein S, et al. A modulatory interleukin-10 response to Staphylococcal peptidoglycan prevents Th1/Th17 adaptive immunity to Staphylococcus aureus. J Infect Dis 2011; 204(2): 253–262. [DOI] [PubMed] [Google Scholar]
- 27. Wang J, Roderiquez G, Norcross MA. Control of adaptive immune responses by Staphylococcus aureus through IL-10, PD-L1, and TLR2. Sci Rep 2012; 2: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yanaba K, Bouaziz JD, Matsushita T, et al. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol 2009; 182(12): 7459–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang K, Tao L, Su J, et al. TLR4 supports the expansion of FasL+CD5+CD1dhi regulatory B cells, which decreases in contact hypersensitivity. Mol Immunol 2017; 87: 188–199. [DOI] [PubMed] [Google Scholar]
- 30. Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011; 117(2): 530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van de Veen W, Stanic B, Yaman G, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol 2013; 131(4): 1204–1212. [DOI] [PubMed] [Google Scholar]
- 32. Stanic B, van de Veen W, Wirz OF, et al. IL-10-overexpressing B cells regulate innate and adaptive immune responses. J Allergy Clin Immunol 2015; 135(3): 771–780. [DOI] [PubMed] [Google Scholar]
- 33. Wawrzyniak M, O’Mahony L, Akdis M. Role of regulatory cells in oral tolerance. Allergy Asthma Immunol Res 2017; 9(2): 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. James J, Weaver V, Cantorna MT. Control of circulating IgE by the vitamin D receptor in vivo involves B cell intrinsic and extrinsic mechanisms. J Immunol 2017; 198(3): 1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo J, Guo H, Liu Z, et al. Analysis of peripheral B cell subsets in patients with allergic rhinitis. Allergy Asthma Immunol Res 2018; 10(3): 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blair PA, Norena LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010; 32(1): 129–140. [DOI] [PubMed] [Google Scholar]
- 37. Salazar A, Casanova-Méndez I, Pacheco-Quito M, et al. Low expression of IL-10 in circulating bregs and inverted IL-10/TNF-α ratio in tears of patients with perennial allergic conjunctivitis: a preliminary study. Int J Mol Sci 2019; 20(5): E1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fu R, Li J, Zhong H, et al. Broncho-Vaxom attenuates allergic airway inflammation by restoring GSK3β-related T regulatory cell insufficiency. PLoS ONE 2014; 9(3): e92912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Janossy G, Shohat M, Greaves MF, et al. Lymphocyte activation. IV. The ultrastructural pattern of the response of mouse T and B cells to mitogenic stimulation in vitro. Immunology 1973; 24(2): 211–227. [PMC free article] [PubMed] [Google Scholar]