Abstract
Human cytomegalovirus (CMV) is a highly prevalent herpesvirus worldwide. According to the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO), CMV infects people of all ages, and by the age of five, approximately one-third of children in the United States are infected. Although the infection is generally asymptomatic, it can cause severe disease in immunocompromised patients, transplant and transfusion recipients, as well as newborn neonates. The objective of this study is to systematically review published literature on CMV in the MENA region to estimate its incidence in the region and describe its epidemiological and clinical significance. The literature was searched through four scientific databases: PubMed, Scopus, Science Direct, and Web of Science. A total of 72 studies from 11 countries satisfied the inclusion criteria, covering a period from 1988–2019. The CMV IgG seroprevalence ranged from 8.7–99.2% (SD = 38.95%). CMV incidence in these countries ranged between 1.22% and 77% in transplant and transfusion recipients, with an increase in incidence with advanced age. However, the incidence rate was unclear for congenital CMV due to the variability of the reporting. This review highlights the need for more robust and well-designed studies to better estimate CMV incidence in the MENA region, standardize diagnostic criteria, and consider prophylactic and pre-emptive treatments to limit the morbidity and mortality of the disease.
Keywords: cytomegalovirus, transplantation, transfusion, congenital, MENA region
1. Introduction
Cytomegalovirus (CMV) is a global herpesvirus that is highly prevalent worldwide. It is a ubiquitous virus with a prevalence of about 100% in both Africa and Asia, and 80% in Europe and North America [1]. It is a member of the family Herpesviridae and genus Cytomegalovirus [2]. CMV is classified as a β-herpesvirus (HHV-5) and considered to be the largest herpesvirus to infect humans, with a genome of nearly 240 kb [3]. According to the Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO), CMV can infect people of all ages; over 50% of adults are infected with CMV by the age of 40, and approximately one in three children are infected with CMV by the age of five in the United States [4]. Although CMV infection is usually asymptomatic, it could lead to severe outcomes in immunosuppressed individuals, particularly transplant recipients and blood transfusion patients [5]. CMV disease can mimic a range of different manifestations and pose significant diagnostic challenges, leading to late or inaccurate diagnosis and adverse health outcomes [6]. Therefore, CMV infection is considered a critical health concern for high-risk populations.
Since CMV is a persistent latent virus, it can manipulate and evade the immune system. Studies have reported multiple genes in CMV that are responsible for immune evasion that profoundly interfere with both the innate and adaptive immunity of the host, thus preventing viral elimination [7]. Nevertheless, following primary infection, CMV and the host’s immune system reach a homeostatic balance, where a lifelong latency is established primarily in cells of the myeloid lineage [8]. Although reactivation phases can occur, their detection is rare in immunocompetent individuals. The continuous commitment of the immune system to control the viral infection throughout the host’s life places a heavy burden on the host’s immune system, which could lead to vascular diseases and immune senescence in the elderly [9]. Significant suppression of the host immune responses against CMV can alter the life-or-death immune surveillance balance, allowing CMV reactivation or primary infection to cause clinical manifestations [10,11,12,13,14]. Such cases could be observed with transplantation patients that need to undergo immune suppression therapy. CMV is one of the most frequently reported opportunistic viral pathogens in immune-deficient patients, including solid organ transplant (SOT) and hematopoietic stem cell transplant (HSCT) recipients [15]. The uncontrolled viral replication and dissemination can be life-threatening, as it could result in end-organ damage [1]. Furthermore, CMV is also a leading cause of congenital diseases worldwide, resulting in about 29 congenital conditions as reported from the United States alone [16].
Notably, there are significant differences in terms of CMV seroepidemiology between different populations and ethnic backgrounds. Since CMV epidemiological studies are scarce in the MENA region, it was of interest to conduct a systemic review to paint a picture of the current status of CMV and evaluate its incidence rate in high-risk sub-populations in the MENA region, namely transplant and transfusion recipients and the newborns.
2. Methods
2.1. Search Strategy
We conducted a systematic review of all literature published on CMV in the MENA region using four databases: PubMed, Scopus, Science Direct, and Web of Science. The search covered all literature within the databases up to March 2019. The databases were queried with the keywords: “CMV”, “Cytomegalovirus”, “human herpesvirus 5”, and “HHV-5”. Each keyword was queried individually with the name of each of the countries in the MENA region. The countries considered as part of the MENA region in this review were: Algeria, Bahrain, Djibouti, Egypt, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Sudan, Syria, Tunisia, United Arab Emirates, and Yemen. In addition to the names of the countries, the terms “west bank”, “Gaza”, “UAE”, “United Arab Emirates”, “Emirates”, “KSA”, “Middle East”, “North Africa”, and “MENA” were used to account for alternate names and ensure complete coverage of the region. PubMed, Science Direct, and Web of Science were searched without filters. Scopus was searched while filtering out books, book chapters, and commentaries. All retrieved citations were imported into EndNote X8, and duplicates were removed using the EndNote X8 built-in “Find Duplicates” feature. Finally, the titles and abstracts of the remaining citations were screened to remove any irrelevant articles, such as plant or animal viruses and human viruses other than CMV.
