Short abstract
Spinal cord injury (SCI), a serious neurological disease, has few therapeutic interventions. A small molecule, P7C3, has been confirmed to play a role in neuroprotection of some neurological diseases. But the effect of P7C3 on acute SCI has not been investigated. Here, we observed the therapeutic effect of P7C3 for alleviating the neurological damage by direct subcutaneous injection after SCI once daily for 7 or 14 days in a rat model. The locomotion and histopathological changes were evaluated. Our results demonstrated that P7C3 treatment contributed to functional recovery and tissue repair following SCI. Improved locomotor function with P7C3 treatment was correlated with increased survival of neurons and oligodendrocytes (OLs) and proliferation of OLs and their progenitors, which resulted in tissue repair and myelination in the injured SC. P7C3 treatment also restored the nicotinamide adenine dinucleotide level in the SC, which was reduced by SCI. These results suggest that P7C3 plays a role in improving neurological outcome following SCI.
Impact statement
Spinal cord injury (SCI), a serious neurological disease, has few therapeutic interventions. This study confirms for the first time that P7C3 is conducive to functional recovery and tissue repair after SCI. P7C3 treatment can rescue the nicotinamide adenine dinucleotide level of the injured spinal cord, which results in more survival of neurons and oligodendrocytes (OLs) and proliferation of OLs and their progenitors, and thus increase myelination and tissue repair. This result indicates that P7C3 plays a role in improving neurological outcome following SCI.
Keywords: P7C3, spinal cord injury, neuroprotection, neuron, oligodendrocyte, functional recovery
Introduction
Spinal cord injury (SCI), a serious neurological disease, gives rise to eternal neurological damage with limited possibility for spontaneous repair.1,2 SCI triggers a variety of cellular and molecular events such as inflammatory response,3 oxidative stress,4,5 and glutamate excitotoxicity6,7 to neurons and oligodendrocytes (OLs), leading to demyelination and further impairment of neurological function. To date, there is no effective treatment strategy for SCI, but therapeutic strategies that promote the survival of neurons and OLs may prevent long-term neurological dysfunction.8,9
As an orally bioavailable and non-toxic small molecule, P7C3 can cross the blood–brain barrier and promote neuronal survival in neurodegenerative animal models and neural cell damage,10–12 and its protective roles have been confirmed in preclinical trials of Parkinson’s disease,13–15 traumatic brain injury,16–18 peripheral nerve injury,19 amyotrophic lateral sclerosis,20 age-associated cognitive decline,11 and neurodegeneration-associated depression.21 However, the effect of P7C3 on acute SCI has not yet been studied.
In this study, we demonstrate that P7C3 enhances the survival of neurons and OLs and the proliferation of OLs and their progenitors, thereby increasing tissue repair and myelination in the injured SC and promoting recovery of neurological function.
Materials and methods
Animals
Two-month-old female Sprague-Dawley rats were used in this study. Experiments were performed according to the Guide for the Laboratory Animals Care and Use (National Research Council, 1996) and validated by the Animal Care and Use Committees of Bengbu Medical College. The animals were housed under standard conditions.
SCI model and administration of P7C3
Rats received anesthesia using pentobarbital (50 mg/kg) prepared in phosphate-buffered saline (PBS) by intraperitoneal injection. A laminectomy at the T10 level was performed. The exposed SC received contused injury using a NYU Impactor (10-g rod and 12.5-mm height).22,23 Then the animals were assigned to the control (ctrl) and P7C3 treatment group randomly. The postoperative nursing was performed according to previous reports.22,23 In P7C3 treatment group, P7C3 (MedChem Express, NJ, USA) was prepared as previously described,16 and animals were intraperitoneally injected with 20 mg/kg P7C3 immediately after SCI and once daily for 7 or 14 days. In the control group, DMSO was injected intraperitoneally. The rats were sacrificed on day 3, 7, 14, or 42 post injury.
Behavioral assessment
Locomotion was evaluated using Basso, Beattie, and Bresnahan (BBB) locomotor scale by two trained investigators24 at 0, 1, 3, 7, 14, 21, 35, and 42 days after injury. A score ranging from 0 to 21 was assigned on the basis of crawling ability of animals.
Tissue preparation
At predetermined time points following SCI, pentobarbital (60 mg/kg) was used to anesthetize the rats. The animals were perfused with PBS and then 4% paraformaldehyde (PFA). The injury site of SC was separated, post-fixed with 4% PFA at 4°C overnight, and cryoprotected in 30% sucrose. Finally, the 20-μm-thick serial transverse sections were cut with a cryostat (Thermo Fisher Scientific) and mounted on gelatin-coated slides for staining.
Histological analysis
Luxol fast blue (LFB), hematoxylin and eosin (H&E), and Nissl staining were performed for histopathological examination of SC tissue sections. Lesion and myelinated areas of SC at six weeks post SCI were measured by H&E and LFB, and motor neurons were quantified with Nissl staining.25 The cavitation and LFB-stained tissue at the injury epicenter and the epicenter to rostral and caudal 1, 2, 3, and 4 mm in axial sections were measured and normalized to the percentage of total stained area.
