Abstract
Study Design:
Ambispective cohort study design.
Objectives:
Cervical spine metastases have distinct clinical considerations. The aim of this study was to determine the impact of surgical intervention (± radiotherapy) or radiotherapy alone on health-related quality of life (HRQOL) outcomes in patients treated for cervical metastatic spine tumours.
Methods:
Patients treated with surgery and/or radiotherapy for cervical spine metastases were identified from the Epidemiology, Process, and Outcomes of Spine Oncology (EPOSO) international multicentre prospective observational study. Demographic, diagnostic, treatment, and HRQOL (numerical rating scale [NRS] pain, EQ-5D (3L), SF-36v2, and SOSGOQ) measures were prospectively collected at baseline, 6 weeks, 3 months, and 6 months postintervention.
Results:
Fifty-five patients treated for cervical metastases were identified: 38 underwent surgery ± radiation and 17 received radiation alone. Surgically treated patients had higher mean spinal instability neoplastic scores compared with the radiation-alone group (13.0 vs 8.0, P < .001) and higher NRS pain scores and lower HRQOL scores compared to the radiation alone group (P < .05). From baseline to 6 months posttreatment, surgically treated patients demonstrated statistically significant improvements in NRS pain, EQ-5D (5L), and SOSGOQ2.0 scores compared with nonsignificant improvements in the radiotherapy alone group.
Conclusions:
Surgically treated cervical metastases patients presented with higher levels of instability, worse baseline pain and HRQOL scores compared with patients who underwent radiotherapy alone. Significant improvements in pain and HRQOL were noted for those patients who received surgical intervention. Limited or no improvements were found in those treated with radiotherapy alone.
Keywords: spine oncology, cervical metastases, surgery, radiotherapy
Introduction
Cervical spine metastases represent only 8% to 15% of all spinal metastases; however, they require a distinct clinical perspective considering the complex biomechanics of the cervical spine and often have worse prognoses compared with thoracic and lumbar metastatic disease.1-6 The intricate neurological, vascular, and visceral anatomy presents unique challenges to both the surgeon and the radiation oncologist for the management of metastases in this region.7-9 The main goal of treatment for patients with metastatic spinal disease is to improve pain and maintain or improve neurological function and health-related quality of life (HRQOL).10 Generally, indications for surgical management are progressive neurological deficits resulting from compression of neural structures, intractable pain, and spinal instability.11-13 The indications for radiotherapy alone include pain without myelopathy or instability.14 The potential collateral damage to critical organs as a result of surgical or radiation treatment limit treatment options and stress the necessity for multidisciplinary management.
To date, there has been a paucity of literature and no prospective studies investigating HRQOL and adverse event outcomes in this unique patient population treated with radiation and/or surgery. Epidemiology, Process, and Outcomes of Spine Oncology (EPOSO) is a comprehensive, prospective clinical observational study aimed at collecting patient, diagnostic and treatment variables along with disease-specific and HRQOL data on consecutively treated patients with metastatic spine tumors. This is a subgroup analysis of the larger EPOSO cohort with the main objective of the study being to determine the impact of surgical intervention (± radiotherapy) and radiotherapy alone on HRQOL outcomes in patients treated for cervical metastatic spine tumours. A secondary aim of this study is to identify treatment specific adverse events during follow-up.
Materials and Methods
Patient Population and Study Design
The AOSpine Knowledge Forum Tumor initiated an international, multicenter, prospective observational study between August 2013 and February 2017 at 10 spine centers across North America and Europe (ClinicalTrials.gov identifier NCT01825161). This study was complete with full ethics approval from the University of British Columbia Clinical Ethics Review Board (Number: H13-00 980). Study population included all consecutively treated patients from participating centers diagnosed with metastatic spine tumors who had undergone surgery and/or radiotherapy for the treatment of spinal metastases from any primary tumor and were aged 18 to 75 years. Patients with a primary spinal bone tumor or central nervous system tumor were excluded. The ethics board of each participating center approved the protocol. All patients provided written informed consent for study participation.
A subgroup of patients with cervical spine metastases treated with either surgical intervention (± radiotherapy) or radiotherapy alone was identified from the above cohort. Demographic, diagnostic information, and disease-specific and generic HRQOL data was collected prospectively and analyzed retrospectively representing an ambispective study design.
All patients enrolled underwent treatment with either surgical intervention (± radiotherapy) or radiotherapy alone as per individual institution protocol. Adverse event and clinical data was collected prospectively and entered into the EPOSO database. All treatment was at the discretion of the most responsible clinician including surgical instrumentation, post-operative management (including wound management and VTE prophylaxis), and dose and duration of radiotherapy.
Outcome Measures
Outcome measures data was collected at enrollment and follow-up scheduled at 6, 12, and 26 weeks following the initiation of treatment for cervical metastases. The pain numerical rating scale (NRS) visual analog scale was used for the evaluation of pain.15-17 Patients were assessed for HRQOL using the EQ-5D (5L)18, and the SF-36v2 Questionnaire.19 Disease-specific HRQOL was evaluated with the Spine Oncology Study Group Outcomes Questionnaire (SOSGOQ2.0).20,21
Statistical Analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary NC). Simple summary statistics were used to report all outcome measures’ endpoints by treatment group. Differences in baseline parameters were analyzed by using a t test or Wilcoxon rank sum test for continuous variables, whereas the chi-square test or Fisher’s exact test were used for categorical variables. A mixed effect model was used to test for differences in outcomes compared to baseline for each treatment and considered missing data during follow-up. Significance was defined as P < .05.
