Abstract
The high yield potential of winter wheats cannot be realized due to disease pressure under field conditions. One of the most harmful of such diseases is stem rust, hence the constant search for sources of resistance and the development of new varieties resistant to stem rust is of great relevance. This study deals with the identification of stem rust resistance genes in a collection of winter wheats grown in Southern Russia. This genepool has not been studied yet. A total of 620 samples of winter soft wheat from various ecological and geographical zones were tested under field conditions. To identify the specific genes or alleles responsible for resistance, all samples were genotyped using PCR. As a result, the groups of resistant samples, carrying the Sr2, Sr31, Sr38 and Sr44 genes in various combinations, were identified. Most of the stem rust resistance was provided by the presence of the effective Sr44 gene. This information can be used in the future breeding work for stem rust resistance.
Keywords: MAS, Sr2, Sr31, Sr38, Sr44, stem rust
1. Introduction
Winter wheat is one of the main sources of food for the population in most countries of the world [1]. It is necessary to obtain stable and high grain yields in order to provide the population with food. The varieties developed by breeders have a high productive potential, which cannot fully be realized because of crop diseases [2].
One of the most harmful winter wheat diseases is stem rust (Puccinia graminis f. sp. tritici). The disease appears after flowering on stems and leaf sheaths, in the form of rust-brown oblong powdering with urediniospore pustules that coalesce in the form of brown stripes and tearing of the epidermis. This disease can reduce winter wheat yields by up to 80% [3]. The Ug99 stem rust emergence in 1999 [4], which overcame the rust resistance of many varieties [5], made it urgent to find sources of resistance and to identify new winter wheat varieties with these genes.
The stem rust resistance genes mostly used in the world breeding are the Sr31 (located in the translocation with the Lr26, Yr9 and Pm8) and Sr38 genes (linked to the gene for adult plant resistance to leaf rust Lr37 and Yr17) [6]. Currently, Ug99 overcomes their resistance. According to Terefe [7] there was virulence to the main stem rust resistance genes Sr5, Sr6, Sr9e and Sr38 of the TTKSF race, which is one of the variations of the highly virulent race Ug99. This race was found in Africa, and it was Boshoff who reported stem rust isolates virulent to Sr38 for the first time [8].
Sibikeev [9] reported the detection of stem rust isolates overcoming the Sr31 gene resistance in the central non-black-earth zone of Russia in the years of 2012 and 2013. However, the Sr31 and Sr38 genes keep providing resistance to local stem rust races in some regions of the world [10,11,12]. In China, the Sr38 and Sr44 genes and their combination with the Sr25 and Sr2 genes proved to be effective stem rust resistance genes [13]. The Sr38, Sr26 and Sr36 genes remain effective against the Indian race of stem rust [11]. In the south of Russia, in the Southern Federal District, gene Sr31 keeps maintaining its effectiveness [14,15].
The Sr2 and Sr44 genes belong to the group of effective genes, resistant to the Ug99 race and its other phenotypes [16]. The Sr2 gene provides partial but long-lasting stem rust resistance for more than 50 years [17,18]. The gene is non-specific and effective against many Puccinia graminis tritici isolates in all wheat-growing regions of the world. [19]. The stem rust resistance gene Sr44 is resistant to the complex Ug99 race, namely TTKSK, TTSKT and TTTSK [20].
We have not studied the genotypes of the resistant winter soft wheat samples collected in our research center before. The purpose of our work was to identify the sources of stem rust resistance genes in the winter soft wheat collection samples of the Federal State Budgetary Scientific Institution “Agricultural Research Centre “Donskoy” (FSBSI “ARC “Donskoy”) for their further use in breeding.
2. Results
The PCR analysis showed a wide variety of samples according to the presence of the studied stem rust resistance genes. For PCR analysis, we used all 620 lines.
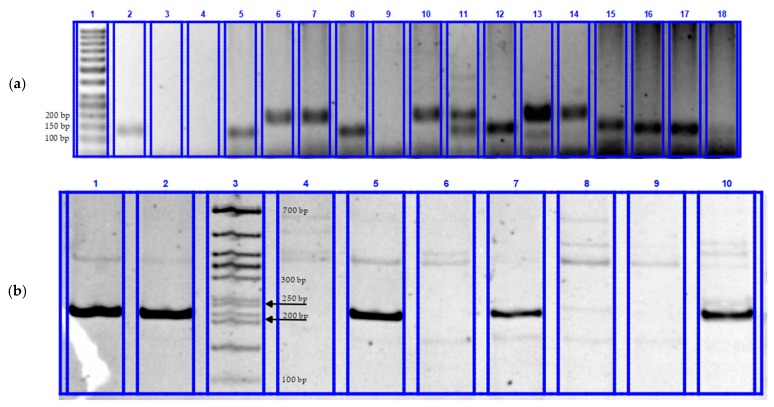
Figure 1 shows fragments of electrophoregrams for the analysis of the Sr2 (Figure 1a), Sr31 (Figure 1b) and Sr38 (Figure 1c) genes, respectively.