Immunohistochemistry
Frozen sections were unfreezed at room temperature and washed twice with PBS. The immunohistochemistry was performed as previously described.25 The primary antibodies and the corresponding secondary antibodies were used as follows: rabbit anti-caspase-3, mouse anti-β III tubulin, and mouse anti-CNP antibodies (all at 1:200 and from Abcam, Cambridge, MA, USA), fluorescein isothiocyanate (FITC)- or rhodamine-conjugated donkey anti-rabbit or mouse IgG (1:200; Jackson Laboratories, West Grove, PA, USA). Photographs were acquired using a fluorescence microscope (Zeiss, Oberkochen, Germany). The number of cells was counted as previously described.25
NAD quantification
For assessment of nicotinamide adenine dinucleotide (NAD) level, the injury site of SC was dissected after animal was sacrificed at 48 h following SCI. The sample was flash frozen immediately. Homogenization of tissue specimens were performed using a Dounce homogenizer in buffer (0.1 M PBS containing 0.125 μg/mL pepstatin, 2.5 μg/mL aprotinin, 1 μg/mL leupeptin, and 0.2 N NaOH) at 4°C, and the homogenates were flash frozen in liquid nitrogen. The NAD+/NADH Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China) was used to quantify NAD level.
BrdU incorporation detection
To evaluate cell proliferation after P7C3 treatment, bromodeoxyuridine (BrdU) incorporation detection was performed as previously described.25 The primary antibodies and the corresponding secondary antibodies were as follows: mouse anti-CNP (1:200; Abcam), mouse anti-O4 (1:50; Chemicon, Temecula, CA, USA), or mouse anti-Iba1 (1:50; Abcam) and rabbit anti-BrdU (1:100; Sigma-Aldrich), rhodamine-conjugated anti-mouse and FITC-conjugated anti-rabbit secondary antibodies (at 1/300 dilution). Fluorescence images were taken using a Zeiss Axio Observer microscope. The percentages of CNP+/BrdU+, O4+/BrdU+, and Iba1+/BrdU+ cells among the total number of CNP+, O4+, or Iba1+ cells were quantified in five random fields per section containing over 500 CNP+, O4+, or Iba1+ cells.
Statistical analysis
The results were evaluated using one-way analysis of variance. Differences were determined significant at P < 0.05. The data are presented as mean ± standard deviation (SD) and analyzed using SPSS v.14.0 (SPSS Inc., Chicago, IL, USA).
Results
P7C3 promotes the recovery of locomotor function following SCI
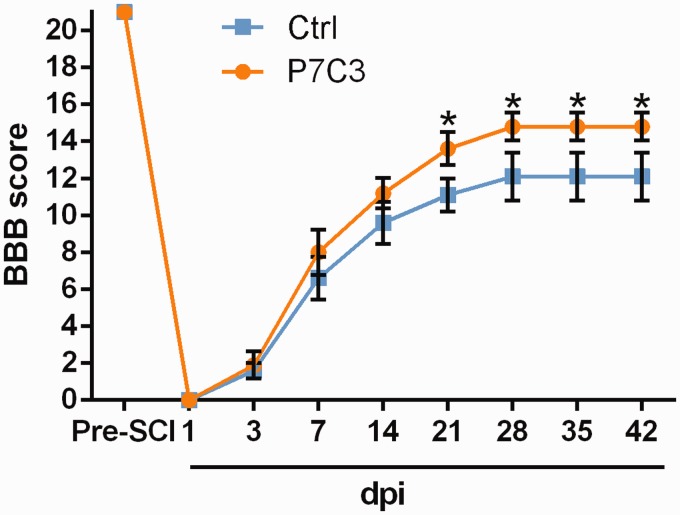
We examined whether P7C3 can improve locomotor function of SCI rats using the BBB scale. The P7C3-treated rats displayed significant improvement of locomotor function relative to the control starting at three weeks post injury that lasted up to the end of the experimental period (P < 0.05; Figure 1). This suggests that P7C3 treatment improves functional recovery from contusive SCI.
Figure 1.
Treatment with P7C3 promotes the recovery of locomotor function after SCI. Locomotor function at three to six weeks post SCI was evaluated using the BBB scale. Data represent mean ± SD (n = 8). *P < 0.05. SCI: spinal cord injury; BBB score: Basso, Beattie, and Bresnahan score. (A color version of this figure is available in the online journal.)
P7C3 increases white matter sparing and reduces tissue damage following SCI
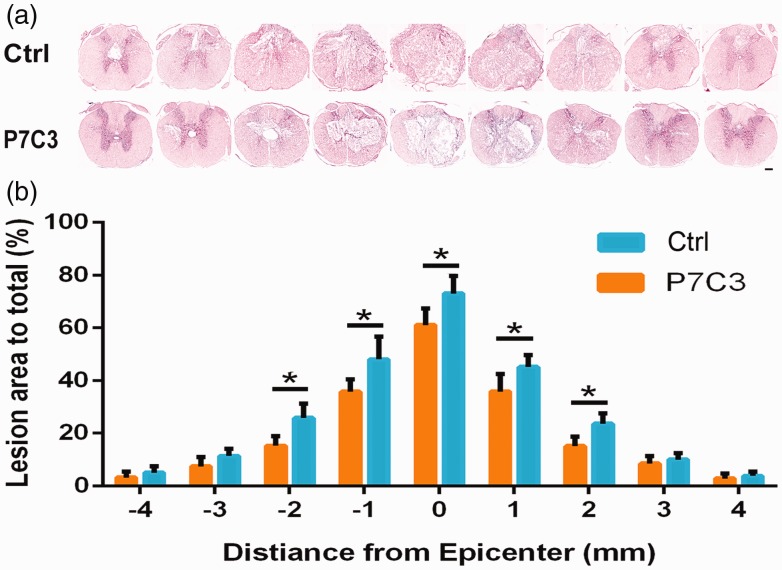
The extent of functional recovery following contusive injury is correlated with the amount of epicenter white matter that is spared.26 To determine whether P7C3 treatment affects lesion size following SCI, the amount of white matter throughout the SC was assessed by H&E staining in the six weeks after injury (Figure 2). P7C3-treated rats had a greater white matter at the lesion site than control rats (P < 0.05; Figure 2(a) and (b)), which is consistent with the time course of locomotor recovery. These results suggest that P7C3 preserves white matter of the lesion site following SCI, which contributes to functional improvement.
