Short abstract
As a common disease, abdominal aortic aneurysm (AAA) features permanently progressively dilated abdominal aorta. Various cytokines are implicated in AAA pathogenesis. Clarification of involved cytokines combined with functional analysis may provide new insights into AAA pathogenesis. Using a mouse model, this study analyzed the cytokine profiles in AAA. Cytokines were measured in AAA tissues of saline control or angiotensin II-treated ApoE−/− mice using an antibody array of 200 cytokines, cytokine receptors, and related proteins. Statistical analysis revealed that 21 of 200 proteins were differentially expressed in AAA. These differentially expressed proteins were subjected to function and pathway enrichment analysis, which revealed that leukocyte migration and positive regulation of cell adhesion were the most significant biological processes. Specific signaling pathways, including Janus kinase/signal transducers and activators of transcription and cytokine–cytokine receptor interaction, were prominent in Kyoto encyclopedia of genes and genomes pathway enrichment analysis. Importantly, our data identified cytokines which had not previously been illustrated in AAA pathogenic pathways. Bivariate correlation analysis between these cytokines and protease activity showed that granulocyte colony-stimulating factor (G-CSF), macrophage inflammatory protein 1 g, cardiotrophin 1, milk fat globule-EGF factor 8 protein, interleukin 33, and periostin were positively correlated with matrix metalloprotease 1 (MMP-1), MMP-9, cathepsin B, and cathepsin L. G-CSF was positively correlated with cathepsin L. In conclusion, these results demonstrate that cytokine profile is significantly altered in AAA, and that the newly identified crucial cytokines may function potentially in AAA pathogenesis.
Impact statement
Various cytokines are known contributors to abdominal aortic aneurysm (AAA) pathologic processes, but the mechanisms underlying the pathogenesis remains unclear. We illustrated the altered cytokine profiles in AAA by high throughput antibody array of 200 cytokines, cytokine receptors and related proteins, as well as bioinformatics analysis of differentially expressed proteins in lesion tissues from AAA mice infused with angiotensin II. Functional analyses of differentially expressed cytokines showed clustering on cell migration and adhesion processes. More importantly, crucial cytokines whose association with AAA formation had not been established were identified. Significant correlations were found between these cytokines and protease activity. This study identifies several crucial markers for further researches on the molecular basis of AAA.
Keywords: Abdominal aortic aneurysm, cytokines, gene ontology, protease, bioinformatics, chemokines
Introduction
Abdominal aortic aneurysm (AAA) features progression of permanent weakening in aortic wall.1 AAA development leads to elevated risk of life-threatening aortic rupture, which is a major mortality cause. Current mainstream treatment for large, symptomatic or ruptured AAAs is surgical or endovascular aortic repair. Except for aortic repair, few medical methods have been developed to prevent or reverse AAA growth, which is partially due to lack of knowledge on its pathogenesis.
Pathologically, AAA is characterized by vascular smooth muscle cell (VSMC) death, adventitial immune cell infiltration (primarily macrophages and lymphocytes), extracellular matrix (ECM) proteolysis, and angiogenesis in aorta wall.2 In AAA progression, matrix metalloproteases (MMPs), as well as serine and cysteine proteases, are key components in degradation of ECM.3 Inflammation is considered a crucial part in AAA pathogenesis, indicated by various cytokine interactions and significant immunocyte infiltration in AAA specimens of human and animal models.4 Elevated levels of circulating cytokines, including interleukin 1 (IL-1), IL-6, interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) in AAA patients have been revealed. Furthermore, studies have demonstrated contributions of specific cytokines in AAA pathogenic processes.5–7 Cytokines modulate biological processes of immunocytes as well as vascular cells. A large number of cytokines are also associated with adhesion of immune cells to endothelial cells and increased reactive oxygen species.8 The production of proteases, including MMPs, serine proteases and cathepsins, can be regulated by cytokines which can therefore modulate AAA progression.9 That is, a considerable number of cytokines are involved in various pathogenetic pathways in AAA. Identifying the key cytokines and pathways in AAA pathogenesis may contribute to development of novel biomarkers and treatment methods.
Our study aimed to investigate the characteristics of cytokines and pathways involved in AAA pathogenesis using advanced protein array. The findings expanded the knowledge on AAA molecular mechanisms, and might expedited discovery of new candidate molecules for clinical application.
Materials and methods
Animal model, histologic analysis, and tissue processing
All animal protocols have received approval by the Ethics Review Board of PUMC Hospital and were conducted in accordance with terms issued by National Institutes of Health. As previously described, angiotensin II (Ang II)-induced AAA model was established in apolipoprotein E deficiency (ApoE−/−) mice with C57 background.10,11 Briefly, 10-week-old male ApoE−/− mice were randomly allocated to control and AAA groups. Control group mice were infused with normal saline (n = 5), and AAA group mice were administrated with Ang II (Sigma, USA, 1000 ng/(kg·min), n = 5). All animals were fed normal mouse chow during the 28-day infusion. Afterwards, the mice were injected with pentobarbital (40 mg/kg). Then anesthetized mice received laparotomy, and abdominal aortas were harvested.
The harvested abdominal aortas were first measured for maximum diameter. The lesion tissues were sliced into serial sections (5 µm thick) for hematoxylin and eosin (HE) staining. Cell lysates were obtained, and concentration of lysate proteins was tested by BCA kit manual (Pierce, USA).
Protein antibody array
Protein lysates of abdominal aorta tissues (100 µL) were quantitatively analyzed on antibody arrays of 200 cytokines, cytokine receptors, and related proteins (details in Supplementary Table S1, GSM-CAA-4000, RayBiotech, USA) following product instructions (https://www.raybiotech.com/mouse-cytokine-array-gs4000). Protein array slides were subsequently incubated with protein lysates, mixed antibodies labeled with biotin, and Cy3-conjugated streptavidin. The plates were scanned with a microarray scanner (InnoScan 300, Innopsys, France) at Cy3 wavelength, and fluorescence intensity data were analyzed with Q-Analyzer Tool specific for this array. The differentially expressed proteins (DEPs) were then identified.
Function enrichment analysis
The identified DEPs in AAA were input to DAVID to analyze the enriched terms concerning gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG).12 Briefly, GO annotations were performed to reveal characteristic biological attributes for DEPs, and KEGG database was consulted to obtain significant pathways for DEPs using clustering algorisms.