Results
Demographics
A total of 55 patients were identified from the EPOSO cohort, 38 (69.1%) of which received surgical intervention (± radiotherapy) and 17 (30.9%) received radiotherapy alone. The mean patient age was 57.4 (SD 10.9) years and 50.9% (n = 28) were male. The 3 most common types of primary cancer were breast (n = 15), lung (n = 15), and kidney (n = 5). The most common location for metastatic disease in the cervical spine was the cervicothoracic junction with 35 (63.6%) of patients having burden of disease between C6 and C7 (Table 1).
Table 1.
Demographic and Clinical Information for All Patients With Cervical Metastatic Disease.
| All (N = 55) | Surgery ± Radiation (n = 38) | Radiation (n = 17) | P | |
|---|---|---|---|---|
| Mean Age (SD) | 57.4 (10.9) | 57.0 (10.9) | 58.4 (7.9) | .641 |
| Gender, n (%) | ||||
| Male | 28 (50.9) | 21 (55.3) | 7 (41.2) | .334 |
| Female | 27 (49.1) | 17 (44.7) | 10 (58.8) | |
| Primary tumor type, n (%) | .126 | |||
| Breast | 15 (27.3) | 10 (26.3) | 5 (29.4) | |
| Lung | 15 (27.3) | 10 (26.3) | 5 (29.4) | |
| Myeloma | 3 (5.5) | 3 (7.9) | 0 (0) | |
| Kidney | 5 (9.1) | 1 (2.6) | 4 (23.5) | |
| Prostate | 3 (5.5) | 2 (5.3) | 1 (5.9) | |
| Other | 14 (25.5) | 12 (31.6)a | 2 (11.8)b | |
| Primary tumor removed, n (%) | ||||
| No | 25 (45.5) | 22 (57.9) | 3 (17.6) | |
| Yes—totally | 23 (41.8) | 11 (28.9) | 12 (70.6) | |
| Only partial | 6 (10.9) | 4 (10.5) | 2 (11.8) | |
| Unknown | 1 (1.8) | 1 (2.6) | 0 (0.0) | |
| Pain type, n (%) | ||||
| None | 7 (12.7) | 1 (2.6) | 6 (35.3) | |
| Axial | 40 (72.7) | 32 (84.2) | 8 (47.1) | |
| Radicular | 28 (50.9) | 22 (57.9) | 6 (35.3) | |
| Location of tumor, n (%) | ||||
| Occiput-C2 | 8 (14.5) | 7 (18.4) | 1 (5.9) | |
| C3-5 | 27 (49.1) | 16 (42.1) | 11 (64.7) | |
| C6-7 | 35 (63.6) | 24 (63.2) | 11 (64.7) | |
| ASIA Impairment Scale (AIS), n (%) | ||||
| A | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| B | 2 (3.7) | 2 (5.3) | 0 (0.0) | |
| C | 5 (9.3) | 5 (13.2) | 0 (0.0) | |
| D | 13 (24.1) | 13 (34.2) | 0 (0.0) | |
| E | 34 (63.0) | 18 (47.4) | 16 (100.0) | |
| Pain type, n (%) | ||||
| Pain-free lesion | 4 (7.4) | 0 (0.0) | 4 (25.0) | <.001 |
| Occasional pain but not mechanical | 10 (18.5) | 4 (10.5) | 6 (37.5) | |
| Mechanical pain | 40 (74.1) | 34 (89.5) | 6 (37.5) | |
| Epidural compression, n (%) | ||||
| No compression (grade 0) | 12 (23.1) | 5 (13.5) | 8 (46.7) | .025 |
| Compression present (grade 1a/b/c, 2, 3) | 40 (76.9) | 32 (86.5) | 9 (53.3) | |
| SINS, mean (SD) | 11.5 (3.6) | 13.0 (2.8) | 8.0 (2.8) | <.001 |
Abbreviations: ASIA, American Spinal Injury Association; SINS, spinal instability neoplastic score.
a 3× myeloma, 6× gastrointestinal, 1× thyroid, 1× uterine, 1× liver, 2× bladder, and 1× oropharynx.
b 1× gastrointestinal and 1× bladder.
Of the 55 patients, 20 (36.4%) of them experienced only axial pain, 8 (14.5%) had only radicular pain, 20 patients (36.4%) had both axial and radicular pain, and 7 (12.7%) experienced no pain. There were no patients with complete neurological deficits (American Spinal Injury Association Impairment Scale [AIS] A) and all those who underwent radiotherapy alone were graded as AIS E (Table 1). Patients who received surgical intervention (± radiotherapy) had a higher mean spinal instability neoplastic score (SINS) than radiotherapy alone (13.0 vs 8.0, P < .001) and presented more often with mechanical neck pain before intervention (89.5% vs 37.5%, P < .001). Two illustrative cases are presented in Figures 1 and 2.
Figure 1.