Figure 2.
Treatment with P7C3 reduces SC tissue damage following contusion. (a) Representative images of the lesion center with H&E staining at six weeks post SCI. (b) Quantification of total area of lesion in axial sections at the lesion epicenter and at 1, 2, 3, and 4 mm rostral (+) and caudal (−) to the epicenter. Data represent mean ± SD (n = 6). *P < 0.05; **P < 0.01. Scale bar = 200 μm. (A color version of this figure is available in the online journal.)
P7C3 preserves myelin following SCI
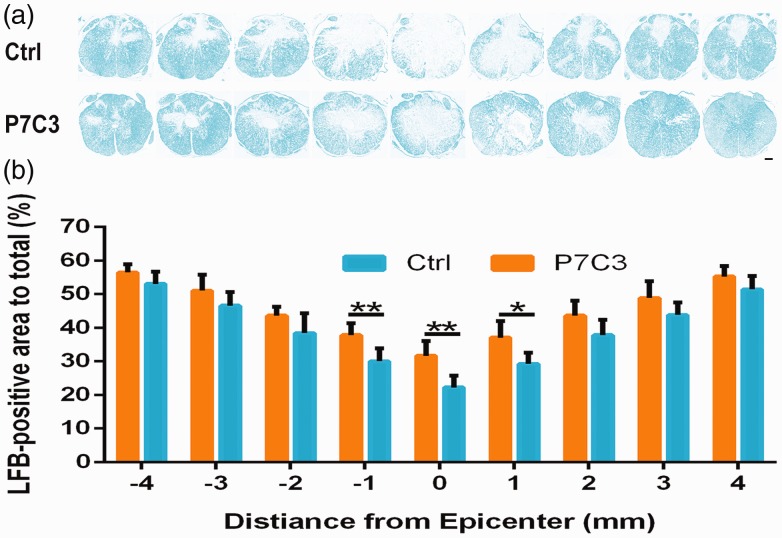
To examine the preservation of white matter by P7C3 in greater detail, we evaluated the degree of myelination of the lesion center by LFB staining. Our results showed that the degree of myelination of the lesion center was higher in animals treated with P7C3 than that in controls (P < 0.05; Figure 3(a) and (b)).
Figure 3.
Treatment with P7C3 increases residual myelination following SCI. (a) Representative images of white matter from the lesion epicenter (0) and at 1, 2, 3, and 4 mm rostral (+) and caudal (−) to the epicenter, as detected by LFB staining. (b) Quantification of LFB-stained areas at various distances from the lesion epicenter. Data represent mean ± SD (n = 6). *P < 0.05; **P < 0.01. Scale bar = 200 μm. (A color version of this figure is available in the online journal.)
P7C3 promotes neuronal survival
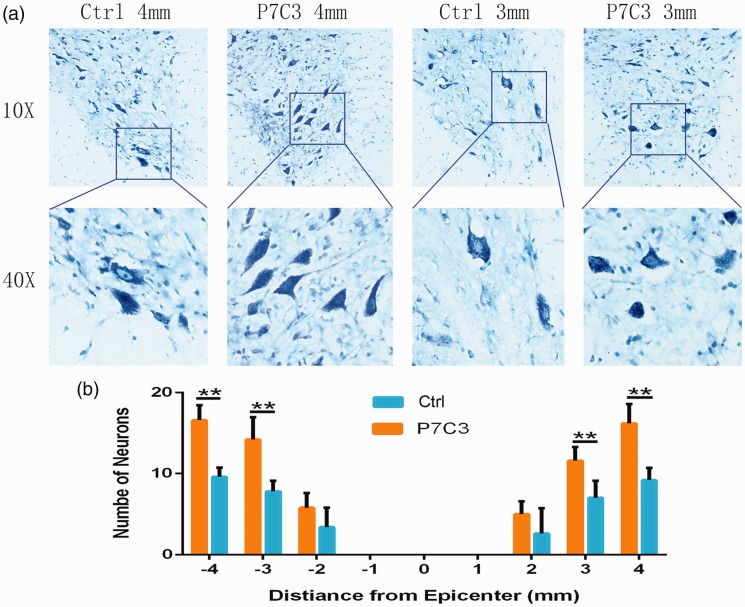
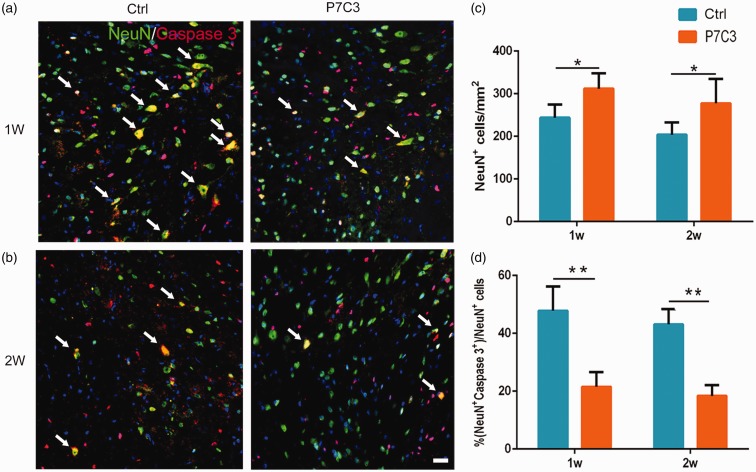
To assess the effect of P7C3 on motor neuron survival, we quantified motor neurons of ventral horn at the epicenter and the epicenter to rostral and caudal 1, 2, 3, and 4 mm at six weeks post SCI. As shown in Figure 4(a) and (b), the residual motor neurons were significantly more in the P7C3 than those in the control (P < 0.01). Activated caspase-3 immunolabeling revealed more NeuN+ neurons at the edge of the lesion center in P7C3-treated rats when compared to control rats at one week (311.78 ± 14.71 vs. 243.68 ± 12.52, P < 0.05; Figure 5(a) and (c)) and two weeks (277.58 ± 21.54 vs. 203.74 ± 11.71, P < 0.05; Figure 5(b) and (c)) post injury. On the other hand, there were fewer caspase-3+ neurons in the P7C3-treated group than in controls at one week (21.50% ± 2.09% vs. 47.84% ± 2.97%, P < 0.01; Figure 5(a) and (d)) and two weeks (18.35% ±1.55% vs. 43.15% ± 2.15%, P < 0.01; Figure 5(b) and (d)).