ELISA examination
ELISAs were used to measure levels of DEPs which had not been studied in AAA formation (RayBiotech, USA), in order to examine the results of protein array. Expressions of MMP-1, MMP-9, and cathepsins B and L were further measured. Protein lysates were incubated in antibody coated plates over 8 h, and subsequently processed for incubation in biotin-conjugated detection antibody (2 h), horseradish peroxidase-conjugated streptavidin (45 min), and 3,3′,5,5′-tetramethylbenzidine (30 min) reagents. Terminal reaction was performed with sulfuric acid. The OD values at 450 nm wave length were immediately obtained using microplate reader (ELx800NB, BioTek, USA).
Real-time RT-PCR analysis
Real-time RT-PCR experiments were conducted routinely.10,11 The primers of thymus-expressed chemokine (TECK; CCL25) are 5′-TTACCAGCACAGGATCAAATGG-3′ (forward), 5′-CGGAAGTAGAATCTCACAGCAC-3′ (reverse). The primers of chemokine receptor 9 (CCR9) are 5′-CTTCAGCTATGACTCCACTGC-3′ (forward), 5′-CAAGGTGCCCACAATGAACA-3′ (reverse).
Statistical analysis
Statistical analysis was performed using SPSS v. 25.0. Results were presented as means ± SD. DEP in AAA group was defined as proteins with fold change >1.5 or <0.67 compared with control group, t test P value <0.05, and fluorescent value >150. Pearson bivariate correlation was used to explore the relationships between specific cytokines and protease activity. A strong correlation was defined according to parameters of correlation coefficient (>0.7) and P value (<0.05).
Results
Measurement and histology of induced AAA
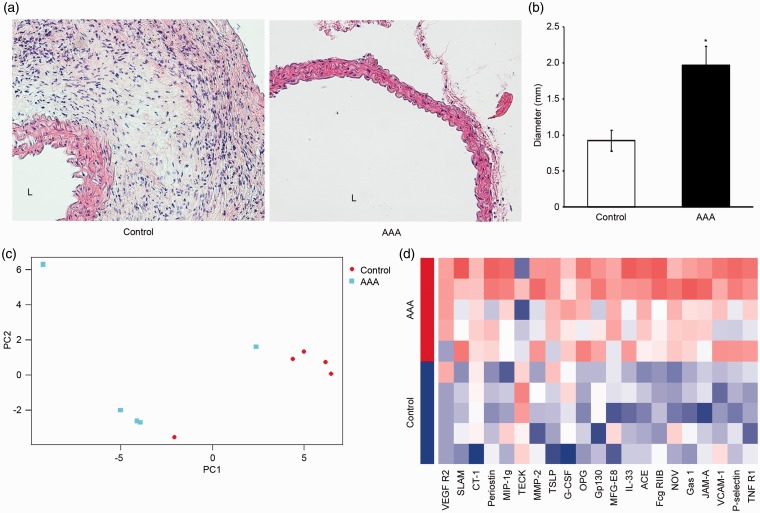
The harvested abdominal aortas of control and AAA groups were measured for maximum diameter and fixed for histological study. The HE stained histological images of aortas from AAA group showed significant thinning of aortic wall and loss of media in contrast with control group (Figure 1(a)). The diameter of aortas from AAA group was significantly larger than those from control group (Figure 1(b)). Therefore, the induction of AAA was successful, and further proteomic studies were initiated.
Figure 1.
Gross anatomic and histological observations of induced abdominal aortic aneurysm (AAA), as well as principle component analysis and hierarchical clustering heatmap based on protein array profiles of 21 differentially expressed proteins in AAA. (a) Representation of hematoxylin–eosin staining in aorta from control and AAA groups (magnification: 200×). L: lumen. (b) Diameter of harvested aortas. The diameter of AAA group aortas was significantly larger than control group (n = 5/group, means ± SD). *P < 0.05 vs. control group. (c) Principal component analysis. Subjects of two groups (control and AAA, n = 5/group) were projected according to the first two principal components of the protein array profile. (d) Hierarchical clustering and heatmap representation of expression levels for 21 cytokines (columns) across 10 subjects (rows). The expression of each cytokine was normalized across all samples. Low expression is denoted by blue and high expression by red.
Identification and visualizations of DEPs
Among the 200 proteins in the array, 21 cytokines fit the criteria of DEP (Table 1). First, principle component analysis (PCA) was conducted on all DEPs between groups (Figure 1(c)). PCA is a robust mathematical method that reduces the dimensionality of data and visualizes the relationships between subjects based on the variation across groups. The first two principal components were plotted to show the difference between groups. The majority of the two groups fell in two separate areas, though there were disperse dots away from the clusters. Therefore, we concluded that cytokine expression significantly differs between two groups, and that the consistence within each group was acceptable.
Table 1.
Twenty-one identified differentially expressed proteins from aortic tissues in the comparison between abdominal aortic aneurysm (AAA) and control mice.
| Entrez gene ID | Official gene symbol/Alternative name | Gene full name | Fold change |
|---|---|---|---|
| 20300 | CCL25/TECKa | Chemokine (C-C motif) ligand 25 | 0.58 |
| 20308 | Ccl9/MIP-1ga | Chemokine (C-C motif) ligand 9 | 1.25 |
| 53603 | TSLP | Thymic stromal lymphopoietin | 1.50 |
| 20344 | SELP/P-selectin | Selectin, platelet | 1.53 |
| 12985 | CSF3/G-CSFa | Colony stimulating factor 3 (granulocyte) | 1.57 |
| 77125 | IL33a | Interleukin 33 | 1.75 |
| 16456 | F11R/JAM-A | F11 receptor | 1.77 |
| 21937 | TNFRSF1A/TNF R1 | Tumor necrosis factor receptor superfamily, member 1a | 1.82 |
| 14451 | GAS1 | Growth arrest specific 1 | 1.91 |
| 17390 | MMP2 | Matrix metallopeptidase 2 | 1.91 |
| 17304 | MFGE8a | Milk fat globule-EGF factor 8 protein | 1.96 |
| 27218 | SLAMF1/SLAM | Signaling lymphocytic activation molecule family member 1 | 2.00 |
| 14130 | Fcgr2b/Fcg RIIB | Fc receptor, IgG, low affinity IIb | 2.02 |
| 22329 | VCAM1 | Vascular cell adhesion molecule 1 | 2.04 |
| 16195 | Il6st/Gp130 | Interleukin 6 signal transducer | 2.22 |
| 18133 | NOV | Nephroblastoma overexpressed gene | 2.63 |
| 50706 | POSTN/Periostina | Periostin, osteoblast specific factor | 2.68 |
| 13019 | CTF1/CT-1a | Cardiotrophin 1 | 3.45 |
| 11421 | ACE | Angiotensin I converting enzyme (peptidyl-dipeptidase A) 1 | 3.66 |
| 16542 | KDR/VEGF R2 | Kinase insert domain protein receptor | 6.73 |
| 18383 | TNFRSF11B/OPG | Tumor necrosis factor receptor superfamily, member 11b | 8.00 |
aNewly identified crucial cytokines which have not been illustrated in AAA formation.