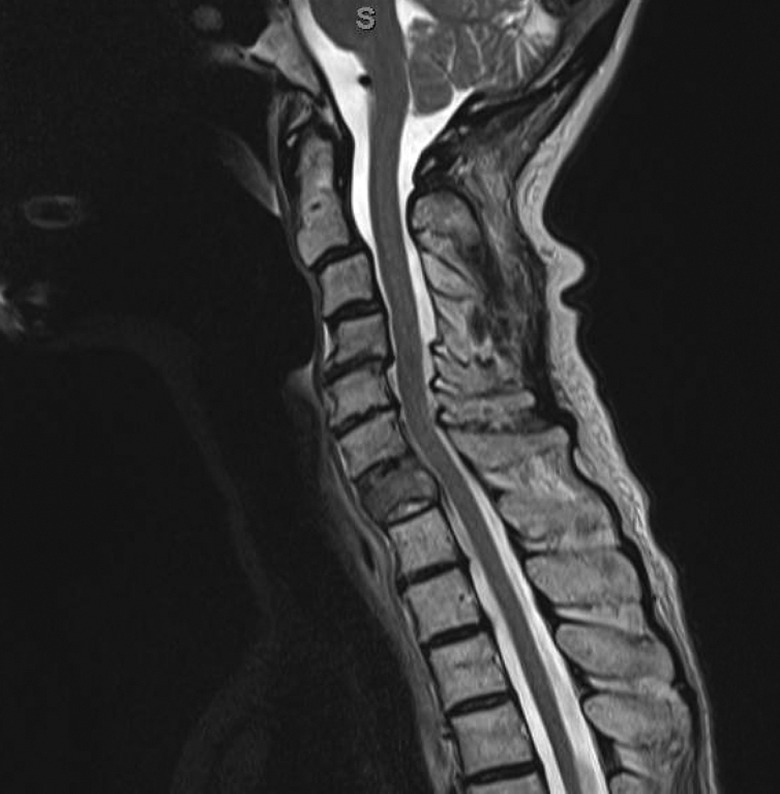
A 75-year-old woman with metastatic breast carcinoma, presenting with neck pain and no neurological symptoms. She received palliative radiation treatment to the cervical spine. She received 1800 cGy in 4 fractions.
Figure 2.
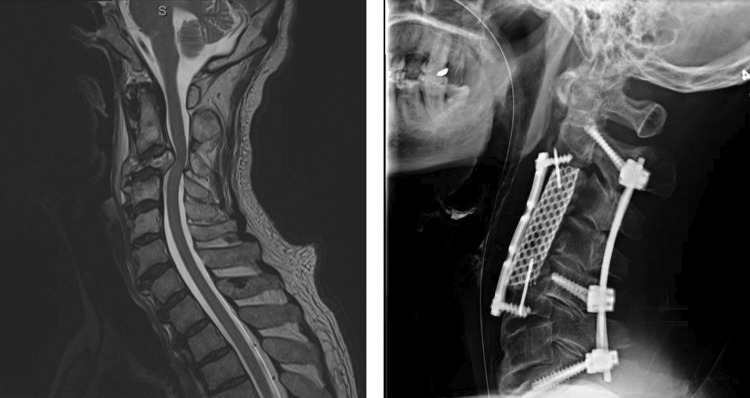
A 63-year-old man with metastatic bladder carcinoma. He presented with mechanical neck pain, bilateral leg paresthesias, and hyperreflexia. Imaging demonstrated destruction of the C3 vertebral body with significant focal kyphosis and posterior subluxation of C2 into C3. He underwent an anterior C3 and C4 vertebral body resection, decompression spinal cord, and osteotomy with anterior correction of kyphosis. Anterior cage C2-5 with cement and plate C2-5.
Surgical Details
A total of 38 patients underwent surgical intervention, 23 (60.5%) patients had a posterior surgical approach, 9 (23.6%) had an anterior approach, and only 6 (15.8%) had a combined approach. The mean operating time was 236.5 (SD 109.8) minutes and included a median of 4 spinal levels instrumented (Table 2). In the surgical treatment group, 25 (65.8%) patients had radiotherapy, of whom 11 (44.0%) had adjuvant radiotherapy, which began at mean 1.5 (SD 1.0) months after surgery.
Table 2.
Surgical Data and Details for Patients Treated With Cervical Metastatic Disease.
| Characteristic | Surgery ± Radiation (N = 38) |
|---|---|
| Approach, n (%) | |
| Anterior | 9 (23.7) |
| Posterior | 23 (60.5) |
| Combined anterior/posterior | 6 (15.8) |
| Surgical levels, median (range) | 4.0 (1-8) |
| Surgical time, min, mean (SD) | 236.5 (109.8) |
| Length of stay, days, median (range) | 8.0 (2-26) |
| Previous radiation therapy, n (%) | 8 (21.1) |
| Adjuvant radiation therapy, n (%) | 11 (28.9) |
| Start of adjuvant radiotherapy, months, mean (SD) | 1.5 (1.0) |
| Intraoperative adverse event, n (%) | 4 (10.5) |
| Dural tear | 1 (2.6) |
| Implant complication requiring revision | 1 (2.6) |
| Massive blood loss | 1 (2.6) |
| Vascular injury | 1 (2.6) |
| Postoperative adverse event, n (%) | 17 (44.7) |
| Dysphasia/dysphonia | 1 (2.6) |
| Pneumonia | 2 (5.3) |
| Thromboembolic event | 4 (10.5) |
| Urinary tract infection | 4 (10.5) |
| Wound drainage | 4 (10.5) |
| Wound infection | 2 (5.3) |
Radiotherapy Details
Considering the 17 patients who only received radiotherapy, 9 (52.9%) underwent conventional radiotherapy and 8 (47.1%) received multifraction stereotactic radiotherapy. The median radiation dose for conventional radiotherapy was 20 Gy in 5 fractions. The median radiation dose in the stereotactic radiation group was 24 Gy in 2 fractions (Table 3).