Figure 1.
Electrophoregrams for screening winter soft wheat samples by determining stem rust resistance genes: (a)—Sr2, agarose gel: 1—GeneRuler 50 + bp (ThermoScientific), 2—WIR64679 (positive control of experience), 3—H2O deionized (negative control of experience), 4—WIR42910, 5—K15-0542, 6—K15-0553, 7—K15-0580, 8—K16-0147, 9—K16-0148, 10—K16-0149, 11—K16-0150, 12—K17-0278, 13—K17-0283, 14—K17-0287, 15—K17-0292, 16—K17-0294, 17—K17-0300, 18—K17-0302; (b)—Sr31, PAGE: 1—TchLr26 (positive control of experience), 2—K17-0216, 3—The molecular weight marker Evrogen 50 + bp (50–700 bp), 4—K17-0218, 5—K17-0224, 6—K17-0223, 7—K17-0232, 8—K17-0217, 9—K17-0219, 10—17-0256; (c)—Sr38, agarose gel: 1—GeneRuler 50 + bp (ThermoScientific), 2—H2O deionized (negative control of experience), 3—TchLr37 (positive control of experience), 4—WIR42910, 5—WIR53496, 6—WIR56750, 7—WIR56753, 8—K15-0672, 9—K15-0588, 10—K15-0604, 11—K15-0605, 12—K15-0606, 13—K15-0680, 14—K16-0002, 15—K16-0004, 16—K16-0007, 17—K16-0018, 18—K16-0143.
Samples 2, 5, 8, 12, 15, 16 and 17 have a diagnostic fragment of the functional allele (120 bp) of the Sr2 gene (Figure 1a). Samples 1, 2, 5, 7, 8, and 10 in Figure 1b have a diagnostic fragment of the functional allele (207 bp) of the Sr31 gene. Samples 3, 8, 10, 11, 12 and 18 (Figure 1c) have a diagnostic fragment of the functional allele of the Sr38 gene. The remaining samples do not have the diagnostic fragments Sr2, Sr31 and Sr38. Only sample 11 from Figure 1a is heterozygous.
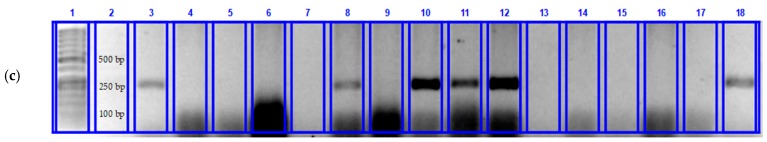
The separation of restriction products on a 2% agarose gel was used to identify the functional allele of the stem rust resistance Sr44 gene. Figure 2 shows electrophoregram fragments of the analysis of the Sr44 gene before (Figure 2a) and after restriction (Figure 2b), respectively.
Figure 2.
Determination of Sr44 gene in winter wheat samples on agarose gel: (a)—before restriction: 1—Thermo Scientific GeneRuler 50 + bp molecular weight marker (50–1000 bp), 2—H2O deionized (negative control of experience), 3—K17-0350, 4—K17-0354, 5—K17-0357, 6—K17-0361, 7—K17-0372, 8—K17-0378, 9—K17-0393, 10—K17-0397, 11—K17-0411, 12—K17-0425, 13—K17-0426, 14—K17-0427, 15—K17-0428, 16—K17-0429, 17—K17-0430, 18—K17-0431; (b)—after restriction: 1—Thermo Scientific GeneRuler 50 + bp molecular weight marker (50–1000 bp), 2—Donskaya Polukarlikovaya (positive control of experience), 3—K17-0350, 4—K17-0354, 5—K17-0357, 6—K17-0361, 7—K17-0372, 8—K17-0378, 9—K17-0393, 10—K17-0397, 11—K17-0411, 12—K17-0425, 13—K17-0426, 14—K17-0427, 15—K17-0428, 16—K17-0429, 17—K17-0430, 18—K17-0431.
All samples in Figure 2a showed an amplicon measuring 874 bp. The amplification of the samples was successful. The samples with the identified amplicon of the Xbe404728 marker were cut using MSpI restriction endonuclease and separated on a 2% agarose gel (Figure 2b). Two separated fragments on the gel show the presence of the functional allele of the stem rust resistance Sr44 gene.
The research data for each of the studied genes (Sr2, Sr31, Sr38 and Sr44) were reduced to a binary form, where ‘1’ is the presence of the stem rust resistance gene, and ‘0’ is its absence.
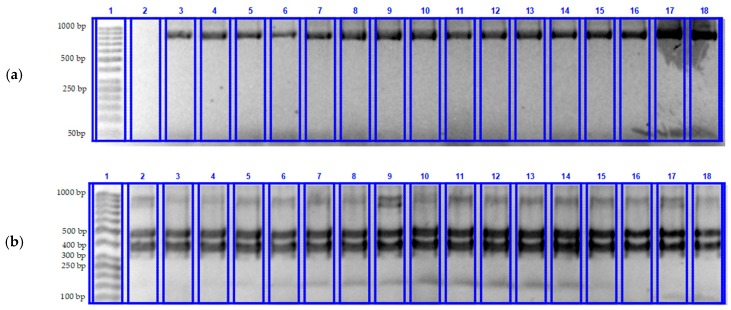
Four groups of samples from the field tests, including susceptible (S), medium susceptible (MS), medium resistant (MR) and resistant (R), were identified (Figure 3).