Clustering heatmap was made using array profiles of DEPs between groups (Figure 1(d)). In the heatmap, subjects in AAA and control groups clustered respectively, and displayed distinct patterns of cytokine expression. Cytokine expression patterns were similar among subjects within each group, and differed between groups.
Functional analysis
The DEPs were uploaded into DAVID platform for retrieval of significant clustering from GO and KEGG. Enrichment of protein function was analyzed with R package “clusterProfiler”.
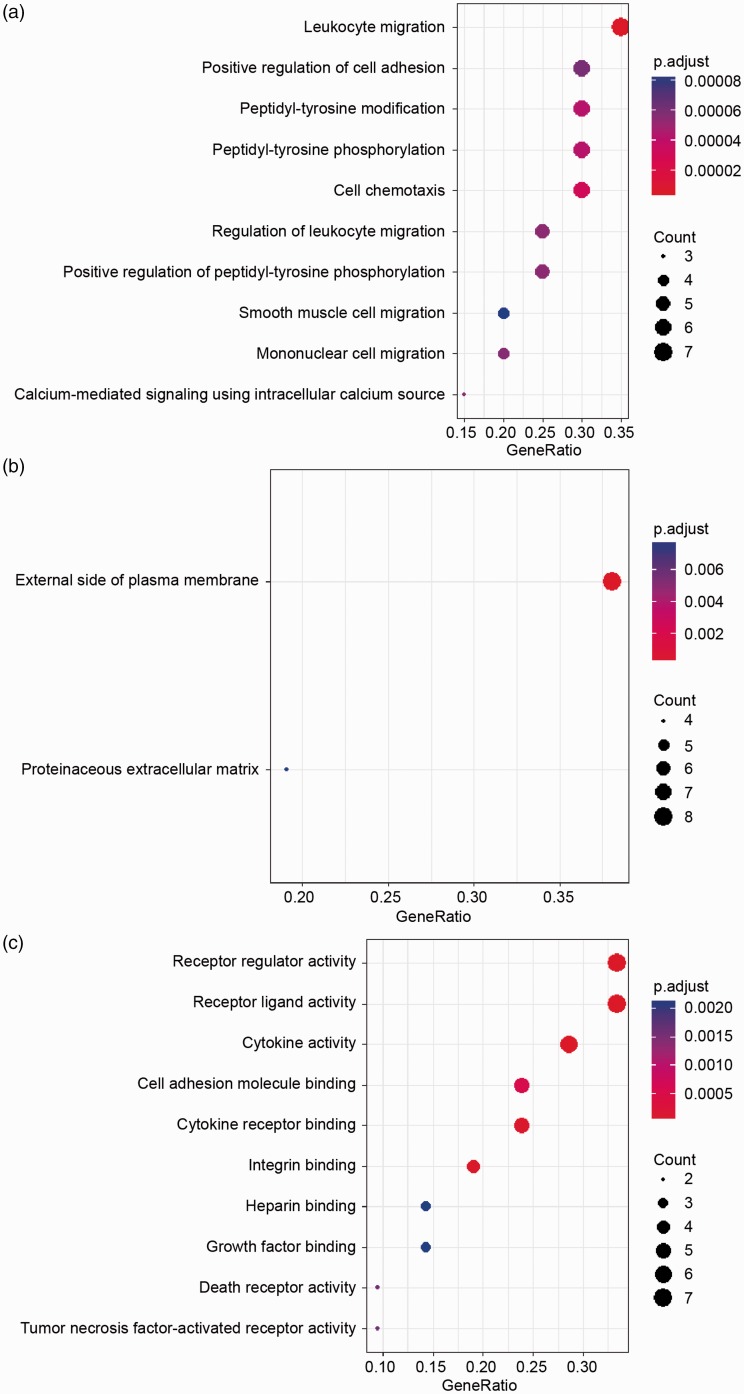
GO biological process analysis results demonstrated that the DEPs were significantly enriched in leukocyte migration, positive regulation of cell adhesion, peptidyl-tyrosine modification and phosphorylation, cell chemotaxis, etc. (Figure 2(a)). Cellular component analysis displayed that the DEPs were significantly enriched on the external side of plasma membrane (Figure 2(b)). In molecular function category, the DEPs were enriched in receptor regulatory and ligand activities, cytokine activity, cell adhesion molecule binding, cytokine receptor binding, etc. (Figure 2(c)). Specific terms and their involved cytokines are shown in Supplementary Table S2. The above results are consistent with established knowledge that inflammatory cell infiltration is significant pathological change in AAA. It also conforms to previous studies on GO annotations of AAA.13
Figure 2.
Gene ontology enrichment analysis of 21 differentially expressed proteins (DEPs) in abdominal aortic aneurysm. (a) Biological process annotation results showed that the DEPs were significantly enriched in leukocyte migration, positive regulation of cell adhesion, etc. (b) Cellular component annotation displayed that the DEPs were significantly enriched on the external side of plasma membrane. (c) Molecular function annotation showed that the DEPs were enriched in receptor regulatory and ligand activities, cytokine activity, cell adhesion molecule binding, cytokine receptor binding, etc.