Table 3.
Radiotherapy Data and Details for Those Who Received Radiation Therapy Only.
| Type, n (%) | Radiation (N = 17) |
|---|---|
| Conventional | 9 (52.9) |
| Total dose, Gy, median (range) | 20.0 (18-30) |
| Number of fractions, median (range) | 5.0 (4-10) |
| Stereotactic | 8 (47.1) |
| Total dose, Gy, median (range) | 24.0 (24 – 28) |
| Number of fractions (range) | 2.0 (2 – 3) |
| Adverse events, n (%) | 8 (47.1) |
| Skin | 3 (17.6) |
| Mucous membrane | 2 (11.8) |
| Pharynx and esophagus | 4 (23.5) |
| Upper gastrointestinal | 3 (17.6) |
| Lower gastrointestinal (including pelvis) | 2 (11.8) |
| Hemoglobin drop | 1 (5.9) |
| Platelet drop | 1 (5.9) |
Patient-Reported Outcomes Assessment
The patients who received surgery (± radiotherapy) had higher pain NRS scores (7.4 vs 3.4, P < .001), worse EQ-5D scores (0.43 vs 0.65, P < .006), and worse SOSGOQ2.0 scores (45.9 vs 61.4, P = .004) at baseline compared to those who received radiotherapy alone. Neither the physical component summary nor the mental component summary of the SF-36v2 demonstrated statistically significant differences postintervention from baseline in the surgical treatment (± radiotherapy) or radiation alone groups at 6 months postintervention (Table 4).
Table 4.
Mixed Effects Model Demonstrating Patient-Reported Outcomes Estimates.a
| Surgery ± Radiation | Radiation | Surgery vs Radiotherapy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (95% CI) | Change (95% CI) | Adjusted Pb | n | Mean (95% CI) | Change (95% CI) | Adjusted Pb | P c | |
| Pain NRS | |||||||||
| Baseline | 35 | 7.4 (6.5; 8.2) | 17 | 3.4 (2.2; 4.5) | <.001 | ||||
| 6 weeks | 25 | 4.5 (3.4; 5.5) | −2.9 (−4.5; −1.2) | <.001 | 12 | 2.3 (0.9; 3.8) | −1.0 (−3.3; 1.2) | .798 | .020 |
| 12 weeks | 17 | 4.7 (3.6; 5.8) | −2.6 (−4.7; −0.5) | .006 | 11 | 3.2 (1.8; 4.5) | −0.2 (−2.9; 2.6) | 1.000 | .079 |
| 26 weeks | 13 | 4.5 (3.3; 5.7) | −2.8 (−4.9; −0.8) | .002 | 7 | 3.5 (1.9; 5.1) | 0.1 (−2.6; 2.9) | 1.000 | .315 |
| EQ-5D (3L) | |||||||||
| Baseline | 34 | 0.43 (0.34; 0.52) | 17 | 0.65 (0.53; 0.78) | .006 | ||||
| 6 weeks | 24 | 0.61 (0.51; 0.70) | 0.17 (0.01; 0.34) | .036 | 12 | 0.68 (0.55; 0.81) | 0.03 (−0.20; 0.26) | 1.000 | .344 |
| 12 weeks | 17 | 0.64 (0.56; 0.73) | 0.21 (0.02; 0.40) | .020 | 11 | 0.70 (0.59; 0.82) | 0.05 (−0.19; 0.30) | .995 | .395 |
| 26 weeks | 13 | 0.67 (0.59; 0.75) | 0.23 (0.07; 0.40) | .003 | 7 | 0.71 (0.60; 0.81) | 0.06 (−0.17; 0.28) | .990 | .551 |
| SF-36v2 PCS | |||||||||
| Baseline | 35 | 30.0 (26.6; 33.5) | 17 | 35.3 (30.4; 40.3) | .086 | ||||
| 6 weeks | 23 | 28.5 (24.2; 32.8) | −1.5 (−9.1; 6.1) | .997 | 12 | 34.0 (28.2; 39.8) | −1.3 (−12; 9.0) | 1.000 | .131 |
| 12 weeks | 17 | 32.8 (28.4; 37.2) | 2.7 (−6.0; 11.5) | .965 | 10 | 34.5 (28.8; 40.1) | −0.9 (−13; 10.8) | 1.000 | .636 |
| 26 weeks | 13 | 31.2 (25.5; 37.0) | 1.2 (−8.8; 11.2) | 1.000 | 7 | 34.9 (27.2; 42.6) | −0.4 (−14; 13.1) | 1.000 | .434 |
| SF-36v2 MCS | |||||||||
| Baseline | 35 | 42.3 (37.8; 46.7) | 17 | 45.7 (39.3; 52.1) | .382 | ||||
| 6 weeks | 23 | 44.8 (39.5; 50.1) | 2.5 (−5.7; 10.8) | .970 | 12 | 47.8 (40.5; 55.2) | 2.1 (−9.3; 13.6) | .998 | .504 |
| 12 weeks | 17 | 44.1 (39.5; 48.7) | 1.8 (−6.4; 10.0) | .996 | 10 | 46.0 (40.0; 52.0) | 0.3 (−11; 11.2) | 1.000 | .612 |
| 26 weeks | 13 | 47.6 (40.9; 54.3) | 5.4 (−7.0; 17.7) | .844 | 7 | 45.5 (36.5; 54.6) | −0.2 (−17; 16.8) | 1.000 | .705 |
| SOSGOQ2.0 | |||||||||
| Baseline | 33 | 45.9 (39.9; 52.0) | 17 | 61.4 (53.0; 69.8) | .004 | ||||