2.2. Study Selection
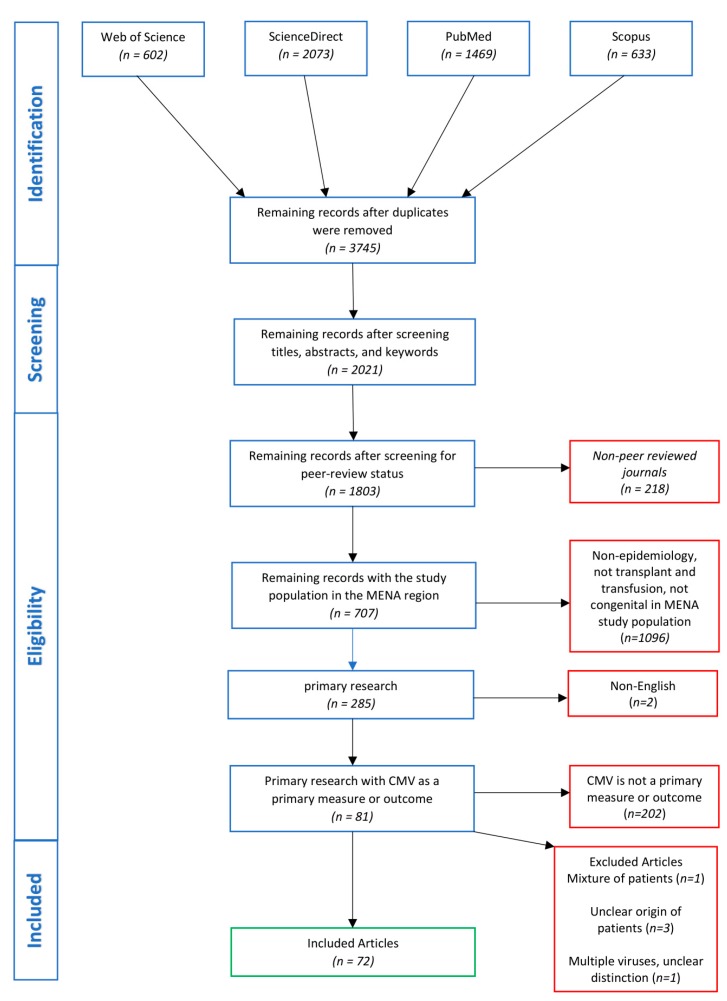
The following inclusion criteria were used in study selection: (i) published in a peer-reviewed journal, (ii) articles studying the epidemiology CMV in the MENA region, (iii) published as primary research articles, (iv) articles published in English or with an abstract in English, and (v) articles in which CMV is a primary measure or outcome of the study. The peer-review status of the published studies was confirmed using Ulrich’s Periodicals Directory (https://www.ulrichsweb.com/). Only studies that satisfied all five inclusion criteria were included in this review. Studies were excluded from the review if: (i) the study consisted of a mixture of patients from within and without the MENA region, and it was not possible to differentiate the data, (ii) it is not clear to differentiate the origins of the patients, and (iii) the study investigated multiple viruses, including CMV, and the data could not be differentiated. A schematic of the search strategy and study selection process is shown in Figure 1.
Figure 1.
Flow diagram of the search strategy and article selection.
2.3. Data Extraction and Analysis
The studies included in this systematic review were analyzed three times by the same individual to ensure accurate capture of the information. The analyzed data included the country of the study, period of study, number of subjects, type of the study, incidence/prevalence of CMV antibodies or infection, symptoms and complications, proposed risk factors and routes of transmission, as well as treatment and prevention.
3. Results
3.1. Search Findings
The search yielded 4777 studies, of which 3745 citations remained after removing duplicates (Figure 1). After screening the titles, abstracts, and keywords, 1724 citations were excluded. The removed citations included irrelevant studies, such as those on animal and plant viruses. The remaining 2021 citations were screened against the eligibility criteria. Of these, 218 studies were removed due to the lack of peer-reviewing, and 1096 were removed for not discussing the epidemiology of CMV in transplant and transfusion patients nor its congenital illness. Furthermore, 422 non-primary research studies were removed (i.e., review articles, editorials, communications, case-reports, etc.), two for being published in languages other than English with no English abstract, and 202 for not having CMV as a measure or outcome of the study. One study was excluded as it was retracted by the authors. Finally, five studies were excluded as they met the exclusion criteria. The remaining 72 studies covered 11 countries (Egypt, Iran, Iraq, Israel, Jordan, Kuwait, Oman, Palestine, Saudi Arabia, Sudan, and Tunisia) over 31 years, from 1988–2019. The highest number of studies was from Iran (n = 27), followed by Israel (n = 21). No studies meeting the eligibility criteria were found from Algeria, Bahrain, Djibouti, Libya, Morocco, Qatar, Syria, the United Arab Emirates, and Yemen.