Figure 3.
Distribution of winter wheat samples by groups of stem rust resistance.
In total, 49 samples (7.90%) were relevant to the susceptible group, 130 samples (20.97%) were relevant to the medium-susceptible group, 105 samples (16.94%) were relevant to the medium-resistant group, and 336 samples (54.19%) were relevant to the resistant group.
The final data table (field tests) is presented in Supplementary Table 1 (Table S1).
Table 1.
Results of analysis of variance.
| Df | Sum Sq | Mean Sq | F Value | Pr(>F) a | |
|---|---|---|---|---|---|
| Group | 3 | 647,279 | 215,760 | 4849 | <2 × 10−16 |
| Residuals | 1236 | 54,993 | 44 |
a Level of significance
The differences between the groups were estimated by variance analysis. The results of the variance analysis, given in Table 1, show the statistical significance of the differences between groups.
There were also identified great differences between the average values in the groups estimated by the Duncan method (Table 2).
Table 2.
Differences between average values of stem rust resistance groups.
| Group | Diff | Lwr.ci | Upr.ci | P-Value a |
|---|---|---|---|---|
| MS-MR | 25.32601 | 24.11186 | 26.54015 | <2 × 10−16 |
| R-MR | −15.92262 | −16.95718 | −14.88805 | <2 × 10−16 |
| S-MR | 56.40136 | 54.71582 | 58.08690 | <2 × 10−16 |
| R-MS | −41.24863 | −42.25491 | −40.24234 | <2 × 10−16 |
| S-MS | 31.07535 | 29.52418 | 32.62652 | <2 × 10−16 |
| S-R | 72.32398 | 70.78409 | 73.86387 | <2 × 10−16 |
a Level of significance
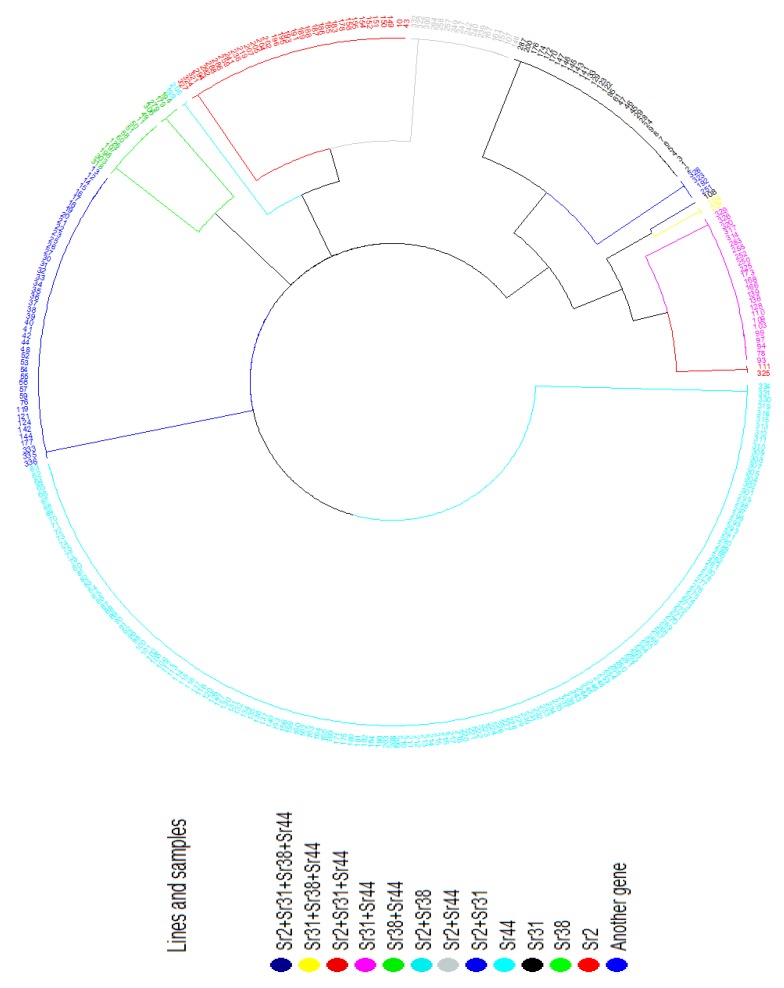
Since stable samples are of the greatest interest in breeding, we have considered the R group in detail. This group was clustered by the binary method because our PCR analysis data were presented as a binary matrix. The samples were divided into 13 clusters (Figure 4).
Figure 4.
Cluster diagram of winter wheat samples of the group R.