Figure 4.
Treatment with P7C3 alleviates motor neuron loss after SCI. (a) Representative Nissl-stained images of neurons in the ventral horn 3 and 4 mm rostral to the lesion at six weeks post SCI. (b) Quantification of the number of ventral horn neurons at the injury epicenter (0) and at 1, 2, 3, and 4 mm rostral (+) and caudal (−) to the epicenter (n = 6). Data represent mean ± SD. *P < 0.01. Scale bar = 20 μm. (A color version of this figure is available in the online journal.)
Figure 5.
Treatment with P7C3 enhances neuronal survival in the injured SC. (a and b) Representative photomicrographs of caspase-3+ cells (red) co-localized with NeuN+ neurons (green) in the SC at one- and two-week post SCI. (c and d) Quantitative analysis of the number and percentage of caspase-3+ neurons. Data represent mean ± SD (n = 6). *P < 0.05; **P < 0.01. Scale bar = 20 μm. (A color version of this figure is available in the online journal.)
P7C 3 protects OLs from SCI-induced cell death
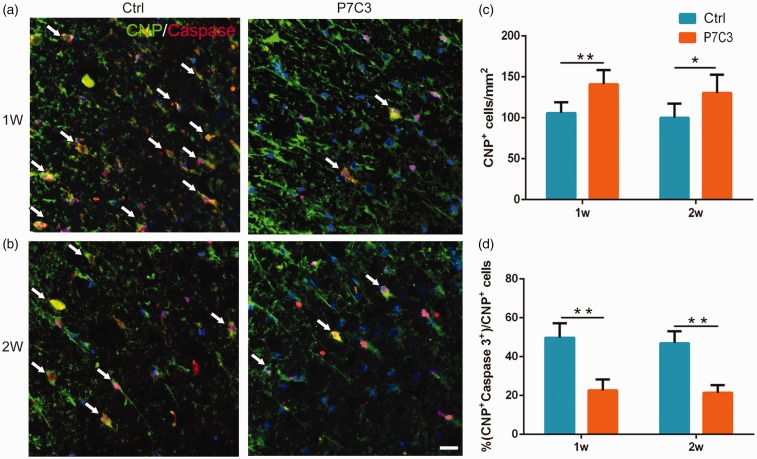
Consistent with our findings in neurons, we observed more CNP+ OLs at the edge of the lesion center in rats with P7C3 treatment relative to control rats at one week (141.13 ± 6.46 vs. 105.82 ± 4.71, P < 0.01; Figure 6(a) and (c)) and two weeks (130.17 ± 9.13 vs. 100.00 ± 7.10, P < 0.05; Figure 6(b) and (c)). The percentages of caspase-3+ OLs were lower in P7C3-treated rats than those in the control group at both time points (1 week: 22.67% ± 2.12% vs. 49.80% ± 2.63%, P < 0.01; Figure 6(a) and (d) and 2 weeks: 21.43% ± 1.50% vs. 46.97% ± 2.49%, P < 0.01; Figure 6(b) and (d)).
Figure 6.
Treatment with P7C3 enhances the survival of OLs in the injured SC. (a and b) Representative photomicrographs of caspase-3+ cells (red) co-localized with CNP+ OLs (green) in the SC at one- and two-week post SCI. (c and d) Quantitative analysis of the number and percentage of caspase-3+ OLs. Data represent mean ± SD (n = 6). *P < 0.05; **P < 0.01. Scale bar = 20 μm. (A color version of this figure is available in the online journal.)