3.2. Epidemiological Findings
The reviewed studies covering CMV incidence in transplant recipients included renal transplants (n = 15), hematopoietic stem cell transplant (HSCT; n = 4), bone marrow transplants (BMT; n = 3), liver transplants (n = 3), and coronary artery bypass graft (n = 1). Country-wise, the majority of the studies were from Iran (n = 19). The remaining studies were published from Saudi Arabia (n = 4), Egypt (n = 2), Israel (n = 2), Kuwait (n = 2), Iraq (n = 1), Jordan (n = 1), Oman (n = 1), Sudan (n = 1), and Tunisia (n = 1). Overall, CMV infection among transplant recipients has been reported with an incidence ranging from 1.22% to 72%, regardless of prophylactic treatment. On the other hand, the anti-CMV IgG seroprevalence rate ranged between 8.7% and 99.2% (SD = 38.95), being mostly reported in Iran (Table 1). Fewer studies reported the IgM seroprevalence (n = 3), which ranged from 1.6% to 9.6%. The design and criteria to describe CMV disease in these studies were inconsistent (Table 1). Furthermore, multiple studies reported an association between increased incidences of CMV and advanced age. Other risk factors that were found include anti-rejection therapy, previous exposure to CMV, serological mismatch between the donor and recipient, as well as, several immunological determinants. However, there was a lack of CMV epidemiological studies from several countries in the region (Algeria, Bahrain, Djibouti, Lebanon, Libya, Morocco, Palestine, Qatar, Syria, the UAE, and Yemen), rendering the actual incidence of CMV across the region undetermined. As for congenital CMV, the studies were also inconsistent with their diagnosis and reporting of the results. Therefore, accurate incidence/prevalence data could not be deduced. Furthermore, the majority of the studies report infected cases with no incidence/prevalence data. The 38 studies with epidemiological information included in this review came from Israel (n = 19), Iran (n = 9), Iraq (n = 2), Egypt (n = 2), Kuwait (n = 1), and Oman (n = 1).
Table 1.
Cytomegalovirus (CMV) in transplant and transfusion recipients in the MENA region.
| Country | Study Type | Study Period | Transplant Type | No. of Patients | Seroprevalence | Median Time of Detection after TX | Symptoms and Complications | Reference |
|---|---|---|---|---|---|---|---|---|
| Iran | Retrospective study | 1984–2002 | Kidney | 1925 | 5.2% | 1 to 9 months | Elevated serum creatinine, fever, thrombocytopenia, nausea, vomiting, elevated alkaline phosphatase, leukocytosis, leukopenia, rarely pneumonia, conjunctivitis, vascular dermatitis. | Pour-Reza-Gholi et al., 2005 [28] |
| Iran | Retrospective study | 1984–2007 | Kidney | 2211 | 2.1% | NM | 1 patient died, 3 lost their allograft function | Nemati et al., 2008 [20] |
| Iran | Cross-sectional study | 1991–2010 | Kidney | 96 | 37.5% | NM | NM | Khameneh et al., 2013 [57] |
| Tunisia | Cohort study | 1994–1998 | Kidney | 18 | 72% | First 30 days | Six patients had acute rejection | Charfeddine et al., 2002 [21] |
| Iran | Retrospective study | 1996–2007 | Liver | 22 | 4.5% | NM | NM | Yaghobi et al., 2010 [58] |
| Saudi Arabia | Cross-sectional | 1996–2014 | Donors | 263 | 13.2% | NM | NM | Alsuhaibani et al., 2015 [51] |
| Iran | Retrospective study | 1998–2014 | Kidney | 725 | 24.6% | First 5 months | Weakness, fever, respiratory symptoms | Babazadeh et al., 2017 [27] |
| Kuwait | Cohort Study | 2000–2005 | Kidney | 54 | 11.11% | NM | NM | Madi et al., 2011 [59] |
| Saudi Arabia | Retrospective study | 2000–2006 | Kidney | 689 | 3.6% | NM | Fever, malaise, leukopenia | Basri et al., 2007 [60] |
| Iran | cohort study | 2001–2002 | BMT | 15 | 53.5% | 4 weeks | Fever; gastrointestinal; skin lesion; retinitis, pneumonia, UTI | Behzad-Behbahani et al., 2004 [24] |
| Egypt | Retrospective study | 2001–2003 | BMT | 28 | 39% | NM | NM | Zekri et al., 2004 [42] |
| Iran | Cross-sectional | 2002–2004 | Kidney | 179 | 17.6% | NA | Fever, malaise, arthralgias, myalgia, leukopenia and/or thrombocytopenia, or tissue-invasive disease | Pourmand et al., 2007 [61] |