| 6 weeks | 22 | 61.8 (53.5; 70.1) | 15.8 (3.0; 28.7) | .008 | 11 | 64.1 (52.6; 75.5) | 2.6 (−14.5; 19.8) | 1.000 | .748 |
| 12 weeks | 16 | 67.9 (60.6; 75.1) | 21.9 (9.1; 34.7) | <.001 | 10 | 66.2 (56.9; 75.5) | 4.8 (−11.7; 21.2) | .979 | .772 |
| 26 weeks | 13 | 69.9 (62.3; 77.4) | 23.9 (9.8; 38.0) | <.001 | 7 | 72.1 (61.8; 82.3) | 10.7 (−8.5; 29.8) | .613 | .724 |
Abbreviations: NRS, numerical rating scale; EQ-5D (3L), EuroQoL-5D (3L); SF-36, Short Form–36; PCS, physical component summary; MCS, mental component summary; SOSGOQV2.0, Spine Oncology Study Group Outcomes Questionnaire version 2.0.
a Boldfaced P values indicate statistical significance (P < .05)
b Adjusted P value by Tukey-Kramer for comparison of change to baseline value per treatment group.
c P value for comparison of mean value of both treatment groups.
Surgery (± Radiotherapy) Patient-Reported Outcomes Assessment
In those who received surgical intervention, there were statistically significant improvements in pain compared with baseline at 6 weeks (7.4 vs 4.5, P < .001), 3 months (7.4 vs 4.7, P = .006), and 6 months (7.4 vs 4.5, P = .002) posttreatment. Also, significant improvements in EQ-5D (5L) scores from baseline at 6 weeks (0.43 vs 0.61, P = .036), 3 months (0.43 vs 0.64, P = .020), and 6 months (0.43 vs 0.67, P = .003) posttreatment were observed. Finally, improvements in the total score of the SOSGOQ2.0 from baseline were seen in the surgery (± radiotherapy) group at 6 weeks (45.9 vs 61.8, P = .008), 3 months (45.9 vs 67.9, P < .001), and at 6 months (45.9 vs 69.9, P < .001) postoperatively.
All individual domains of the SOSGOQ2.0 showed improvement for the surgery (± radiotherapy) group, including physical function, pain, social function, and mental health, except for the neurological function. The pain domain demonstrated the greatest degree of improvement between all domains with an observed median of 30 points improvement at 6 months posttreatment.
Radiation Patient-Reported Outcomes Assessment
Those patients who received radiotherapy alone demonstrated no significant changes in NRS pain scores from baseline at 6 weeks (3.4 vs 2.3, P = .798), 3 months (3.4 vs 3.2, P = 1.000), and 6 months (3.4 vs 3.5, P = 1.000) posttreatment (Table 4). There were no significant improvements in ED-5D (5L) scores noted at 6 weeks (0.65 vs. 0.68, P = 1.0), 3 months (0.65 vs 0.70, P = .995), and 6 months (0.65 vs 0.71, P = .990) posttreatment. Finally, no improvement was seen in the total score of SOSGOQ2.0 from baseline in the radiotherapy only group at 6 weeks (61.4 vs 64.1, P = 1.000), 3 months (61.4 vs 66.2, P = .979), and 6 months (61.4 vs 72.1, P = .613) (Table 4).
Adverse Events
Postoperative adverse events occurred in 17 out of 38 (44.7%) and intraoperative events occurred in 4 (10.5%) of surgical patients. Intraoperative events included dural tear, implant complication and bleeding complications. Postoperative events included thromboembolic events, wound complications, infections, neurologic deterioration, and dysphasia (see Table 2). Radiation adverse events occurred in 11 out of 25 (44.0%) surgical patients and 8 out of 17 (47.1%) radiation only patients with the three most reported being gastrointestinal symptoms, dermatologic manifestations, and blood dyscrasias (see Table 3).
Dropout and Death
At 6-month follow-up, 5 (13.2%) patients were lost to follow-up and 14 (36.8%) had died in the surgery (± radiation group). In the radiation alone group, 3 (17.6%) were lost to follow-up and 4 (23.5%) had passed away at the time of 6-month follow-up.