To get further insights into the pathways in which the DPEs are involved, analysis of KEGG pathway was performed. As shown, signaling pathway, including Janus kinase/signal transducers and activators of transcription (Jak-STAT) and cytokine-cytokine receptor interaction were most clustered, which is consistent with previous findings (Table 2). Furthermore, these cytokines are shown integratedly in the illustration of these pathways (Supplementary Figure S2). It is well established that cytokines are key agents in cellular and protein interactions in AAA. Jak-STAT pathway can be activated by Ang II and may contribute to AAA progression.14
Table 2.
Pathway enrichment analysis of differentially expressed proteins in abdominal aortic aneurysm.
| Pathway | Gene count (%) | P value | Proteins |
|---|---|---|---|
| Cytokine–cytokine receptor interaction | 8 (38.1%) | 0.00000042 | CTF1, CCL25, CCL9, CSF3, IL6ST, TSLP |
| Jak-STAT signaling pathway | 4 (19%) | 0.0037 | CTF1, CSF3, IL6ST, TSLP |
| Malaria | 3 (14.3%) | 0.0049 | CSF3, SELP, VCAM1 |
| Leukocyte transendothelial migration | 3 (14.3%) | 0.027 | F11R, MMP2, VCAM1 |
| Osteoclast differentiation | 3 (14.3%) | 0.031 | FCGR2B, TNFRSF11B, TNFRSF1A |
| Cell adhesion molecules (CAMs) | 3 (14.3%) | 0.049 | F11R, SELP, VCAM1 |
Verification of crucial cytokines
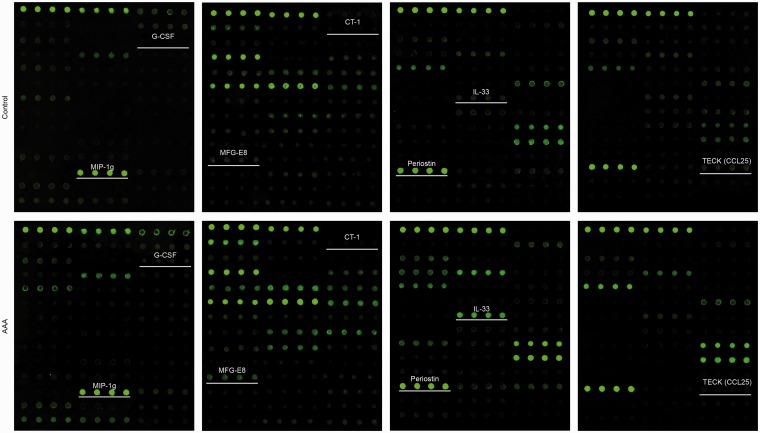
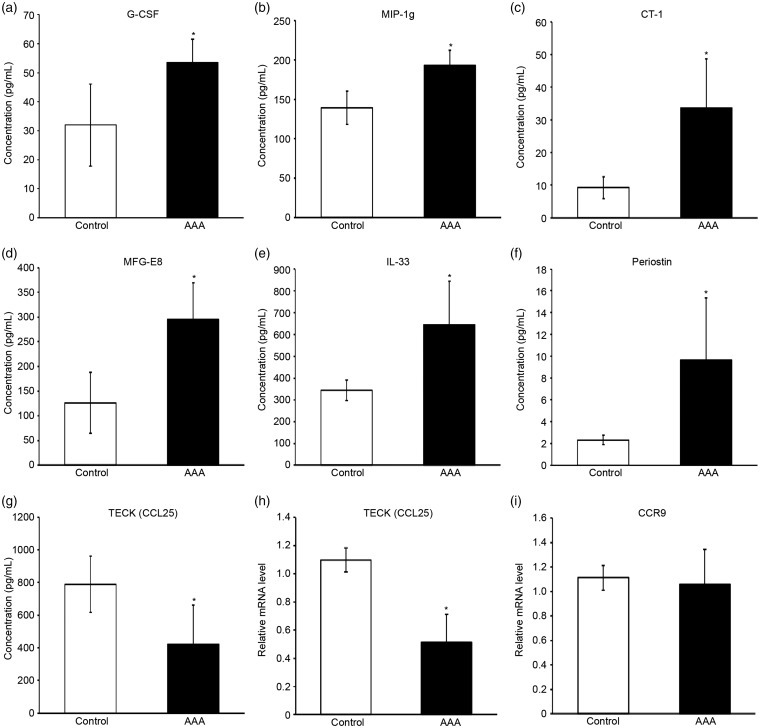
Among the 21 DEPs, 7 cytokines have not previously been studied in AAA formation. Quantified fluorescence intensity data were obtained from the scanned images of the array slides (Figure 3). There was significant difference of fluorescence intensity between AAA and control groups (Supplementary Figure S1). In order to confirm these altered cytokine expression profiles, ELISA was performed for TECK, periostin, IL-33, milk fat globule-EGF factor 8 protein (MFG-E8), cardiotrophin 1 (CT-1), macrophage inflammatory protein 1 g (MIP-1g), and granulocyte colony-stimulating factor (G-CSF). ELISA results showed that G-CSF, MIP-1g, CT-1, MFG-E8, IL-33, and periostin were significantly upregulated in AAA group, and that TECK was significantly downregulated (Figure 4(a)–(g)). These results agreed with the data of protein array, and validated the involvement of these DEPs in AAA pathobiology.
Figure 3.
Fluorescence dot images of the array slides with newly identified crucial cytokines which have not been illustrated in abdominal aortic aneurysm formation. The cytokines include granulocyte colony-stimulating factor (G-CSF), macrophage inflammatory protein 1 g (MIP-1g), cardiotrophin 1 (CT-1), milk fat globule-EGF factor 8 protein (MFG-E8), interleukin-33 (IL-33), periostin, and thymus-expressed chemokine (TECK, also known as chemokine (C-C motif) ligand 25, CCL25).
Figure 4.
ELISA-derived concentrations of newly identified crucial cytokines which have not been illustrated in abdominal aortic aneurysm formation, and quantitative real-time RT-PCR derived relative mRNA level of the only downregulated cytokine and its receptor. The newly identified crucial cytokines include (a) granulocyte colony-stimulating factor (G-CSF), (b) macrophage inflammatory protein 1 g (MIP-1g), (c) cardiotrophin 1 (CT-1), (d) milk fat globule-EGF factor 8 protein (MFG-E8), (e) interleukin-33 (IL-33), (f) periostin, and (g) thymus-expressed chemokine (TECK, also known as chemokine (C-C motif) ligand 25, CCL25; n = 5/group, means ± SD). Additional real-time RT-PCR detected the relative mRNA levels of (h) TECK (CCL25), and its receptor (i) C-C chemokine receptor type 9 (CCR9; n = 3/group, means ± SD). *P < 0.05 vs. control group.