| Iran | Retrospective study | 2002–2006 | BMT | 104 | IgG seroprevalence: 8.7% IgM Seroprevalence: 9.6% | 1st–10th weeks | NA | Ramzi et al., 2009 [62] |
| Saudi Arabia | Retrospective study | 2002–2007 | Cord blood | 73 | 58.9% | NM | NM | Al-Hajjar et al., 2011 [34] |
| Israel | Cohort study | 2003 | Liver | 81 | 8.5% | NA | Fever, disturbed liver functions in all patients, one patient had concurrent CMV pneumonitis and one CMV retinitis | Shibolet et al., 2003 [29] |
| Iran | Retrospective study | 2005–2008 | Kidney | 925 | IgG seroprevalence: 100% | NA | NA | Saghafi et al., 2009 [63] |
| Saudi Arabia | Retrospective study | 2005–2011 | HSCT | 82 | 1.22% | NA | NA | Al-Sweedan et al., 2017 [64] |
| Jordan | Retrospective study | 2005–2013 | HSCT | 72 | 31% | 23 days (12–31) post-transplantation | None of the patients developed CMV disease | Hussain et al., 2015 [65] |
| Sudan | Retrospective study | 2006 | Kidney | 98 | 32.7% | 2–3 months after kidney transplantation | Fever, diarrhea, hepatitis, neutropenia and/or thrombocytopenia. | Enan et al., 2011 [25] |
| Iran | Prospective study | 2006–2008 | Kidney | 40 | Infection a: 82.5% Disease b: 25% |
Infection: 4.7 weeks Disease: 11 weeks | CMV disease, nine patients manifested with elevated serum creatinine values and one, elevated liver enzymes | Taherimahmoudi et al., 2009 [41] |
| Iran | Retrospective study | 2006–2013 | Liver | 145 | 32% | 12 to 445 days post transplantation | Only 1 patient (2%) developed CMV disease | Davoudi et al., 2014 [66] |
| Oman | Retrospective study | 2006–2015 | Kidney | 703 | 14.5% | 21 months (15 days–84 months) | Fever, diarrhea, pneumonitis, lymphopenia, anemia, thrombocytopenia | Siddiqui et al., 2017 [26] |
| Iraq | Cross-sectional study | 2007–2008 | Kidney | 43 | 97.7% | NA | NA | Al-Alousy et al., 2011 [67] |
| Iran | Cross-sectional | 2007–2010 | Transfusion | 96 | IgG Seroprevalence: 77.4% IgM seroprevalence: 7.1% |
NA | NA | Sepehrvand et al., 2010 [54] |
| Israel | Retrospective study | 2007–2012 | HSCT | 121 | 61% | NA | First CMV infection with myeloablative conditioning and acute GVHD | Cohen et al., 2015 [22] |
| Iran | Prospective study | 2008 | Kidney | 68 | 70.6% | NA | 19 cases of acute rejection | Khameneh et al., 2008 [68] |
| Iran | Cross section | 2009–2010 | Kidney | 91 | 34.4% | 30 days | NA | Nasiri et al., 2011 [69] |
| Iran | Retrospective study | 2011–2013 | HSCT | 126 | 34% | 40 days (3–77) after transplantation | GI, dermal symptoms with hepatic involvement * 9 cases develop GVHD |
Valadkhani et al., 2016 [23] |
| Iran | Case-control study | 2012–2013 | coronary artery bypass graft (CABG) | 110 | CMV DNA in Cases: 14.5% CMV DNA in Controls: 4% | NM | CMV in aortic plaques associated with increased risk of atherosclerosis | Heydar et al., 2015 [30] |
| Iran | Cross-sectional | 2012–2013 | Kidney Graft | 96 | 19.8% | NM | NM | Khameneh et al., 2013 [57] |
| Iran | Cross-sectional | 2012–2013 | Donors | 1008 | IgG seroprevalence: 99.2% IgM seroprevalence: 1.6% | NA | NA | Safabakhsh et al., 2013 [53] |
| Kuwait | Retrospective study | 2012–2014 | Kidney | 1168 | 15.4% | NA | 41% have graft rejection, 34.4% develop systemic CMV disease, 24.5% develop CMV syndrome, 1.6% died | Madi et al., 2015 [19] |
| Iran | Cohort study | 2013 | Kidney | 82 | 49% | Four months post-transplantation | The study aimed to correlate CMV infection with decreasing in vitamin D level. | Saber et al., 2015 [70] |
| Egypt | Cross-sectional | 2016 | Donors | 88 | IgG Seroprevalence: 96.6% | NA | NA | Gawad et al., 2016 [56] |
* NM: Not mentioned; NA: Not applicable, due to the subjects being healthy individuals. a: Infection was defined by the presence of anti-CMV IgG, anti-CMV IgM, CMV DNA, or a positive result for the pp65 antigenemia assay. b: Disease was defined by the presence of symptoms.
4. Discussion
To the best of our knowledge, this is the first systematic review study that investigated the epidemiology and status of CMV in immunocompromised patients in the MENA region. A total of 72 out of 3745 screened studies, covering 11 countries, were examined transplantation and blood transfusion recipients as well as the congenital CMV.