P7C3 promotes OPCs and OLs proliferation
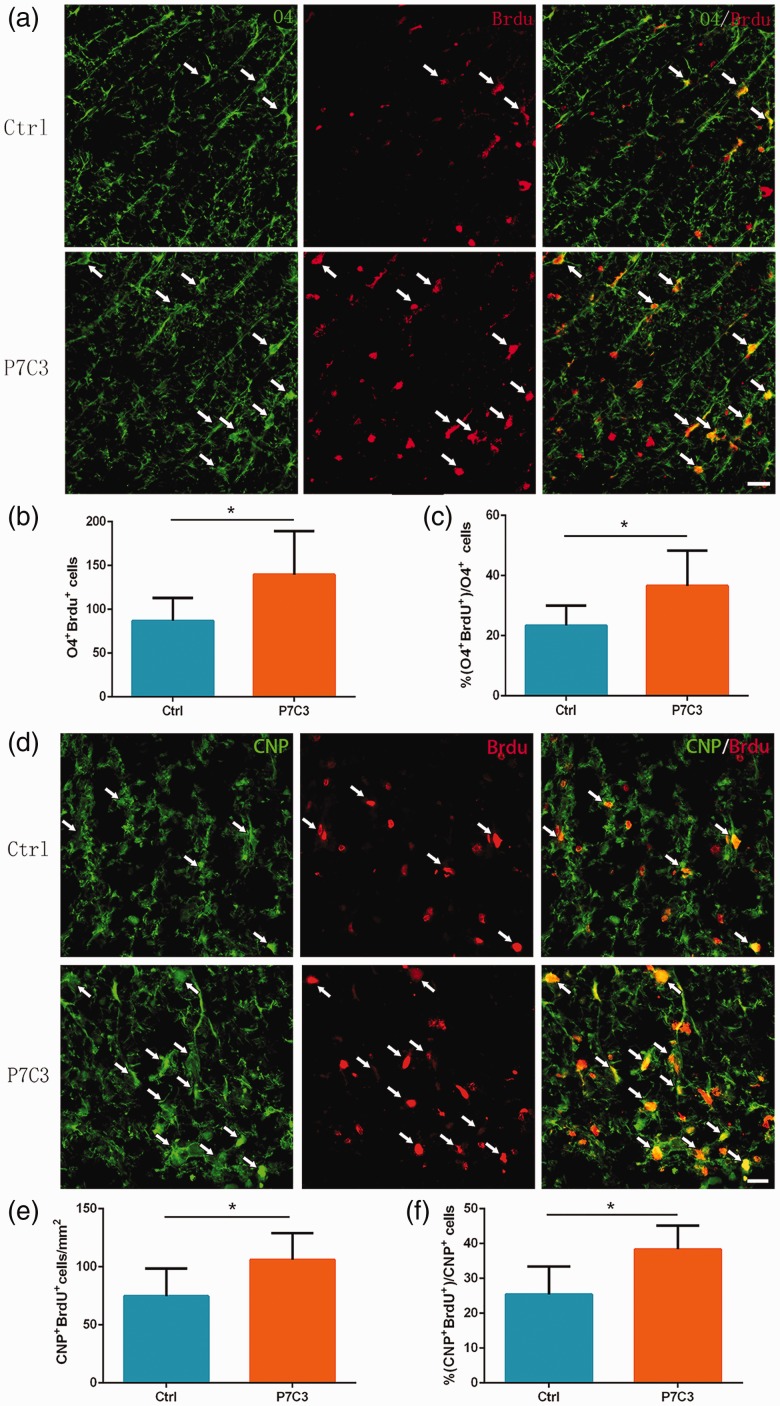
The BrdU incorporation detection at one week post SCI displayed a greater number of BrdU+/O4+ OPCs (139.70 ±20.28 vs. 86.70 ± 9.97, P < 0.05; Figure 7(a) and (b)) and BrdU+/CNP+ OLs (106 ± 9.37 vs. 74.71 ± 9.73, P < 0.05; Figure 7(d) and (e)) in the lesion center of P7C3-treated group. Accordingly, there were higher percentage of BrdU+ cells out of O4+ cells (36.56% ± 4.45% vs. 23.34% ±2.52%, P < 0.05; Figure 7(a) and (c)) and CNP+ cells (38.35% ± 2.77% vs. 25.39% ± 3.27%, P < 0.05; Figure 7(d) and (f)) in the lesion center of the P7C3-treated group. These data confirm that P7C3 treatment promotes endogenous oligodendrocyte progenitor cells (OPCs) and OLs proliferation following SCI.
Figure 7.
Proliferation of OPCs and OLs in the injured SC. (a and d) Representative photomicrographs of BrdU+ cells (red) co-localized with O4+ OPCs (green) or CNP+ OLs (red) in the SC at one-week post SCI. Quantitative analysis of the number and percentage of BrdU+ OPCs (b and c) and BrdU+ OLs (e and f). Data represent mean ± SD (n = 6). *P < 0.05. Scale bar = 20 μm. (A color version of this figure is available in the online journal.)
P7C3 treatment increases NAD level in the injured SC
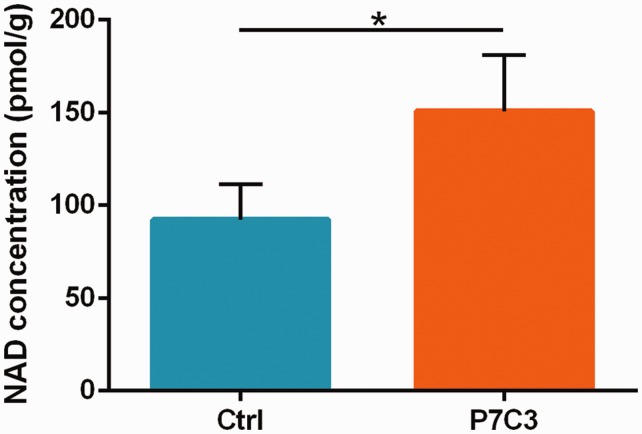
P7C3 enhances flux in the NAD+ salvage pathway in in vitro cultured mammalian cells.12 To determine whether P7C3 treatment helps to maintain this process after SCI, we examined NAD level at 48 h following SCI which refers to previous study.27 We found that NAD level in the SC of injured rat significantly reduced relative to the control animals (P < 0.05). However, this reduction was rescued by P7C3 treatment (P < 0.05; Figure 8).