The largest number of samples in the R group (156 samples (46.43%)) was included in the cluster with the presence of the single stem rust resistance gene Sr44 (Table 3). The resistance of 10.42% in the samples (35 pcs.) was due to the presence of the Sr2 gene.
Table 3.
Distribution of winter wheat samples in the clusters of the R group.
| No of Cluster | Cluster | Number of Samples | The Percentage of Samples in the Group | No of Cluster | Cluster | Number of Samples | The Percentage of Samples in the Group |
|---|---|---|---|---|---|---|---|
| 1 | Sr44 | 156 | 46.43 | 9 | Sr31_Sr2 | 4 | 1.19 |
| 2 | Sr31 | 32 | 9.52 | 10 | Sr38_Sr2 | 2 | 0.60 |
| 3 | Sr38 | 11 | 3.27 | 11 | Sr44_Sr31_Sr38 | 2 | 0.60 |
| 4 | Sr2 | 35 | 10.42 | 12 | Sr44_Sr38_Sr2 | 0 | 0 |
| 5 | Sr44_Sr31 | 24 | 7.14 | 13 | Sr44_Sr31_Sr2 | 2 | 0.60 |
| 6 | Sr44_Sr38 | 4 | 1.19 | 14 | Sr31_Sr38_Sr2 | 0 | 0 |
| 7 | Sr44_Sr2 | 16 | 4.76 | 15 | Sr44_Sr31_Sr38_Sr2 | 1 | 0.30 |
| 8 | Sr31_Sr38 | 0 | 0 | 16 | Another Gene | 47 | 13.99 |
The Sr31 gene was represented in 32 samples (9.52%), and the Sr38 gene was represented in 11 samples (3.27%).
Twenty-four samples (7.14%) were in Cluster 5 with a combination of the Sr44 and Sr31 stem rust resistance genes. Clusters 6 (Sr44 and Sr38 genes) and 9 (Sr31 and Sr2 genes) contained four samples (1.19%). Two samples (0.60%) belonged to Clusters 10, 11, and 13.
Cluster 15 included only 1 sample (0.30%) carrying all four stem rust resistance genes (K16-0147).
In the R group, there were no winter soft wheat samples identified belonging to Clusters 8, 12 and 14.
Cluster 16 had included 47 samples (13.99%), which indicates the presence of other effective resistance genes that we have not identified yet.
The cluster distribution of the samples was unbalanced. The relationship of the resistance of the samples with clusters was estimated by the Matthews correlation coefficient (Table 4) [21].
Table 4.
The Matthews correlation coefficients.
| Cluster | Correlation Coefficient | Cluster | Correlation Coefficient |
|---|---|---|---|
| 1 | 0.36 | 9 | 0.07 |
| 2 | 0.14 | 10 | 0.05 |
| 3 | −0.01 | 11 | 0.05 |
| 4 | 0.04 | 13 | 0.05 |
| 5 | 0.18 | 15 | 0.04 |
| 6 | 0.02 | 16 | −0.56 |
| 7 | 0.06 |
A weak positive correlation was identified for Clusters 2 and 5. The average negative correlation for Cluster 16 was determined (r = −0.56). The average positive correlation for Cluster 1 was identified (r = 0.36). Therefore, the presence of the Sr44 gene affects the stability of winter soft wheat samples. The presence of another gene that we have not studied may have a partial effect on plant resistance. It can be either the stability gene already known in the world, or one not studied. Further research is needed.
We have identified the winter wheat samples with group stem rust resistance, having two or more effective resistance genes in various combinations (Table 5).
Table 5.
Winter soft wheat samples with the group of stem rust resistance.
| Genotype | Samples |
|---|---|
| Sr44 + Sr2 | 107, 148, 157, 161, 167, 239, 240, 242, 247, 249, 257, 258, 284, 300, 322 and 332 |
| Sr44 +Sr31 + Sr38 | 104 and 201 |
| Sr44 + Sr31 + Sr2 | 111 and 325 |
| Sr44 + Sr31 + Sr38 + Sr2 | 108 |
In total, 21 samples possessed group stem rust resistance. Most of these samples were the lines developed in the FSBSI “ARC “Donskoy”. The sample № 111was the cultivar ‘Don 85’ (WIR58516). The sample № 148 was the cultivar ‘Voyazh ‘. These varieties were developed in our Center and are actively used in breeding work. The varieties’ progeny may also have stem rust resistance genes Sr2, Sr44 and Sr31.
3. Discussion
The Sr31 gene has lost its stem rust resistance but is still a valuable resource. The gene is in translocation with resistance genes to other diseases. According to Yu (Yu et al.) [16], three QTLs found in the 1BS chromosome, which is homologous to 1RS, may be due to the residual effect of Sr31, or another gene obtained from rye translocation. According to Miroshnichenko [15], the Sr31 gene keeps maintaining its effectiveness in Southern Russia, and the Sr38 gene is low level effective, which has been proven by our results, as 32 winter wheat samples of the R group (9.52%) had the Sr31 gene and 11 samples (3.27%) had the Sr38 gene.