4.1. CMV in Transplantation Recipients in the MENA Region
CMV infects up to 60–100% of people in adulthood, and it is one of the main agents involved in infectious complications after transplantation [17]. It threatens the survival of transplant recipients and the function of the transplanted organ. Graft rejection and graft-versus-host disease (GVHD) are multisystem disorders that are common complications of transplantation. GVHD occurs when immune cells transplanted from a non-identical donor (the graft) recognize the transplant recipient (the host) as foreign, thereby initiating an immune reaction that causes disease in the transplant recipient [18]. A less well-established risk factor for GVHD is the CMV status of the donor and host [19,20,21,22,23]. Fortunately, it rarely results in mortality, with only one study reporting severe effects [20]. Generally, infection with CMV results in systemic viral syndrome, as shown in Table 1. The main manifestations are characterized by fever, malaise, vomiting, leukopenia, thrombocytopenia, and elevated liver enzymes. Upper digestive tract symptoms and pain are also common [23,24,25,26]. Furthermore, respiratory illnesses, including pneumonia, have been reported in a few studies [24,27,28,29]. Few studies reported other illnesses including hepatitis [25], urinary tract infection [24], rhinitis [28,29], skin conditions [23,24], and rarely, aortic plaques [30]. The disease caused by post-transplant CMV occurs due to the transplantation of an infected organ, reactivation of latent infection, or after primary infection in seronegative transplant patients [31,32]. The occurrence of disease caused by CMV in transplanted patients varies according to the organ transplanted, the serological match between recipient and donor, the immunosuppressive drugs used, and, most importantly, the interference of any additional diseases [17]. The incidence of CMV infection is 50% to 75% in patients undergoing heart-lung or lung transplantation and 50% in patients undergoing pancreas or kidney-pancreas transplantation. The incidence of CMV infection is 9% to 23% after heart transplantation, and 22% to 29% after liver transplantation [33]. In addition, 30% of patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) and approximately 5% of patients undergoing autologous HSCT develop CMV disease [34]. Moreover, a study on CMV infections in the context of HSCT showed that 68% of pediatric patients who received umbilical cord blood transplantation were CMV seropositive [34].
Multiple studies investigated the association between the risk of CMV infection in transplant patients’ and their demographics, including age, gender, and source of transplantation; however, results were inconsistent. A recently published study from Iran reported that CMV was diagnosed in 178 out of 725 (24.6%) kidney recipients, and showed that the incidence of CMV disease in kidney transplant patients within the age group 41–60 was four-fold more compared to those under 20 years old [27]. Similarly, a study conducted on HSCT in Iran revealed a positive correlation between the age of the recipients and CMV antigenemia [23]. On the other hand, other studies, such as in Saudi Arabia, failed to show this correlation [35]. These observations, along with those from other countries around the globe [36], indicate that age was a risk factor for CMV infection, especially in transplant patients [37,38]. For instance, a study reported that the risk of death was significantly increased in patients >38 years old, who underwent transplantation with peripheral blood, with an unrelated or mismatched donor, and who developed a CMV infection [39]. In addition, a study conducted on 200 allografted patients showed that recipients aged over 16 years were found to be at significant risk of CMV reactivation compared with younger patients (p = 0.007) [40].
In addition to age, several other factors have been identified in association with CMV incidence in SOT and HSCT recipients. One of the most significant risk factors is the extensive use of immunosuppressive drugs for patients undergoing organ transplantation. For instance, Taherimahmoudi et al. suggested that immunosuppression therapy using lymphocyte-depleting antibodies, namely anti-thymocyte globulin (ATG) therapy, is considered as one of the leading risk factors for CMV disease [41]. Charfeddine et al. reported that 12 out of the 16 patients who received kidney transplants suffered from CMV infections. Additionally, 6 out of those 12 patients developed acute rejection episodes due to CMV infection after administrating additional immunosuppressive treatment [21]. Such high frequency of infection was attributed to intensive antirejection therapy (including azathioprine, prednisone, cyclosporine, and ATG), among other factors such as previous exposure to CMV prior to transplantation [42], serological mismatch between both donor and recipient (e.g., CMV-negative recipients (R−)) received grafts from CMV-positive donors (D+)), and immune system factors.
Several immune correlates were identified as predictors for post-transplant CMV infection. One of them is mannose-binding-lectin (MBL), an innate humoral immunity protein that is important for pathogen opsonization and activation of complement pathways. One study documented a positive correlation between the infection and low levels of MBL. MBL deficiency compromises the innate immune response, resulting in a higher risk of developing post-transplant CMV disease and increasing the necessity of prophylactic treatment [43]. Other factors include CD 56+ T-cell levels, HLA mismatch, and GVHD. CD56+ T-cells (also known as NK-like T cells or cytokine-induced killer cells) have a potent cytotoxic effect on CMV, and a significant increase in their levels in renal transplant patients is suggestive of current CMV infection [44]. As for HLA, CMV disease was found to be influenced by more than one HLA allele mismatch [45]. Moreover, some studies have shown that specific alleles can promote or protect from post-transplant CMV disease. For instance, Khalifa et al. reported that 33.3% of CMV pp65 antigenemia positive patients in SOT have the HLA-A * 02 genotype, while patients with HLA-A * 01 (57.1%) had a protective effect against CMV infection [46]. Another study showed that kidney recipients with the HLA-B44 allele are more susceptible to CMV infection after transplantation, while carriers of the HLA-B8 allele are naturally protected from CMV infection [47]. Furthermore, a study showed that specific single-nucleotide polymorphisms (SNPs) in the loci of co-stimulatory molecules CTLA4 and CD28, which function in the regulation of T-cell activation, influence active CMV infection in kidney transplant patients [48]. Overall, these studies suggest the use of HLA typing as a predictor for CMV infection within the context of personalized medicine for prophylactic post-transplantation treatment.