TECK was the only downregulated cytokine among the newly identified crucial cytokines in our study. The official name of TECK is chemokine (C-C motif) ligand 25 (CCL25). CCL25 can regulate leukocyte and cancer cell proliferation, migration, and apoptosis.15 It has been revealed that the receptor of CCL25 is CCR9. To further investigate their expression in aortic tissues, the mRNA level was analyzed. Relative mRNA amount of TECK (CCL25) in aortic tissues from AAA group was significantly lower than control group, which is consistent with ELISA-derived concentration data (Figure 4(h)). However, CCR9 mRNA level did not significantly differ between two groups.
Correlations between newly identified crucial cytokines and protease activity
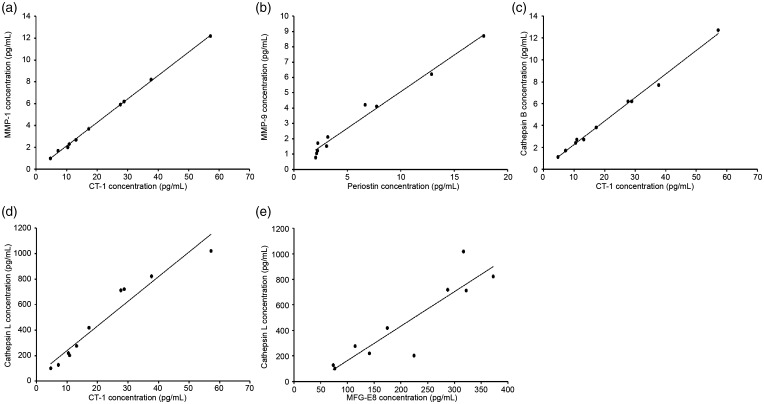
Protease activity is known to play significant roles in AAA development.16 To determine the possible connections of newly identified crucial cytokines with AAA development, we calculated bivariate correlation between these cytokines and protease activity in aortic tissues of all subjects. As shown in Table 3, MMP-1 was positively correlated with MIP-1g, CT-1, MFG-E8, IL-33, and periostin. MMP-9 was positively correlated with MIP-1g, CT-1, MFG-E8, IL-33, and periostin. Cathepsin B was positively correlated with MIP-1g, CT-1, MFG-E8, IL-33, and periostin. Cathepsin L was positively correlated with G-CSF, MIP-1g, CT-1, MFG-E8, IL-33, and periostin. The most significant linear correlations between specific cytokines and protease activity are shown in scatter plots with linear fittings (Figure 5). These results indicate that the newly identified crucial cytokines may participate in AAA pathogenesis by directly or indirectly influencing protease activities.
Table 3.
Pearson bivariate correlation coefficients (P values) between newly identified crucial cytokines which have not been illustrated in abdominal aortic aneurysm formation and protease activity.a
| MMP-1 | MMP-9 | Cathepsin B | Cathepsin L | |
|---|---|---|---|---|
| G-CSF | 0.604 (0.065) | 0.425 (0.221) | 0.600 (0.067) | 0.688* (0.028) |
| MIP-1g | 0.881** (0.001) | 0.776** (0.008) | 0.878** (0.001) | 0.896** (0.000) |
| CT-1 | 1.000** (0.000) | 0.796** (0.006) | 0.998** (0.000) | 0.968** (0.000) |
| MFG-E8 | 0.831** (0.003) | 0.763* (0.010) | 0.819** (0.004) | 0.900** (0.000) |
| IL-33 | 0.830** (0.003) | 0.813** (0.004) | 0.823** (0.003) | 0.774** (0.009) |
| Periostin | 0.800** (0.006) | 0.990** (0.000) | 0.767** (0.010) | 0.780** (0.008) |
| TECK (CCL25) | −0.493 (0.147) | −0.225 (0.532) | −0.504 (0.137) | −0.537 (0.109) |
aThe cytokines include granulocyte colony-stimulating factor (G-CSF), macrophage inflammatory protein 1 g (MIP-1g), cardiotrophin 1 (CT-1), milk fat globule-EGF factor 8 protein (MFG-E8), interleukin 33 (IL-33), periostin, and thymus-expressed chemokine (TECK, also known as chemokine (C-C motif) ligand 25, CCL25). Tested proteases include matrix metalloprotease 1 (MMP-1), MMP-9, cathepsin B, and cathepsin L. In brief, G-CSF, MIP-1g, CT-1, MFG-E8, IL-33, and periostin were positively correlated with MMP-1, MMP-9, cathepsin B, and cathepsin L. G-CSF was positively correlated with cathepsin L.
*P < 0.05 (double-tail).
**P < 0.01 (double-tail).
Figure 5.
Significant linear correlations were revealed between specific cytokines and protease activity in scatter plots with linear fittings: (a) cardiotrophin 1 (CT-1) and matrix metalloprotease 1 (MMP-1), (b) periostin and MMP-9, (c) CT-1 and cathepsin B, (d) CT-1 and cathepsin L, (e) milk fat globule-EGF factor 8 protein (MFG-E8) and cathepsin L.
Discussion
Initiation of AAA is associated with local inflammatory response that entails infiltration of monocytes, polymorphonuclear leukocytes, and T and B lymphocytes. Inflammatory response in AAA is positively regulated by various cytokines, leukotrienes, and immunoglobulins, enforced by upregulation of local adhesion molecules.16 Aortic wall inflammation contributes to VSMC apoptosis, ECM degeneration, and neovascularization. Various molecules, cells, and pathways are implicated in AAA pathogenesis.
Elucidating the molecular basis of AAA is crucial for developing diagnostic and therapeutic options. Protein array can examine expression of hundreds of cytokines in AAA tissues simultaneously. In this study, cytokine arrays were used to determine crucial biomarkers in aortic wall of Ang II-induced AAA samples from ApoE−/− mice. Analysis of the array data revealed 21 DEPs in AAA, including crucial cytokines that have not been reported in AAA pathobiology.