The stem rust resistance gene Sr2 was recommended by Haile and Roder [22] for use in breeding programs in combination with other stem rust resistance genes. Rutkoski et al. have found that the locus of adult plant resistance, which includes the Sr2 gene, plays an important role in the studied germplasm CIMMYT, and the use of genotypes as fixed effects in genomic breeding can give a good projection [23]. In our study, we identified 35 samples (10.42%) of winter soft wheat with this gene. These samples can be used as a testing group using genomic selection.
The Sr44 gene is effective in Russia and in the world [24,25]. According to Baranova et al. [26] the gene is available in the cultivar ‘Donskaya Polukarlikovaya’, which is included in the breeding background of the studied lines. Since the Sr44 gene has been identified in these lines, the cultivar ‘Donskaya Polukarlikovaya’ is a valuable donor for breeding for stem rust resistance.
The results of our study have shown that the Sr44 gene and its combinations with other effective genes play a significant role for the stem rust resistance of winter wheat samples cultivated in Southern Russia. The varieties ‘Don 85’ and ‘Voyazh’ with the Sr2 and Sr44 genes identified in the study can be used as resistance sources against stem rust Ug99 in the breeding programs. These lines differ in other valuable indicators. Using one of these sources should be sufficient to transmit the resistance genes Sr2 and Sr44. The cultivar “Voyage”, having the genes Sr2 and Sr44 and not having the gene Sr31 that is affected by Ug99, will be more useful for breeding work. This information can be used in future breeding work for stem rust resistance.
4. Materials and Methods
4.1. Plant Material
The objects of the study were 620 collection samples of winter soft wheat. These samples were obtained from the VIR (Federal Research Center N. I. Vavilov All-Russian Institute of Plant Genetic Resources) and other scientific institutions as a result of the exchange of breeding material. Some of the lines were created in our center by the method of crossing and selecting breeding material. The studied samples have different ecological and geographical origins. A total of 63.69% of samples were from Russia, 9.08% from Ukraine, 7.38% from Germany, 7.08% from the USA, 10.62% from European countries, 1.69% from China and 0.46% from Kazakhstan.
In total, 139 of the samples (22.42%) were winter soft wheat developed in the Federal State Budgetary Scientific Institution “Agricultural Research Centre “Donskoy” (FSBSI “ARC “Donskoy”).
The lines stored in VIR are available to any breeder upon request. Breeding lines created in our breeding center may be partially accessible.
4.2. Field Test
We studied all of the samples in an infectious background in the fields of the FSBSI “ARC “Donskoy” (46°50′42″ N, 40°18′30″ E) in 2016–2018. The most favorable conditions for the development of stem rust were in the year 2017.
The infection of the wheat varieties was carried out in the booting stage by dusting with a mixture of viable urediniospores of the stem rust race native to our region using the talcum powder method (flour used as a base). The spores were collected from infected plants against an infectious background. The spores were stored at room temperature, with a moisture content of 20–30%. [27]. The estimation of the stem rust response of the wheat varieties was carried out using the Peterson’s scoring scale [28]. The data obtained were divided into 4 groups according to the system for monitoring diseases, pests and weeds in cereal crops by the FAO: (The Food and Agriculture Organization of the United Nations) susceptible (S), medium susceptible (MS), medium resistant (MR) and resistant (R) [29].
4.3. DNA Extraction and Study
The genomic DNA was isolated from the leaves of 5–7-day-old seedlings using the modified CTAB protocol [30]. The quantity and quality of the DNA were assessed on an Implen NP80 spectrophotometer (Implen GmbH, Germany), and adjusted to 50 ng/μL. The primers used in PCR were synthesized by the Evrogen (Russia) and Syntol (Russia) companies (Table 6). PCR was performed in a volume of 25 μL.
Table 6.
Markers linked to the resistance genes Sr2, Sr31, Sr38 and Sr44 with their forward and reverse primers.
| Genes | Marker | Forward Primer | Reverse Primer | References |
|---|---|---|---|---|
| Sr2 | Xgwm533 | 5′-GTTGCTTTAGGGGAAAAGCC | 5′-AAGGCGAATCAAACGGAATA | [31] |
| Sr31 | SCM9 | 5′-TGACAACCCCCTTTCCCTCGT | 5′-TCATCGACGCTAAGGAGGACCC | [32] |
| Sr38 | VENTRIUP-LN2 | 5′-AGGGGCTACTGACCAAGGCT | 5′-TGCAGCTACAGCAGTATGTACACAAAA | [33] |
| Sr44 | Xbe404728 | 5′-GGTGGTGCCTGTCAAGATT | 5′-TTGATGGATCCTGGCTTAGG | [20] |
We separated the amplification products on a 2% agarose or 8% polyacrylamide gel, and stained them with ethidium bromide fluorescent dye. GeneRuler 50 + bp (50–1000 bp) (Thermo Scientific, USA) and Evrogen 50 + bp (50–700 bp, Evrogen, Russia) were used as molecular weight markers. To identify the Sr2 stem rust resistance gene, we used the molecular SSR marker Xgwm533, which is closely linked to this gene for use by authors [31,34,35,36]. The diagnostic Xgwm533 marker fragment was 120 bp. Identification of the Sr31 gene was performed with the SCM9 marker, its diagnostic fragment was 207 bp. The VENTRIUP-LN2 marker was used to identify the Sr38 gene. A diagnostic fragment size of 259 bp indicated the presence of this gene.