The high risk of CMV infection and transmission through organ transplantation is universal. The prevalence across the globe ranges from 45% in developed countries to near 100% in developing countries [1]. The health burden of CMV infection and its manifestations seen in the MENA region is similar to that seen in other populations [49,50]. Even though the risk is high for transplant patients in the MENA region, only a few studies suggested screening for CMV in donors. For instance, in Saudi Arabia, screening for CMV and other pathogens before transplantation, especially in HSCT, was found to improve patient safety and mitigate the risk of accidental CMV infection [51]. One of the suggested approaches to protect from post-transplant CMV infection is the treatment with antibodies targeting CMV antigens (pp. 28, 150), which was shown to be effective in kidney transplantation [52]. On the other hand, Shibolet et al. demonstrated the development of late CMV disease and the occurrence of rejection episodes in liver transplantation recipients, regardless of the early use of antiviral prophylaxis [29]. In conclusion, due to the potential abnormalities associated with CMV infection and various morbidities, the establishment of preventive measures, especially vulnerable populations, including transplant recipients, is required.
4.2. CMV in Blood Transfusion Recipients in the MENA Region
CMV infection not only compromises transplantation but can also compromise the effectiveness of blood-transfusions, leading to transfusion-transmitted CMV infection, especially in immunocompromised patients. Transfusion-transmitted CMV infection occurs mainly due to the re-activation of the latent virus in WBCs. Many studies were performed to assess the seropositivity rates in different MENA region countries. Only a few studies, conducted mainly in Iran and Egypt, reported CMV infection after blood transfusion in the MENA region. The type of transfusion mainly discussed by the articles included in this review is whole blood transfusion; this is because most of the patients that need regular and frequent whole blood donations are anemic and thalassemic patients such as transfusion of other blood products like plasma transfusion, which is safer since most of the time it is autologous. That is why all research done on CMV seroprevalence was about patients with thalassemia who received multiple types of blood from different donors that could have been positive for the virus. A recent study in Mashhad, Iran, showed that out of 1008 blood samples that were tested for CMV antibodies, 99.2% were found to be positive [53]. In another study from Iran, Sepehrvand et al. reported that the high incidence of CMV antibodies that were present in transfusion patients’ blood is a result of receiving blood from CMV infected donors [54].
Furthermore, Mahmoud et al. showed that frequent blood transfusions among thalassemic children in Upper Egypt exposed them to a higher risk of transfusion-transmitted infections, including CMV. In this study, high rates of CMV infections were reported in children receiving a blood transfusion, and the infection was positively correlated with increasing age and the duration of the thalassemia [55]. These high rates of CMV infections are attributed to the high CMV seroprevalence among blood donors in Egypt, as reported by Gawad et al. In their study, 96.6% of blood donors (out of 88 tested blood samples) were CMV seropositive [56].
Overall, these findings demonstrate a high rate of prevalence of CMV infections in the MENA region, and as such, posing serious implications for the blood transfusion practices if proper screening measures are not implemented. High CMV positivity in transfusion patients, highlighted clearly in some of MENA countries (Table 1), assures that there is a crucial need for mitigation plans to reduce CMV transmission in transfusion patients.
4.3. Congenital CMV (cCMV) in the MENA Region
The reviewed studies (n = 38) documented a total of 1271 fetuses and infants from eight countries. The subjects were either confirmed CMV cases with vertical transmission of the virus or unconfirmed seropositive cases [71,72,73,74,75,76,77]. Clinical CMV manifestations were assessed either with antenatal ultrasound or postnatal cCMV associated complications. The majority of the studies came from Israel (n = 19), where the primary clinical features included abnormal ultrasound, severe brain dysmorphology (mainly in the first and second trimesters), hearing abnormalities, as well as occult CNS symptoms that are associated with neurologic sequelae, abortion, premature deaths, and congenital malformations [71,74,78,79,80,81,82,83,84,85,86]. On the other hand, some studies reported asymptomatic cCMV infections [87,88].