GO biological process analysis demonstrated that these DEPs were primarily involved in leukocyte migration and cell adhesion, which is consistent with established theory that inflammatory cell infiltration is a major pathobiological character of AAA. Innate immune cells such as macrophages, neutrophils, mast cells and dendritic cells, as well as adaptive immune cells such as T and B lymphocytes have been found in AAA aorta walls.17 Previous studies have found upregulated cytokines that facilitates leukocyte migration, cell adhesion, and chemotaxis in AAA tissues.18,19 The present study further validated the roles of specific cytokines related to immune cell infiltration in AAA pathogenesis.
The array results of cytokines that have not been reported in association with AAA were validated by ELISA quantifications, which derived consistent results. Although the exact roles of these newly identified crucial cytokines have not been elucidated, most of these cytokines have been found related to vascular inflammatory or degenerative diseases. It is our inference from previous literature that these cytokines may affect AAA through similar mechanisms, considering inflammation and degeneration in aortic walls are well-studied theories of AAA development. Further studies on these cytokines may discover the pathways that connect them to AAA, and provide new targets for studies on AAA diagnosis and treatment.
We observed 1.57-fold elevation of G-CSF level in AAA tissues. G-CSF has been found elevated in human AAA walls compared with normal aortic tissues in another protein array study.18 The underlying mechanisms have not been studied. Its upregulated expression in aortic dissection has been shown to trigger recruitment and activation of neutrophils, which causes inflammatory process by IL-6 signaling pathways in adventitia and aorta dilation and rupture.20 In light of this theory, G-CSF may have similar pro-inflammatory functions in AAA that remains to be examined.
In the present study, periostin level elevated 2.68 folds in AAA tissues. Periostin belongs to fasciclin I family. It localizes in cellular matrix and interacts with integrin at cellular membrane and participates in tissue development and remodeling pathways.21 Changes of periostin expression in human AAA lesions have been noticed, but without statistical significance.22 Another study of human AAA specimen found significantly increased periostin expression compared to normal aorta, and that periostin was correlated with immunocyte infiltration and elastin destruction.23 Furthermore, periostin can trigger MMP production in cardiomyocytes.24 The discoveries, combined with our observations, leads to the hypothesis that periostin may be related in inflammatory infiltration and protease activity in AAA.
According to our data, CT-1 level in AAA tissues was 3.45 folds higher than in control group. CT-1 belongs to IL-6 cytokine family, and has not been reported in AAA formation. CT-1 has been shown to exert stimulatory effects on atherosclerosis, and atherosclerotic disease is an independent risk factor of aneurysm.25 CT-1 promotes inflammatory cytokine and proatherogenic molecule production in endothelial cells and monocytes, leading to monocyte migration and adhesion.26,27
Recent study reported that human aortic endothelial cells overproduced MMP-1 at stimulation with CT-1.28 These mechanisms resemble those contributing to AAA formation and progression, where inflammation and protease activity that leads to ECM degradation are major pathobiology changes. Therefore, it can be inferred that CT-1 may cast similar effects on AAA development.
We observed 1.96-fold increase of MFG-E8 level in AAA tissues. MFG-E8 induces cell death and angiogenesis in aorta, and is considered associated with aging-related vascular diseases.29,30 Since older age is a risk factor for AAA, and adventitial angiogenesis is a known pathobiological change in AAA, MFG-E8 possibly plays regulatory roles in AAA pathogenesis pathways.
IL-33 level elevated 1.75 folds in AAA tissues. As a member of IL-1 family, IL-33 is a key regulator of innate and adaptive immunity. IL-33 can regulate homeostasis and response to stress.31,32 IL-33 is associated with vascular inflammation by promoting adhesion process and chemotaxis.31 Roles of IL-33 in AAA remain unclear, but these evidences indicate that IL-33 may influence inflammatory responses in AAA formation.
Small inflammatory cytokines (8–12 kDa) are also known as chemokines. Chemokines mainly function as director of cell recruitment and migration to locations of injury or inflammation. Among the four families of chemokines, C-C chemokine family is the largest one. C-C chemokines can activate immunocytes, including neutrophils and monocytes.33 The level of MIP-1g, officially named CCL9, was found elevated 1.25 folds. The mechanisms underlying these changes are unknown. Few studies showed its association with AAA previously. However, an experiment in rats has shown elevated CCL9 mRNA expressions in the early inflammatory phase of left ventricular hypertrophy tissues.34 It is possible that MIP-1g regulates AAA development by promoting inflammatory process and vascular remodeling.
According to our data, TECK, also known as CCL25, presented 0.58-fold decreased expression in AAA tissues. Further investigations of real-time RT-PCR revealed significantly decreased CCL25 mRNA level in aortic tissues from AAA group, but the mRNA level of its receptor, CCR9, had no significant difference between groups. Considering mRNA level may not necessarily be consistent with protein concentration, the level of CCR9 protein in aortic tissue remains to be studied. CCL25/CCR9 signaling pathway regulates thymocytes proliferation and migration. It can adjust CD4+ T lymphocyte chemotaxis and regulate immune hemostasis.35,36 Its role in allergic diseases has been revealed.37 It has been revealed that CCL25 is associated with asthma, neonatal necrotizing enteritis, ulcerative colitis, and experimental autoimmune encephalomyelitis by regulating T lymphocyte infiltration.38 Specifically, it has been demonstrated that TLR4 mediates recruitment of Th17 cells via CCL25 pathway.38,39 In addition to regulation of immune cell infiltration, CCL25/CCR9 exerts proatherogenic activity, an important risk factor of AAA.1,40
Based on previous findings, it is likely that TECK (CCL25) may be upregulated and mediate inflammatory activities in AAA. However, contradictory to our speculations, TECK was downregulated in AAA tissues in the present study. This contradiction suggests that AAA may present distinct mode of inflammation from other diseases, particularly, atherosclerosis, of which AAA was traditionally considered an end stage.1 Although both atherosclerosis and AAA are characterized by inflammatory infiltration, recent biological, epidemiological, and clinical evidences suggest that they each have distinct characters. For example, IL-5, IL-6, and IFN-γ play opposing roles in these diseases.41 The downregulation of a proatherogenic cytokine TECK (CCL25) in AAA further promotes the idea that the molecular basis of these diseases differs in inflammatory features. Therefore, TECK can be a key target to study the distinct inflammation pattern of AAA from other inflammatory diseases, and may start a new chapter of AAA research.