The samples successfully amplified with the Xbe404728 marker (Sr44 gene) were used for restriction with endonuclease MSpI (NEB, USA). The composition of the reaction mixture was 10 μL PCR products, 5 μL separating buffer (1.5 μL 10 × Tango buffer; 3.4 μL H2O; 0.1 μL MSpI). The samples were incubated at 37 °C for 2 h and then evaluated on a 2% agarose gel. In the absence of the Sr44 gene in the source material for analysis, the restriction did not occur. In the presence of the Sr44 gene, 2 bands are observed.
4.4. Data Analysis
The winter soft wheat samples were assessed for stem rust resistance on the infectious background in the conditions of Southern Russia. After that, we studied the representation of stem rust resistance genes in our region (Sr2, Sr31, Sr38 and Sr44) in 620 winter wheat samples by the PCR method.
The data analysis was carried out in the programming language R v. 3.5.1 [37] in the RStudio [38] program, with the help of specialized packages “agricolae” (estimation of differences between group mean values by the Duncan method) [39], “ape” (dendrogram analysis) [40], “cyclize” (circular visualization) [41], “dendextend” (visualizing and adjusting tree of hierarchical clustering by the binary method) [42], “DescTools” (ANOVA analysis) [43] and “dplyr” (data grouping and counting) [44].
Acknowledgments
We would like to thank the anonymous reviewers for their constructive comments and suggestions.
Supplementary Materials
The following is available online at https://www.mdpi.com/2223-7747/8/12/559/s1, Table S1: Stem rust resistance data in winter wheat samples.
Author Contributions
Conceptualization, N.N.V. and E.V.I.; Formal analysis, N.N.V.; Funding acquisition, A.V.A.; Investigation, N.T.K. and N.V.S.; Methodology, N.N.V.; Project administration, E.V.I.; Resources, D.M.M.; Supervision, A.V.A. and E.V.I.; Validation, N.N.V. and S.N.V.; Visualization, N.N.V.; Writing—original draft, N.N.V. and E.V.I.; Writing—review & editing, N.N.V. and E.V.I.
Funding
This work was funded by Russian Academy of Sciences (0706-2015-0001; 0706-2019-0003).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Shewry P., Hey S. The contribution of wheat to human diet and health. Food Energy Secur. 2015;4:178–202. doi: 10.1002/fes3.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vyska M., Cunniffe N., Gilligan C. Trade-off between disease resistance and crop yield: A landscape-scale mathematical modelling perspective. J. R. Soc. Interface. 2016;13:20160451. doi: 10.1098/rsif.2016.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis C., Persoons A., Bebber D., Kigathi R., Maintz J., Findlay K., Bueno-Sancho V., Corredor-Moreno P., Harrington S., Kangara N., et al. Potential for re-emergence of wheat stem rust in the United Kingdom. Commun. Biol. 2018;1:s42003–s42018. doi: 10.1038/s42003-018-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pretorius Z.A., Singh R.P., Wagoire W.W., Payne T.S. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. Tritici in Uganda. Plant Dis. 2000;84:203. doi: 10.1094/PDIS.2000.84.2.203B. [DOI] [PubMed] [Google Scholar]
- 5.Soko T., Bender C., Prins R., Pretorius Z. Yield Loss Associated with Different Levels of Stem Rust Resistance in Bread Wheat. Plant Dis. 2018;102:2531–2538. doi: 10.1094/PDIS-02-18-0307-RE. [DOI] [PubMed] [Google Scholar]
- 6.Management of Wheat and Barley Diseases. [(accessed on 25 September 2019)]; Available online: https://books.google.ru/books?id=xKU5DwAAQBAJ.