Figure 8.
Treatment with P7C3 restores NAD levels in the injured SC. NAD levels in SC tissue were evaluated at 48 h post SCI. Data represent mean ± SD (n = 5). *P < 0.05. NAD: nicotinamide adenine dinucleotide. (A color version of this figure is available in the online journal.)
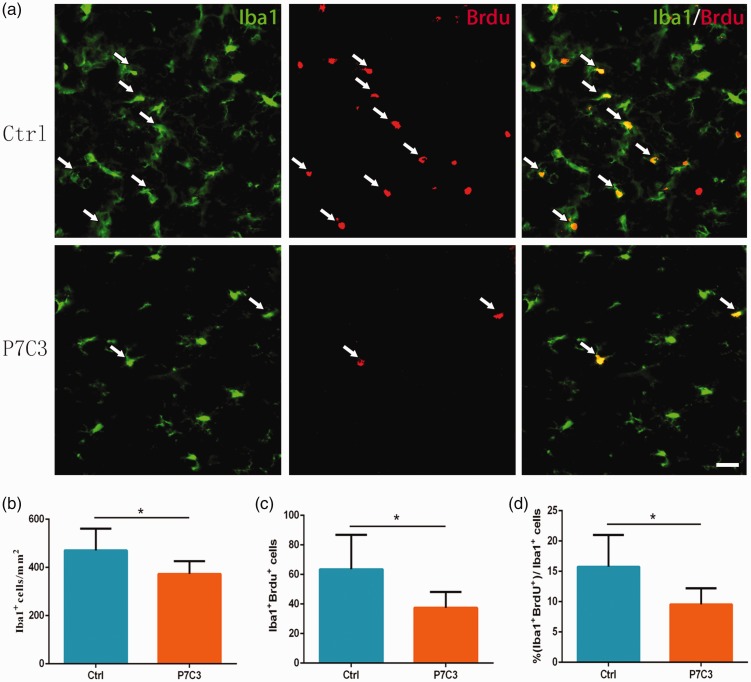
P7C3 suppresses microglia and macrophage proliferation in the injured SC
Using Iba1 immunolabeling to identify microglia/macrophages,28 we found that there were fewer Iba1+ cells at the circumference of the lesion center in the P7C3-treated than those in the control rats (373.30 ± 21.52 vs. 470.90 ±31.98, P < 0.05; Figure 9(a) and (b)) at one week after SCI. Double immunolabeling for Iba1 and BrdU showed that P7C3-treated rats had fewer BrdU+ and Iba1+ cells at the lesion center at one week post SCI than those in control group (36.73 ± 3.58 vs. 63.51 ± 9.54, P < 0.05; Figure 9(a) and (c)). Consistent with this finding, there was also a significant difference of BrdU+ cells among Iba1+ cells between the P7C3-treated and control groups (9.56% ±1.00% vs. 15.75% ± 1.85%, P < 0.05; Figure 9(a) and (d)). These indicate that treatment with P7C3 suppresses microglia/macrophage proliferation following SCI.
Figure 9.
Treatment with P7C3 suppresses the proliferation of microglia and macrophages in the injured SC. (a) Representative photomicrographs of double immunofluorescence labeling of BrdU+ (red) and Iba1+ (green) microglia and macrophages in the SC at one-week post SCI. (b, c, d) Quantitative analysis of the number and percentage of BrdU+ Iba1+ cells. Data represent mean ± SD (n = 6). *P < 0.05. Scale bar = 20 μm. (A color version of this figure is available in the online journal.)
Discussion
In this study, using an SCI model, we assess the neuroprotective role of P7C3. The result of BBB score displayed no obvious difference before two weeks following SCI in animals with or without P7C3 administration. However, the BBB score showed significant improvements in the P7C3 group starting from the third week post SCI, demonstrating that locomotor function was improved by P7C3 treatment.
We next examined whether P7C3 administration facilitated tissue repair. A significant decrease of the SC lesion area in P7C3-treated rats, as compared to controls, indicates that P7C3 stimulates tissue repair post SCI that leads to functional improvement.
Myelination preservation builds foundation of functional recovery after SCI.29,30 In this study, more residual myelin was observed in rats treated with P7C3 than in control group at six weeks following SCI, suggesting that P7C3 preserves residual myelin in the injured SC.
P7C3 was shown to suppress the peripheral neuron death and increase functional recovery in a neonatal nerve injury model19; it was also found to promote survival of mature cortical neurons following traumatic brain injury.16 Neurons and OLs died massively after SCI.31–33 However, it remains unclear whether P7C3 affects the survival of neurons following SCI. In P7C3-treated rats, there were more residual motor neurons than those in controls. Furthermore, activated caspase-3 staining displayed that P7C3 administration protected neurons from SCI-caused apoptosis. The death of OLs is a key factor contributing to functional impairment after SCI; P7C3 administration also protected OLs from apoptosis after SCI in our study. Thus, P7C3 promotes the survival of SC neurons and OLs following contusion injury.
We found that P7C3 administration stimulated the proliferation of OPCs, as evidenced by a higher rate of BrdU incorporation. The differentiation of these OPCs resulted in an increase of the number of myelin-forming OLs, which promoted the recovery of motor function.