In terms of disease morbidity, one of the common complications of cCMV observed in the MENA region is abnormal brain sonography. Hadar et al. reported a 67% occurrence of abnormal brain sonography in infants born to mothers with primary maternal infection, and 8.3% in infants born to mothers with non-primary infection [89]. Another common complication of cCMV is hearing loss. A retrospective study in Israel correlated early cCMV infection with hearing loss [79]. In addition, cCMV could lead to hepatic damage, including hepatitis and cholestatic disease [88]. The frequency of which (6.6%) was found to be less than was previously expected and is far out shadowed by CNS involvement (84.6%) and hearing loss (53.8%) [88]. In all cases, however, rapid antiviral treatment showed improvement in symptoms, albeit over prolonged periods [79,88]. As for mortality, higher rates were found in association with primary cCMV infections compared to recurrent infections, and abortions were reported at all pregnancy stages [73,90]. Jahromi et al. found that there is an association between seropositivity and abortion rate [91].
Nevertheless, there is substantial variability in the definitions of symptomatic and asymptomatic cCMV infection, study designs, and the methods of determining CMV infection. This variability, along with the variability in study design and detection methods, created difficulty in assessing the overall status of cCMV in the region (Table 2). Similar variations are also seen in the US and Germany, suggesting complicated disease manifestations, depending on the genetic, health, and nourishment status of the infants and their mothers [92,93,94,95]. Still, the incidence rate of cCMV in the MENA region seems to be higher than in other populations. Schlesinger et al. reported an incidence of 945/135,000 (0.7%) births in 2005 in Israel [96,97]. This incidence rate is similar to that found in the United States (0.6–0.7%) [98]. The rate is, however, higher than in Europe, to which Israel’s healthcare system is more similar. For instance, Sweden and the UK have incidence rates of 4.6/1000 births (0.46%) and 3.2/1000 births (0.32%), respectively, and a decades-long prospective study in Denmark found the incidence rate to be 0.4% [99,100].
Table 2.
CMV in the MENA region.
| Country | Study Type | Study Period | No. of Patients | CMV Results | Symptoms and Complications | Reference |
|---|---|---|---|---|---|---|
| Kuwait | Experimental study | 1988 | 575 infants | 2.6% positive IgM | NM | El-Mekki et al., 1988 [101] |
| Israel | Retrospective study | 1993–1997 | 63 pregnant women | 34.8% showed vertical transmission | Abnormal ultrasound, neurologic sequelae | Lipitz et al., 1997 [76] |
| Iraq | Prospective-follow up until delivery | 1999 | 60 pregnant women | 10% CMV IgM in cord blood | Congenital malformations, microcephaly | Al-Ali et al., 1999 [86] |
| Israel | Retrospective study | 1999–2008 | 59 primary Periconceptional CMV infection | 18.6% CMV infections | NM | Hadar et al., 2010 [73] |
| Israel | Retrospective study | 2001–2012 | 9845 infants | 0.57% CMV infection | Abnormal hearing | Barkai et al., 2014 [81] |
| Iran | case-control study | 2002–2003 | 95 with sensory hearing loss | 34.6% CMV infection | Sensorineural hearing loss | Pasternak et al., 2018 [102] |
| Iran | This case-control study | 2003–2004 | 250 women with a history of abortion and 200 matched with no abortion | 5% positive for CMV | Abortion | Jahromi et al., 2010 [91] |
| Israel | A prospective study | 2005 | 70 infants who received breast milk from seropositive mothers | 5.7% acquired CMV by the second or third week of pregnancy | NM | Miron et al., 2005 [103] |
| Israel | Experimental study | 2005 | 5000 Newborns | 81.5–85% serum IgM 0.7% had cCMV infection | NM | Ziyaeyan et al., 2007 [97] |
| Israel | Retrospective study | 2005–2013 | 149 | 36% CMV infection | Severe hearing loss | Bilavsky et al., 2016 [104] |
| Israel | Retrospective-cohort study | 2005–2012 | 210 infants with cCMV | 75% symptomatic 25% asymptomatic |
Prematurity, abnormal hearing, lenticulostriate vasculopathy | Bilavsky et al., 2015 [72] |
| Israel | Retrospective study | 2005–2013 | 284 infants with cCMV | 69.7% symptomatic 30.3% asymptomatic |
Hepatitis, cholestatic disease | Bliavsky et al., 2015 [88] |
| Palestine | Retrospective study | 2006–2012 | 249 newborns | 4 out of 249 newborns with cCMV born to mothers with positive CMV DNA in urine | NM | Neirukh et al., 2013 [105] |
| Israel | Retrospective case-control study | 2006–2015 | 138 infants with cCMV | 66.67% positive with amniocentesis | Abnormal hearing, developmental delay | Bilavsky et al., 2016 [106] |
| Iran | Experimental study | 2007 | 92 pregnant women with caesarian section | 98% of women had positive serum IgG 5.4% of women had positive serum IgM Neonates from IgG positive mothers had positive IgM |
NM | Townsend et al., 2013 [107] |