- 7.Terefe T., Visser B., Pretorius Z. Variation in Puccinia graminis f. sp tritici detected on wheat and triticale in South Africa from 2009 to 2013. Crop Prot. 2016;86:9–16. doi: 10.1016/j.cropro.2016.04.006. [DOI] [Google Scholar]
- 8.Boshoff W.H.P., Pretorius Z.A., Van Niekerk B.D., Komen J.S. First report of virulence in Puccinia graminis f. sp. tritici to wheat stem rust resistance genes Sr8b and Sr38 in South Africa. Plant Dis. 2002;86:922. doi: 10.1094/PDIS.2002.86.8.922B. [DOI] [PubMed] [Google Scholar]
- 9.Sibikeev S.N., Markelova T.S., Baukenova E.A., Druzhin A.E. Likely threat of the spread of race UG99 of Puccinia graminis f. sp. tritici on wheat in Southeastern Russia. Russ. Agric. Sci. 2016;42:145–148. doi: 10.3103/S1068367416020154. [DOI] [Google Scholar]
- 10.Yadav P., Mishra V., Arun B., Chand R., Vishwakarma M., Vasistha N., Mishra A., Kalappanavar I., Joshi A. Enhanced resistance in wheat against stem rust achieved by marker assisted backcrossing involving three independent Sr genes. Curr. Plant Biol. 2015;2:25–33. doi: 10.1016/j.cpb.2015.05.001. [DOI] [Google Scholar]
- 11.Singh R., Hodson D., Huerta-Espino J., Jin Y., Njau P., Wanyera R., Herrera-Foessel S., Ward R., Sparks D. Will stem rust destroy the world’s wheat crop? Adv. Agron. 2008;98:271–309. doi: 10.1016/S0065-2113(08)00205-8. [DOI] [Google Scholar]
- 12.Xu X., Yuan D., Li D., Gao Y., Wang Z., Liu Y., Wang S., Xuan Y., Zhao H., Li T., et al. Identification of stem rust resistance genes in wheat cultivars in China using molecular markers. PeerJ. 2018;6:e4882. doi: 10.7717/peerj.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X., Li D., Liu Y., Gao Y., Wang Z., Ma Y., Yang S., Cao Y., Xuan Y., Li T. Evaluation and identification of stem rust resistance genes Sr2, Sr24, Sr25, Sr26, Sr31 and Sr38 in wheat lines from Gansu Province in China. PeerJ. 2017;5:e4146. doi: 10.7717/peerj.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkova G.V., Sinyak E.V. Stem rust of wheat. Prot. Quar. Plants. 2011;11:14–16. [Google Scholar]
- 15.Miroshnichenko O.O., Volkova G.V. Efficacy of the known genes of resistance (Sr) to wheat stem rust (pathogene Puccinia graminis pers.) in the south of Russia; Proceedings of the Biologicheskaya zashchita rastenii–osnova stabilizatsii agroekosistem: Mater. Mezhdunar. nauch.prakt. konf (Biological Protection of Plants is the Basis of the Stabilization of AgroEco systems: Proc. Int. Sci.Pract. Conf.); Krasnodar, Russia. 11–13 September 2018; pp. 358–360. [Google Scholar]
- 16.Yu L., Barbier H., Rouse M., Singh S., Singh R., Bhavani S., Huerta-Espino J., Sorrells M. A consensus map for Ug99 stem rust resistance loci in wheat. Theor. Appl. Genet. 2014;127:1561–1581. doi: 10.1007/s00122-014-2326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hare R.A., McIntosh R.A. Genetic and cytogenetic studies of durable adult plant resistances in Hope and related cultivars to wheat rusts. J. Plant Breed. 1979;83:350–367. [Google Scholar]
- 18.McIntosh R.A., Wellings C.R., Park R.F. Wheat Rusts, An Atlas of Resistance Genes. CSIRO Publications; East Melbourne, Australia: 1995. pp. 93–99. [Google Scholar]
- 19.Malik R., Parveen S., Saharan M.S., Kumar R., Sharma A.K., Bhardwaj S.C., Sharma I. Characterization of stem rust resistance gene Sr2 in Indian wheat varieties using polymerase chain reaction (PCR) based molecular markers. Afr. J. Biotechnol. 2013;12:2353–2359. doi: 10.5897/AJB12.1521. [DOI] [Google Scholar]
- 20.Liu W., Danilova T., Rouse M., Bowden R., Friebe B., Gill B., Pumphrey M. Development and characterization of a compensating wheat-Thinopyrum intermedium Robertsonian translocation with Sr44 resistance to stem rust (Ug99) Theor. Appl. Genet. 2013;126:1167–1177. doi: 10.1007/s00122-013-2044-6. [DOI] [PubMed] [Google Scholar]
- 21.Matthews B.W. Comparison of the predicted and observed secondary structure of T4 phage lysozyme. BBA Protein Struct. 1975;405:442–451. doi: 10.1016/0005-2795(75)90109-9. [DOI] [PubMed] [Google Scholar]
- 22.Haile J.K., Roder M. Status of genetic research for resistance to Ug99 race of Puccinia graminis F. sp. tritici: A review of current research and implications. Afr. J. Agric. Res. 2013;8:6670–6680. doi: 10.5897//AJAR2013.7257. [DOI] [Google Scholar]
- 23.Rutkoski J.E., Poland J.A., Singh R.P., Huerta-Espino J., Bhavani S., Barbier H., Rouse M.N., Jannink J.L., Sorrells M.E. Genomic Selection for Quantitative Adult Plant Stem Rust Resistance in Wheat. Plant Genome. 2014;7:3. doi: 10.3835/plantgenome2014.02.0006. [DOI] [Google Scholar]