During AAA progression, increased activity of MMPs, as well as serine and cysteine proteases, augments degradation of ECM.16 MMPs have been extensively studied in AAA. Cathepsins are crucial cysteine proteases which is linked to ECM remodeling and AAA pathogenesis.42,43 Significant correlations found between cytokines and protease activity may provide directions for future studies on the roles of cytokines in AAA progression. Our study revealed the correlations between the newly identified crucial cytokines and protease activity, while the causation remains to be elucidated.
The current study has limitations that need mentioning. The sample size was limited. Among the 200 tested cytokines, around 80 proteins were upregulated or downregulated in AAA tissues, only 21 cytokines fit the criteria of DEP, which limited the extent of GO and KEGG pathway enrichment analysis.
Conclusion
In conclusion, our data provide a comprehensive protein array study and bioinformatics analysis of DEPs in Ang II induced AAA in ApoE−/− mice, as well as discovery of crucial cytokines which might be involved in the progress of AAA. Furthermore, significant correlations were found between newly identified crucial cytokines and protease activity. This study identifies several crucial markers for further researches on the molecular basis of AAA. Further experiments are needed to elucidate the functions of these newly identified crucial cytokines in AAA.
Supplemental Material
Supplemental material, EBM885101 Supplemetal Material for Discovery of crucial cytokines associated with abdominal aortic aneurysm formation by protein array analysis by Yuan Li, Dan Yang, Bo Sun, Xu Zhang, Fangda Li, Zhili Liu and Yuehong Zheng: for the ALICE (All-Literature Investigation of Cardiovascular Evidence) Group in Experimental Biology and Medicine
Author contributions
All authors designed and conducted the experiments, analyzed the data and reviewed the manuscript; DY, BS, and XZ conducted the experiments, YL and DY composed the manuscript, and FDL, ZLL, and YHZ provided critical reviews on the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Natural Science Foundation of China (81770481), Natural Science Foundation of Beijing (7172171), CAMS Innovation Fund for Medical Sciences (CIFMS, 2017-I2M-1-008), and Fundamental Research Funds for the Central Universities (3332018166).
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol 2019; 16:225–42 [DOI] [PubMed] [Google Scholar]
- 2.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol 2011; 8:92–102 [DOI] [PubMed] [Google Scholar]
- 3.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2006; 26:987–94 [DOI] [PubMed] [Google Scholar]
- 4.MA3RS-Study-Investigators. Aortic wall inflammation predicts abdominal aortic aneurysm expansion, rupture, and need for surgical repair. Circulation 2017; 136:787–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjala H, Airaksinen J, Leinonen M, Saikku P, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 1997; 17:2843–7 [DOI] [PubMed] [Google Scholar]
- 6.Rohde LE, Arroyo LH, Rifai N, Creager MA, Libby P, Ridker PM, Lee RT. Plasma concentrations of interleukin-6 and abdominal aortic diameter among subjects without aortic dilatation. Arterioscler Thromb Vasc Biol 1999; 19:1695–9 [DOI] [PubMed] [Google Scholar]
- 7.Pearce WH, Sweis I, Yao JS, McCarthy WJ, Koch AE. Interleukin-1 beta and tumor necrosis factor-alpha release in normal and diseased human infrarenal aortas. J Vasc Surg 1992; 16:784–9 [PubMed] [Google Scholar]
- 8.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 2009; 78:539–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu K, Libby P, Mitchell RN. Local cytokine environments drive aneurysm formation in allografted aortas. Trends Cardiovas Med 2005; 15:142–8 [DOI] [PubMed] [Google Scholar]
- 10.Li FD, Nie H, Tian C, Wang HX, Sun BH, Ren HL, Zhang X, Liao PZ, Liu D, Li HH, Zheng YH. Ablation and inhibition of the immunoproteasome catalytic subunit LMP7 attenuate experimental abdominal aortic aneurysm formation in mice. J Immunol 2019; 202:1176–85 [DOI] [PubMed] [Google Scholar]
- 11.Ren H, Li F, Tian C, Nie H, Wang L, Li HH, Zheng Y. Inhibition of proteasome activity by low-dose bortezomib attenuates angiotensin II-induced abdominal aortic aneurysm in apo E(-/-) mice. Sci Rep 2015; 5:15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4:44–57 [DOI] [PubMed] [Google Scholar]
- 13.Choke E, Cockerill GW, Laing K, Dawson J, Wilson WR, Loftus IM, Thompson MM. Whole genome-expression profiling reveals a role for immune and inflammatory response in abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg 2009; 37:305–10 [DOI] [PubMed] [Google Scholar]
- 14.Qin Z, Bagley J, Sukhova G, Baur WE, Park HJ, Beasley D, Libby P, Zhang Y, Galper JB. Angiotensin II-induced TLR4 mediated abdominal aortic aneurysm in apolipoprotein E knockout mice is dependent on STAT3. J Mol Cell Cardiol 2015; 87:160–70 [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Liu Z, Xu Z, Wu X, Zhang D, Zhang Z, Wei J. The role of chemokine receptor 9/chemokine ligand 25 signaling: from immune cells to cancer cells. Oncol Lett 2018; 16:2071–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maegdefessel L, Dalman RL, Tsao PS. Pathogenesis of abdominal aortic aneurysms: microRNAs, proteases, genetic associations. Annu Rev Med 2014; 65:49–62 [DOI] [PubMed] [Google Scholar]
- 17.Abdul-Hussien H, Hanemaaijer R, Kleemann R, Verhaaren BF, van Bockel JH, Lindeman JH. The pathophysiology of abdominal aortic aneurysm growth: corresponding and discordant inflammatory and proteolytic processes in abdominal aortic and popliteal artery aneurysms. J Vasc Surg 2010; 51:1479–87 [DOI] [PubMed] [Google Scholar]
- 18.Middleton RK, Lloyd GM, Bown MJ, Cooper NJ, London NJ, Sayers RD. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: a protein array study. J Vasc Surg 2007; 45:574–80 [DOI] [PubMed] [Google Scholar]