Discussion
The goal of treatment for metastatic disease to the cervical spine is that of neurologic and functional recovery with relief of pain to improve or maintain HRQOL. The results of this study demonstrate that those selected for surgical treatment (± radiotherapy) for cervical spine metastatic disease achieve improvement in pain and HRQOL at all time points up to and including 6 months follow-up. It also demonstrates that those who received radiotherapy alone in cervical metastatic disease did not show improvement in pain or HRQOL at any follow-up time point and overall maintain these scores at 6 months. This is probably related to different treatment indications and outcome objectives. For example, local control may be a primary indication for radiation, to prevent worsening symptoms down the road.
Indications for surgical intervention have traditionally included neurologic compromise from neural compression, spinal instability, and intractable pain. In the surgical group from the current study, patients demonstrated worse baseline pain and function scores and were likely selected for surgical treatment based on mechanical pain, instability, and associated neurologic compromise. Patients who received surgical intervention demonstrated significant improvements in pain and HRQOL (EQ-5D (5L) and SOSGOQ2.0) and maintained these improvements over 6 months. This is consistent with what has been reported in numerous retrospective studies demonstrating improvements in pain and HRQOL with surgical intervention for cervical metastases, but none using prospectively collected data.8,13,22-31 This study represents a large international collaboration and one of the first prospective cohorts that has used validated and reliable generic and disease-specific HRQOL measures to follow patients with spinal metastases.
The purpose of this study was not to directly compare radiation with surgery for cervical metastases as the 2 groups are not similar at baseline. Surgical intervention is thus an appropriate option and beneficial to patients who demonstrate symptoms of spinal instability and neurologic compromise, improving overall patient quality of life and pain symptoms.
Indications for radiation therapy alone are pain with no signs of myelopathy or mechanical instability.11 There have been retrospective studies and systematic reviews that have demonstrated both improvement and maintenance of HRQOL and pain in patients who receive radiotherapy alone.32-37 Little literature has been published on the outcomes of radiotherapy for metastatic disease in the cervical spine alone. This study is the first to describe a cohort of patients treated for metastatic disease specifically in the cervical spine with radiotherapy alone in which the data was collected prospectively. These patients demonstrated better HRQOL and pain scores at baseline compared to those who underwent surgery, and overall maintained these scores at 6 months follow-up. Thus, there is a need to better predict which patients are unlikely to respond to radiotherapy alone and initiate other treatment options. Several studies have attempted this using the SINS to predict response to radiotherapy with improvement in pain.38,39 Those who had higher SINS scores treated with radiotherapy alone were less likely to show improvements in pain and a 1-point increase in SINS score increased the likelihood of radiation failure by 30%.40 Further studies are necessary to evaluate whether other treatment modalities may provide improvement in quality of life, disability, and pain for those who fail to respond to radiotherapy.
Cervical spine metastasis poses a significant challenge to clinicians due to numerous factors including complex anatomy, proximity to neurovascular structures, and diverse patient populations with varying prognoses.11,28,41 Differences with regard to anatomy and complexity exist based on region in the cervical spine which include occipito-cervical junction (C0-C2), subaxial spine (C3-6), and the cervico-thoracic junction (C7-T2).10,23,29,30,41 Because of this complexity, surgery and radiotherapy can lead to significant adverse events. In this study, those who received surgery had a postoperative complication rate of 44.7%. These included pneumonia, urinary tract infection, wound complications, thromboembolic events, and others. In the literature, complication rates for surgical management of cervical metastatic spine disease range from 10% to 52% and are consistent with our findings.8,13,23,25,28,31 Those in the radiation alone group demonstrated adverse events in 47.1% of patients and 44.0% of patients in the surgical group who received radiotherapy. These included upper gastrointestinal symptoms, dermatologic manifestations, and blood dyscrasias. The complications from surgical intervention, such as pulmonary embolism or wound complication, have the potential for significant repercussions including harm to life or need for reoperation, while complications from radiation therapy, report no major complications.42,43 However, in patients treated surgically for metastatic spine disease, the complications for surgical intervention have been shown to not impact mortality post-intervention and that the 30-day morbidity rate was substantially higher in those receiving radiation therapy.44 Thus, the decision to undergo surgical intervention must take into consideration the adverse event profile and is best approached by a multidisciplinary team including spine surgeons, radiation oncologists, and medical oncologists in discussion with patients’ and families’ wishes.
While this is an international multicenter collaboration involving 10 centers, a limitation of this study is the small sample size of only 55 patients with cervical metastases, making it difficult to evaluate and limit the ability to adjust for confounding variables such as the differences in SINS, pain, and HRQOL scores at baseline between the two treatment groups. This study was not a comparative study and selection for treatment was based on the discretion of the treating physician. Thus, the 2 groups must be looked at individually and baseline differences between the two treatment groups should be considered when interpreting the results. In addition, the differences within the anatomical regions of the cervical spine could not be further evaluated as a small sample size made interpretation of the results challenging. Finally, the choice of surgical technique and approach could not be standardized and is often based on surgical experience, location of the tumor, and guided by the literature. Specifics surrounding the type of instrumentation used, construct design, and implant technology were not available for review from the data collection and cannot be explicitly commented on.