- 24.Lapochkina I., Baranova O., Gainullin N., Kuzmich M., Polyakova S., Polityko P., Mamedov R., Voronov S. Genetic improvement of bread wheat for stem rust resistance in the Central Federal Region of Russia: results and prospects. In: Fahad S., Basir A., editors. Global Wheat Production. IntechOpen Limited; London, UK: 2018. pp. 184–203. [Google Scholar]
- 25.Aktar-Uz-Zaman M., Tuhina-Khatun M., Hanafi M.M., Sahebi M. Genetic analysis of rust resistance genes in global wheat cultivars: An overview. Biotechnol. Biotechnol. Equip. 2017;31:431–445. doi: 10.1080/13102818.2017.1304180. [DOI] [Google Scholar]
- 26.Baranova O.A., Lapochkina I.F., Anisimova A.V., Gajnullin N.R., Iordanskaya I.V., Makarova I.Y. Identification of Sr genes in new common wheat sources of resistance to stem rust race Ug99 using molecular markers. Russ. J. Genet. Appl. Res. 2016;6:344–350. doi: 10.1134/S2079059716030011. [DOI] [Google Scholar]
- 27.Roelfs A.P., Singh R.P., Saari E.E. Rust Diseases of Wheat: Concepts and Methods of Disease Management. CIMMYT; Mexico City, Mexico: 1992. p. 81. [Google Scholar]
- 28.Peterson R.F., Campbell A.B., Hannah A.E. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can. J. Res. 1948;26:297–311. doi: 10.1139/cjr48c-033. [DOI] [Google Scholar]
- 29.Koyshybaev M., Muminjanov H. Guidelines for Monitoring Diseases, Pests and Weeds in Cereal Crops. Food and Agriculture Organization of the United Nations; Ankara, Turkey: 2016. p. 42. [Google Scholar]
- 30.Murray M.G., Thompson W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4326. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayden M., Kuchel H., Chalmers K. Sequence tagged microsatellites for the Xgwm533 locus provide new diagnostic markers to select for the presence of stem rust resistance gene Sr2 in bread wheat (Triticum aestivum L.) Theor. Appl. Genet. 2004;109:1641–1647. doi: 10.1007/s00122-004-1787-5. [DOI] [PubMed] [Google Scholar]
- 32.Weng Y., Azhaguvel P., Devkota R., Rudd J. PCR-based markers for detection of different sources of 1AL.1RS and 1BL.1RS wheat-rye translocations in wheat background. Plant Breed. 2007;126:482–486. doi: 10.1111/j.1439-0523.2007.01331.x. [DOI] [Google Scholar]
- 33.Helguera M., Khan I., Kolmer J., Lijavetzky D., Zhong-qi L., Dubcovsky J. PCR assays for the Lr37-Yr17-Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Sci. 2003;43:1839–1847. doi: 10.2135/cropsci2003.1839. [DOI] [Google Scholar]
- 34.McNeil M., Kota R., Paux E., Dunn D., McLean R., Feuillet C., Li D., Kong X., Lagudah E., Zhang J., et al. BAC-derived markers for assaying the stem rust resistance gene, Sr2, in wheat breeding programs. Mol. Breed. 2008;22:15–24. doi: 10.1007/s11032-007-9152-4. [DOI] [Google Scholar]
- 35.Yu L., Liu S., Anderson J., Singh R., Jin Y., Dubcovsky J., Brown-Guidera G., Bhavani S., Morgounov A., He Z., et al. Haplotype diversity of stem rust resistance loci in uncharacterized wheat lines. Mol. Breed. 2010;26:667–680. doi: 10.1007/s11032-010-9403-7. [DOI] [Google Scholar]
- 36.Kokhmetova A.M., Atishova M.N. Identification of sources of resistance to wheat stem rust using molecular markers. Russ. J. Genet. Appl. Res. 2012;2:486–493. doi: 10.1134/S2079059712060081. [DOI] [Google Scholar]
- 37.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2018. [Google Scholar]
- 38.RStudio Team . RStudio: Integrated Development Environment for R. RStudio, Inc.; Boston, MA, USA: 2016. [Google Scholar]
- 39.Mendiburu F. Agricolae: Statistical Procedures for Agricultural Researc. Comprehensive R Arch. Network; 2019. [(accessed on 25 September 2019)]. R package version 1.3–1. Available online: https://CRAN.R-project.org/package=agricolae. [Google Scholar]
- 40.Paradis E., Claude J., Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 41.Gu Z.G., Gu L., Eils R., Schlesner M., Brors B. circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 42.Galili T. Dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics. 2015;31:3718–3720. doi: 10.1093/bioinformatics/btv428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Signorell A. DescTools: Tools for Descriptive Statistics. Comprehensive R Arch. Network; 2019. [(accessed on 25 September 2019)]. R package version 0.99.28. Available online: https://cran.r-project.org/package=DescTools. [Google Scholar]
- 44.Wickham H., Francois R., Henry L., Muller K. Dplyr: A Grammar of Data Manipulation. Comprehensive R Arch. Network; 2018. [(accessed on 25 September 2019)]. R package version 0.7.6. Available online: https://CRAN.R-project.org/package=dplyr. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.