- 19.Wanhainen A, Mani K, Golledge J. Surrogate markers of abdominal aortic aneurysm progression. Arterioscler Thromb Vasc Biol 2016; 36:236–44 [DOI] [PubMed] [Google Scholar]
- 20.Anzai A, Shimoda M, Endo J, Kohno T, Katsumata Y, Matsuhashi T, Yamamoto T, Ito K, Yan X, Shirakawa K, Shimizu-Hirota R, Yamada Y, Ueha S, Shinmura K, Okada Y, Fukuda K, Sano M. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ Res 2015; 116:612–23 [DOI] [PubMed] [Google Scholar]
- 21.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci 2011; 68:3201–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Didangelos A, Yin X, Mandal K, Saje A, Smith A, Xu Q, Jahangiri M, Mayr M. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: a proteomics approach. Mol Cell Proteomics 2011; 10:M111.008128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita O, Yoshimura K, Nagasawa A, Ueda K, Morikage N, Ikeda Y, Hamano K. Periostin links mechanical strain to inflammation in abdominal aortic aneurysm. PLoS One 2013; 8:e79753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, Yozu R, Shukunami C, Hiraki Y, Kudo A, Ogawa S, Fukuda K. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest 2010; 120:2292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konii H, Sato K, Kikuchi S, Okiyama H, Watanabe R, Hasegawa A, Yamamoto K, Itoh F, Hirano T, Watanabe T. Stimulatory effects of cardiotrophin 1 on atherosclerosis. Hypertension 2013; 62:942–50 [DOI] [PubMed] [Google Scholar]
- 26.Ichiki T, Jougasaki M, Setoguchi M, Imamura J, Nakashima H, Matsuoka T, Sonoda M, Nakamura K, Minagoe S, Tei C. Cardiotrophin-1 stimulates intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 in human aortic endothelial cells. Am J Physiol Heart Circ Physiol 2008; 294:H750–63 [DOI] [PubMed] [Google Scholar]
- 27.Fritzenwanger M, Meusel K, Foerster M, Kuethe F, Krack A, Figulla HR. Cardiotrophin-1 induces interleukin-6 synthesis in human monocytes. Cytokine 2007; 38:137–44 [DOI] [PubMed] [Google Scholar]
- 28.Tokito A, Jougasaki M, Ichiki T, Hamasaki S. Cardiotrophin-1 induces matrix metalloproteinase-1 in human aortic endothelial cells. PLoS One 2013; 8:e68801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Z, Wang M, Everett A, Lakatta E, Van Eyk J. Can proteomics yield insight into aging aorta?. Proteomics Clin Appl 2013; 7:477–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Z, Wang M, Gucek M, Zhang J, Wu J, Jiang L, Monticone RE, Khazan B, Telljohann R, Mattison J, Sheng S, Cole RN, Spinetti G, Pintus G, Liu L, Kolodgie FD, Virmani R, Spurgeon H, Ingram DK, Everett AD, Lakatta EG, Van Eyk JE. Milk fat globule protein epidermal growth factor-8: a pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circ Res 2009; 104:1337–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altara R, Ghali R, Mallat Z, Cataliotti A, Booz GW, Zouein FA. Conflicting vascular and metabolic impact of the IL-33/sST2 axis. Cardiovasc Res 2018; 114:1578–94 [DOI] [PubMed] [Google Scholar]
- 32.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol 2016; 16:676–89 [DOI] [PubMed] [Google Scholar]
- 33.Ridiandries A, Tan JT, Bursill CA. The role of CC-chemokines in the regulation of angiogenesis. Int J Mol Sci 2016; 17:E1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemska S, Monassier L, Gassmann M, Frossard N, Tavakoli R. Kinetic mRNA profiling in a rat model of left-ventricular hypertrophy reveals early expression of chemokines and their receptors. PLoS One 2016; 11:e0161273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnamoorthy V, Carr T, de Pooter RF, Emanuelle AO, Gounari F, Kee BL. Repression of Ccr9 transcription in mouse T lymphocyte progenitors by the notch signaling pathway. J Immunol 2015; 194:3191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol 2002; 168:2811–9 [DOI] [PubMed] [Google Scholar]
- 37.Tubo NJ, Wurbel MA, Charvat TT, Schall TJ, Walters MJ, Campbell JJ. A systemically-administered small molecule antagonist of CCR9 acts as a tissue-selective inhibitor of lymphocyte trafficking. PLoS One 2012; 7:e50498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Han J, Wu M, Xu L, Wang Y, Yuan W, Hua F, Fan H, Dong F, Qu X, Yao R. Toll-Like receptor 4 promotes Th17 lymphocyte infiltration via CCL25/CCR9 in pathogenesis of experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol 2019; 14:493--502 [DOI] [PubMed] [Google Scholar]
- 39.Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, Lu P, Ma C, Branca MF, Weyandt S, Fulton WB, Niño DF, Prindle T, Jr, Ozolek JA, Hackam DJ. Toll-like receptor 4–mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest 2016; 126:495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abd Alla J, Langer A, Elzahwy SS, Arman-Kalcek G, Streichert T, Quitterer U. Angiotensin-converting enzyme inhibition down-regulates the pro-atherogenic chemokine receptor 9 (CCR9)-chemokine ligand 25 (CCL25) axis. J Biol Chem 2010; 285:23496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peshkova IO, Schaefer G, Koltsova EK. Atherosclerosis and aortic aneurysm – is inflammation a common denominator? FEBS J 2016; 283:1636–52 [DOI] [PubMed] [Google Scholar]
- 42.Abisi S, Burnand KG, Waltham M, Humphries J, Taylor PR, Smith A. Cysteine protease activity in the wall of abdominal aortic aneurysms. J Vasc Surg 2007; 46:1260–6 [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Sukhova GK, Yang JT, Sun J, Ma L, Ren A, Xu WH, Fu H, Dolganov GM, Hu C, Libby P, Shi GP. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis 2006; 184:302–11 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, EBM885101 Supplemetal Material for Discovery of crucial cytokines associated with abdominal aortic aneurysm formation by protein array analysis by Yuan Li, Dan Yang, Bo Sun, Xu Zhang, Fangda Li, Zhili Liu and Yuehong Zheng: for the ALICE (All-Literature Investigation of Cardiovascular Evidence) Group in Experimental Biology and Medicine