Conclusions
This ambispective analysis of patients who underwent surgical and/or radiation treatment for cervical metastatic spine disease demonstrates that patients who received surgical intervention have worse baseline disease-specific and generic HRQOL and pain scores when compared to those who underwent radiotherapy only. Those who received surgical treatment for cervical metastases have significant improvements after surgery at 6 weeks with sustained results at 3 and 6 months posttreatment. Therefore, in patients presenting with cervical metastatic spine disease that is amenable to surgery, surgical intervention can significantly improve pain and HRQOL. Future studies should focus on identifying which patients are best selected for surgical intervention and evaluating the variations in outcomes for discrete anatomic regions of the cervical spine.
Acknowledgments
The authors would like to thank Christian Knoll (AOCID Statistician) for statistical analysis support. We are grateful to the collaborating centers’ local clinical research personnel and support staff for their active participation in EPOSO.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Fisher reports personal fees from Medtronic, and Nuvasive, grants from OREF, and receieves fellowship support paid by Medtronic and AOSpine. Dr Sahgal reports advisor with Abbvie, past educational seminars with Elekta AB, Accuray Inc., Varian medical systems, and BrainLAB, with research grant support from Elekta AB, and Travel accommodations/expenses by Elekta, Varian, BrainLAB. Dr Sahgal also belongs to the Elekta MR Linac Research Consortium. Dr Sciubba reports personal fees from Medtronic, Depuy-Synthes, Stryker, Nuvasive, K2M, Baxter, Misonix, outside the submitted work. Dr Versteeg reports personal fees from AOSpine International, outside the submitted work. All other authors have no disclosures to report.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was organized and funded by AOSpine International, through the AOSpine Knowledge Forum Tumor, a pathology-focused working group of up to 10 international spine experts acting on behalf of AOSpine in the domain of scientific expertise. A research grant was received from the Orthopedic Research and Education Foundation (OREF). Study support was provided directly through AOSpine’s Research department and AO’s Clinical Investigation and Documentation unit.
ORCID iD: Daniel M. Sciubba, MD  https://orcid.org/0000-0001-7604-434X
https://orcid.org/0000-0001-7604-434X
Michael G. Fehlings, PhD  https://orcid.org/0000-0002-5722-6364
https://orcid.org/0000-0002-5722-6364
References
- 1. Bartels RH, Feuth T, van der Maazen R, et al. Development of a model with which to predict the life expectancy of patients with spinal epidural metastasis. Cancer. 2007;110:2042–2049. [DOI] [PubMed] [Google Scholar]
- 2. Ortiz Gómez JA. The incidence of vertebral body metastases. Int Orthop. 1995;19:309–311. [DOI] [PubMed] [Google Scholar]
- 3. Jenis LG, Dunn EJ, An HS. Metastatic disease of the cervical spine. A review. Clin Orthop Relat Res. 1999;(359):89–103. [DOI] [PubMed] [Google Scholar]
- 4. Mesfin A, Buchowski JM, Gokaslan ZL, Bird JE. Management of metastatic cervical spine tumors. J Am Acad Orthop Surg. 2015;23:38–46. [DOI] [PubMed] [Google Scholar]
- 5. North RB, LaRocca VR, Schwartz J, et al. Surgical management of spinal metastases: analysis of prognostic factors during a 10-year experience. J Neurosurg Spine. 2005;2:564–573. [DOI] [PubMed] [Google Scholar]
- 6. Sciubba DM, Gokaslan ZL, Suk I, et al. Positive and negative prognostic variables for patients undergoing spine surgery for metastatic breast disease. Eur Spine J. 2007;16:1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacobs WB, Perrin RG. Evaluation and treatment of spinal metastases: an overview. Neurosurg Focus. 2001;11:e10. [DOI] [PubMed] [Google Scholar]
- 8. Quan GM, Vital JM, Pointillart V. Outcomes of palliative surgery in metastatic disease of the cervical and cervicothoracic spine. J Neurosurg Spine. 2011;14:612–618. [DOI] [PubMed] [Google Scholar]
- 9. Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine. 2010;13:94–108. [DOI] [PubMed] [Google Scholar]
- 10. Fehlings MG, Nater A, Tetreault L, et al. Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: results of the prospective multicenter AOSpine study. J Clin Oncol. 2016;34:268–276. [DOI] [PubMed] [Google Scholar]
- 11. Fehlings MG, David KS, Vialle L, Vialle E, Setzer M, Vrionis FD. Decision making in the surgical treatment of cervical spine metastases. Spine (Phila Pa 1976). 2009;34(22 suppl):S108–S117. [DOI] [PubMed] [Google Scholar]
- 12. Aebi M. Spinal metastasis in the elderly. Eur Spine J. 2003;12(suppl 2):S202–S213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heidecke V, Rainov NG, Burkert W. Results and outcome of neurosurgical treatment for extradural metastases in the cervical spine. Acta Neurochir (Wien). 2003;145:873–880. [DOI] [PubMed] [Google Scholar]
- 14. Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol. 2008;7:459–466. [DOI] [PubMed] [Google Scholar]
- 15. Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976). 2005;30:1331–1334. [DOI] [PubMed] [Google Scholar]
- 16. Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil. 2008;89:69–74. [DOI] [PubMed] [Google Scholar]