NAD plays an important role in regulating mitochondrial metabolism.34 In mammalian cells, P7C3 enhances the flux of the NAD+ salvage pathway.12 NAD is depleted rapidly during cerebral ischemia, and the increase of NAD level has been suggested as a strategy to treat the stroke.35 Extended treatment of P7C3 also inhibited the death of newborn neuron, thereby enhancing neurogenesis.11 NAD is also a source of energy for proliferation of progenitor cell36 and plays a key role in postnatal neurogenesis.37,38 In our study, we found that SCI reduced NAD level, but this was rescued by P7C3 treatment. Thus, a novel therapeutic approach for SCI is to use P7C3 to increase NAD level and in this manner promote two important processes that are vital for SCI protection: survival of neurons and OLs, and OPC proliferation.
Following SCI, resident microglia are activated and macrophages rapidly infiltrate into the SC where they secrete reactive oxygen species and inflammatory factors, resulting in the death of neurons and OLs.39–41 In this study, we found that there was fewer microglia/macrophage infiltration at the lesion site of SC in P7C3-treated animals compared with controls. Moreover, P7C3 administration reduced the number of Iba1+/BrdU+ cells (newborn microglia/macrophages) at the lesion site after SCI. On base of these observations, we conclude that P7C3 suppresses the inflammatory response of SCI by inhibiting the proliferation of microglia/macrophages.
Conclusions
In conclusion, P7C3 treatment promotes histological and functional recovery following SCI. Our study also show that P7C3 promoted neuron and OL survival post SCI and increased OPC proliferation, thereby contributing to tissue repair and myelination of the injured SC and restoring neurological function. These results suggest P7C3 treatment as a promising strategy for improving the long-term outcome of SCI.
Authors’ contributions
JGH and HZL designed the experiments and edited the manuscript. FXD and YJS conducted the experiments, analyzed the results, and wrote the manuscript. JC, SQD, and FCW elucidated the data and made the figures. JT, RW, LS, JX, and QQ conducted the experiments and analyzed the results. FXD and YJS contributed equally to this paper.
Availability of supporting data
The data-sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by the National Natural Science Foundation of China (grant no. 81771343).
References
- 1.Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers 2017; 3:17018. [DOI] [PubMed] [Google Scholar]
- 2.Quencer RM, Bunge RP. The injured spinal cord: imaging, histopathologic clinical correlates, and basic science approaches to enhancing neural function after spinal cord injury. Spine 1996; 21:2064–6 [DOI] [PubMed] [Google Scholar]
- 3.Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med 2004; 10:580–3 [DOI] [PubMed] [Google Scholar]
- 4.Fatima G, Sharma VP, Das SK, Mahdi AA. Oxidative stress and antioxidative parameters in patients with spinal cord injury: implications in the pathogenesis of disease. Spinal Cord 2015; 53:3–6 [DOI] [PubMed] [Google Scholar]
- 5.Lam T, Chen Z, Sayed-Ahmed MM, Krassioukov A, Al-Yahya AA. Potential role of oxidative stress on the prescription of rehabilitation interventions in spinal cord injury. Spinal Cord 2013; 51:656–62 [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Chen J, Jiang T, Li W, Huang Y, Lu X, Liu Z, Zhang W, Zhou Z, Ding Q, Santos HA, Yin G, Fan J. Biodegradable spheres protect traumatically injured spinal cord by alleviating the glutamate-induced excitotoxicity. Adv Mater 2018; 30:e1706032. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Xu GY, Pan E, McAdoo DJ. Neurotoxicity of glutamate at the concentration released upon spinal cord injury. Neuroscience 1999; 93:1383–9 [DOI] [PubMed] [Google Scholar]
- 8.Papastefanaki F, Matsas R. From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia 2015; 63:1101–25 [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Ji H, Zhang M, Liu Z, Lao L, Deng C, Chen J, Zhong G. An agonist of the protective factor SIRT1 improves functional recovery and promotes neuronal survival by attenuating inflammation after spinal cord injury. J Neurosci 2017; 37:2916–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pieper AA, McKnight SL, Ready JM. P7C3 and an unbiased approach to drug discovery for neurodegenerative diseases. Chem Soc Rev 2014; 43:6716–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, Capota M, Britt JK, Kotti T, Ure K, Brat DJ, Williams NS, MacMillan KS, Naidoo J, Melito L, Hsieh J, De Brabander J, Ready JM, McKnight SL. Discovery of a proneurogenic, neuroprotective chemical. Cell 2010; 142:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper AA, Ready JM, McKnight SL. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 2014; 158:1324–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Jesus-Cortes H, Miller AD, Britt JK, DeMarco AJ, De Jesus-Cortes M, Stuebing E, Naidoo J, Vazquez-Rosa E, Morlock L, Williams NS, Ready JM, Narayanan NS, Pieper AA. Protective efficacy of P7C3-S243 in the 6-hydroxydopamine model of Parkinson’s disease. NPJ Parkinson’s Dis 2015; 1:15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Jesus-Cortes H, Xu P, Drawbridge J, Estill SJ, Huntington P, Tran S, Britt J, Tesla R, Morlock L, Naidoo J, Melito LM, Wang G, Williams NS, Ready JM, McKnight SL, Pieper AA. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc Natl Acad Sci U S A 2012; 109:17010–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naidoo J, De Jesus-Cortes H, Huntington P, Estill S, Morlock LK, Starwalt R, Mangano TJ, Williams NS, Pieper AA, Ready JM. Discovery of a neuroprotective chemical, (S)-N-(3-(3,6-dibromo-9H-carbazol-9-yl)-2-fluoropropyl)-6-methoxypyridin-2-amine [(-)-P7C3-S243], with improved druglike properties. J Med Chem 2014; 57:3746–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaya MO, Bramlett HM, Naidoo J, Pieper AA, Dietrich WD. Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury. J Neurotrauma 2014; 31:476–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutca LM, Stasheff SF, Hedberg-Buenz A, Rudd DS, Batra N, Blodi FR, Yorek MS, Yin T, Shankar M, Herlein JA, Naidoo J, Morlock L, Williams N, Kardon RH, Anderson MG, Pieper AA, Harper MM. Early detection of subclinical visual damage after blast-mediated TBI enables prevention of chronic visual deficit by treatment with P7C3-S243. Invest Ophthalmol Vis Sci 2014; 55:8330–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin TC, Britt JK, De Jesus-Cortes H, Lu Y, Genova RM, Khan MZ, Voorhees JR, Shao J, Katzman AC, Huntington PJ, Wassink C, McDaniel L, Newell EA, Dutca LM, Naidoo J, Cui H, Bassuk AG, Harper MM, McKnight SL, Ready JM, Pieper AA. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Rep 2014; 8:1731–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemp SW, Szynkaruk M, Stanoulis KN, Wood MD, Liu EH, Willand MP, Morlock L, Naidoo J, Williams NS, Ready JM, Mangano TJ, Beggs S, Salter MW, Gordon T, Pieper AA, Borschel GH. Pharmacologic rescue of motor and sensory function by the neuroprotective compound P7C3 following neonatal nerve injury. Neuroscience 2015; 284:202–16 [DOI] [PubMed] [Google Scholar]
- 20.Tesla R, Wolf HP, Xu P, Drawbridge J, Estill SJ, Huntington P, McDaniel L, Knobbe W, Burket A, Tran S, Starwalt R, Morlock L, Naidoo J, Williams NS, Ready JM, McKnight SL, Pieper AA. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 2012; 109:17016–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker AK, Rivera PD, Wang Q, Chuang JC, Tran S, Osborne-Lawrence S, Estill SJ, Starwalt R, Huntington P, Morlock L, Naidoo J, Williams NS, Ready JM, Eisch AJ, Pieper AA, Zigman JM. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol Psychiatry 2015; 20:500–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma 1992; 9:123–6; discussion 26–8 [DOI] [PubMed] [Google Scholar]
- 23.Yao ZF, Wang Y, Lin YH, Wu Y, Zhu AY, Wang R, Shen L, Xi J, Qi Q, Jiang ZQ, Lu HZ, Hu JG. Transplantation of PDGF-AA-Overexpressing oligodendrocyte precursor cells promotes recovery in rat following spinal cord injury. Front Cell Neurosci 2017; 11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995; 12:1–21 [DOI] [PubMed] [Google Scholar]
- 25.Guo XY, Duan FX, Chen J, Wang Y, Wang R, Shen L, Qi Q, Jiang ZQ, Zhu AY, Xi J, Lu HZ, Hu JG. Subcutaneous administration of PDGF-AA improves the functional recovery after spinal cord injury. Front Neurosci 2019; 13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 2006; 23:635–59 [DOI] [PubMed] [Google Scholar]
- 27.Morris-Blanco KC, Cohan CH, Neumann JT, Sick TJ, Perez-Pinzon MA. Protein kinase C epsilon regulates mitochondrial pools of Nampt and NAD following resveratrol and ischemic preconditioning in the rat cortex. J Cereb Blood Flow Metab 2014; 34:1024–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khayrullina G, Bermudez S, Byrnes KR. Inhibition of NOX2 reduces locomotor impairment, inflammation, and oxidative stress after spinal cord injury. J Neuroinflammation 2015; 12:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 2005; 25:4694–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu XM, Onifer SM. Transplantation-mediated strategies to promote axonal regeneration following spinal cord injury. Respir Physiol Neurobiol 2009; 169:171–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res 2002; 137:37–47 [DOI] [PubMed] [Google Scholar]
- 32.Stenudd M, Sabelstrom H, Frisen J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol 2015; 72:235–7 [DOI] [PubMed] [Google Scholar]
- 33.Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiologic Rev 2018; 98:881–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 2008; 10:179–206 [DOI] [PubMed] [Google Scholar]
- 35.Wang P, Miao CY. NAMPT as a therapeutic target against stroke. Trends Pharmacologic Sci 2015; 36:891–905 [DOI] [PubMed] [Google Scholar]
- 36.Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J 2014; 33:1321–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loris ZB, Pieper AA, Dietrich WD. The neuroprotective compound P7C3-A20 promotes neurogenesis and improves cognitive function after ischemic stroke. Exp Neurol 2017; 290:63–73 [DOI] [PubMed] [Google Scholar]
- 38.Wang SN, Xu TY, Li WL, Miao CY. Targeting nicotinamide phosphoribosyltransferase as a potential therapeutic strategy to restore adult neurogenesis. CNS Neurosci Ther 2016; 22:431–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res 2015; 1619:1–11 [DOI] [PubMed] [Google Scholar]
- 40.Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, Tetzlaff W. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol 2014; 117:54–72 [DOI] [PubMed] [Google Scholar]
- 41.Kroner A, Rosas Almanza J. Role of microglia in spinal cord injury. Neurosci Lett 2019; 709:134370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data-sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.