- 17. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S240–S252. [DOI] [PubMed] [Google Scholar]
- 18. EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 19. Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Street J, Lenehan B, Berven S, Fisher C. Introducing a new health-related quality of life outcome tool for metastatic disease of the spine: content validation using the International Classification of Functioning, Disability, and Health; on behalf of the Spine Oncology Study Group. Spine (Phila Pa 1976). 2010;35:1377–1386. [DOI] [PubMed] [Google Scholar]
- 21. Versteeg AL, Sahgal A, Rhines LD, et al. Psychometric evaluation and adaptation of the Spine Oncology Study Group Outcomes Questionnaire to evaluate health-related quality of life in patients with spinal metastases. Cancer. 2018;124:1828–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atanasiu JP, Badatcheff F, Pidhorz L. Metastatic lesions of the cervical spine. A retrospective analysis of 20 cases. Spine (Phila Pa 1976). 1993;18:1279–1284. [DOI] [PubMed] [Google Scholar]
- 23. Cho W, Chang UK. Neurological and survival outcomes after surgical management of subaxial cervical spine metastases. Spine (Phila Pa 1976). 2012;37:E969–E977. [DOI] [PubMed] [Google Scholar]
- 24. Liu JK, Rosenberg WS, Schmidt MH. Titanium cage-assisted polymethylmethacrylate reconstruction for cervical spinal metastasis: technical note. Neurosurgery. 2005;56(1 suppl):E207. [DOI] [PubMed] [Google Scholar]
- 25. Miller DJ, Lang FF, Walsh GL, Abi-Said D, Wildrick DM, Gokaslan ZL. Coaxial double-lumen methylmethacrylate reconstruction in the anterior cervical and upper thoracic spine after tumor resection. J Neurosurg. 2000;92(2 suppl):181–190. [DOI] [PubMed] [Google Scholar]
- 26. Perrin RG, Laxton AW. Metastatic spine disease: epidemiology, pathophysiology, and evaluation of patients. Neurosurg Clin N Am. 2004;15:365–373. [DOI] [PubMed] [Google Scholar]
- 27. Placantonakis DG, Laufer I, Wang JC, Beria JS, Boland P, Bilsky M. Posterior stabilization strategies following resection of cervicothoracic junction tumors: review of 90 consecutive cases. J Neurosurg Spine. 2008;9:111–119. [DOI] [PubMed] [Google Scholar]
- 28. Oda I, Abumi K, Ito M, et al. Palliative spinal reconstruction using cervical pedicle screws for metastatic lesions of the spine: a retrospective analysis of 32 cases. Spine (Phila Pa 1976). 2006;31:1439–1444. [DOI] [PubMed] [Google Scholar]
- 29. George B, Archilli M, Cornelius JF. Bone tumors at the cranio-cervical junction. Surgical management and results from a series of 41 cases. Acta Neurochir (Wien). 2006;148:741–749. [DOI] [PubMed] [Google Scholar]
- 30. Yang J, Jia Q, Peng D, et al. Surgical treatment of upper cervical spine metastases: a retrospective study of 39 cases. World J Surg Oncol. 2017;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caspar W, Pitzen T, Papavero L, Geisler FH, Johnson TA. Anterior cervical plating for the treatment of neoplasms in the cervical vertebrae. J Neurosurg. 1999;90(1 suppl):27–34. [DOI] [PubMed] [Google Scholar]
- 32. Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94:269–275. [DOI] [PubMed] [Google Scholar]
- 33. Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol). 2003;15:211–217. [DOI] [PubMed] [Google Scholar]
- 34. Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–967. [DOI] [PubMed] [Google Scholar]
- 35. Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24:3388–3393. [DOI] [PubMed] [Google Scholar]
- 36. Rao S, Badani K, Schildhauer T, Borges M. Metastatic malignancy of the cervical spine. A nonoperative history. Spine (Phila Pa 1976). 1992;17(10 suppl):S407–S412. [DOI] [PubMed] [Google Scholar]
- 37. Rief H, Heinhold M, Bruckner T, et al. Quality of life, fatigue and local response of patients with unstable spinal bone metastases under radiation therapy—a prospective trial. Radiat Oncol. 2014;9:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huisman M, van der Velden JM, van Vulpen M, et al. Spinal instability as defined by the spinal instability neoplastic score is associated with radiotherapy failure in metastatic spinal disease. Spine J. 2014;14:2835–2840. [DOI] [PubMed] [Google Scholar]
- 39. Sahgal A, Atenafu EG, Chao S, et al. Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol. 2013;31:3426–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gallizia E, Apicella G, Cena T, Di Genesio Pagliuca M, Deantonio L, Krengli M. The spine instability neoplastic score (SINS) in the assessment of response to radiotherapy for bone metastases. Clin Transl Oncol. 2017;19:1382–1387. [DOI] [PubMed] [Google Scholar]
- 41. Bilsky MH, Shannon FJ, Sheppard S, Prabhu V, Boland PJ. Diagnosis and management of a metastatic tumor in the atlantoaxial spine. Spine (Phila Pa 1976). 2002;27:1062–1069. [DOI] [PubMed] [Google Scholar]
- 42. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life.” JAMA. 2008;299:937–946. [DOI] [PubMed] [Google Scholar]
- 43. Zaikova O, Fosså SD, Bruland OS, Giercksky KE, Sandstad B, Skjeldal S. Radiotherapy or surgery for spine metastases? A population-based study of 903 patients in the south-eastern region of Norway. Acta Orthop. 2011;82:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. [DOI] [PubMed] [Google Scholar]