| Iran | Experimental study | 2008 | 844 pregnant women | 93% had positive serum IgG 5% had positive serum IgM |
Congenital disorders | Arapour et al., 2008 [83] |
| Israel | Observational study | 2008 | Twenty-eight pregnant mothers primary CMV infection acquired after 25 weeks of gestation | 21 neonates had a vertical transmission with no symptoms One pregnancy was terminated in 36 weeks with apparent symptoms |
All 21 infected neonate showed clinical symptoms of CMV infection | Gindes et al., 2008 [75] |
| Israel | Retrospective study | 2009 | All pregnant mothers with positive IgM & high IgG Avidity | 79 women with CMV IgM-high IgG avidity combination (indicate past infection) | NM | Kanengisser -Pines et al., 2009 [108] |
| Israel | Retrospective study | 2009–2010 | 8105 infants | 0.28% prevalence | CNS involvement, abnormal hearing | Barkai et al., 2013 [82] |
| Israel | Cohort study | 2010 | 27 cCMV infected fetuses | Temporal lobe volumes were significantly smaller in fetuses infected with CMV compared to uninfected fetuses | Severe brain dysmorphology in first and second trimesters. | Hoffman et al., 2010 [74] |
| Egypt | Experimental study | 2011 | 33 neonate and mothers | Four neonates with positive IgM, two of which had mothers with positive IgM | Gastrointestinal complications | Abu Faddan et al., 2011 [109] |
| Israel | Prospective study behavioral studies of LSV symptoms | 2011 | 92 infants with congenital CMV | 50 cases had lenticulostriate vasculopathy and hearing loss. | CNS impairment, abnormal hearing | Amir et al., 2011 [80] |
| Israel | Retrospective study | 2011 | Infected infants (CMV DNA positive) | NM | Abnormal white matter | Farkas et al., 2011 [78] |
| Oman | Retrospective review | 2011–2012 | 373 infants | 34 positives cases | Death, prolonged PICU stay, respiratory complication | Abdelmogheth et al., 2014 [90] |
| Kuwait | Prevalence study-follow up until pregnancy | 2013 | 983 pregnant mothers | 9% positive cord blood IgM 0.9% positive urine PCR. Seven of the nine cases had a high viral load |
NA | Al-Awadhi et al., 2013 [110] |
| Egypt | Cross-sectional study | 2013 | 546 pregnant women | 100% positive serum IgG 7.3% positive serum IgM with an intermediate IgG avidity index. Of these, 50% had higher avidity indices after the 3rd trimester. |
NM | Kamel et al., 2014 [111] |
| Israel | Prospective cohort study | 2013 | 142 pregnant women with primary CMV infection and vertical transmission in the 1st and second trimesters | The primary infection occurred in the 1st (50%) and second (50%) trimester Seven pregnancies terminated with neurologic sequelae one neonate died due to neurologic complications |
Auditory damage or neurodevelopmental disabilities | Lipitz et al., 2013 [77] |
| Iran | Prevalence study | 2013–2014 | 100 symptomatic infants less than 3-weeks old | 58% with cCMV | Hearing loss | Ebrahimi-Rad et al., 2017 [96] |
| Iran | Cross-sectional study | 2014 | 225 pregnant women and their newborns | 100% of mothers had positive IgG, of which 2.7% had positive IgM Women with normal deliveries showed low IgG compared to caesarian section |
CMV infection by radiological evaluation (CT scan) | Erfanianahmadpoor et al., 2014 [112] |
| Israel | Retrospective study | 2014–2015 | 178 infants with hearing disability | 2.2% with cCMV | CNS symptoms | Ari-Even Roith et al., 2017 [79] |
| Iran | Prospective study | 2014–2016 | 1617 neonate | 0.49% with cCMV | Short-term growth impairment | Karimian et al., 2016 [84] |
| Sudan | Experimental study | 2015 | 50 infants | 8% with cCMV | Congenital anomalies | Ebrahimet al., 2015 [113] |
| Sudan | Experimental study | 2016 | 90 pregnant women | 98.9% had positive serum IgG 1.1% had positive serum IgM |
NA | Altayeb et al., 2016 [114] |
| Israel | Retrospective cohort study | 2016 | 98 infants from infected mothers | 52 received antiviral upon delivery | Lenticulostriate vasculopathy on postnatal US, Sensorineural hearing loss | Amir et al., 2016 [71] |
| Iran | Case control study | 2016 | 81 pregnant women who aborted | Anti-CMV IgM was higher compared to controls (25.9% compared to 12.2%; OR = 12.2, p = 0.019) | Early abortion | Rasti et al., 2016 [85] |
| Israel | Retrospective cohort study | 2017 | 107 infants with cCMV 95 of which are from mothers with primary infection, 12 from mothers with non-primary infection | Incidence of abnormal brain sonographic findings high in infants of mothers with primary infection was 67%, compared to non-primary infection 8.3% | Infant’s Mothers acquired gestational hypertensive disorder and GDM | Hadar et al., 2010 [89] |
| Iraq | This prospective study | 2019 | 24 neonates | 96% with CMV infection | Jaundice-, hepatosplenomegaly | Alwan et al., 2019 [115] |
| Iran | Pilot study | January 2012 to March 2012 | 620 infants | 0.32% positive for CMV DNA | Infected infants showed no symptoms | Fahimzad et al., 2013 [87] |
* NM: Not mentioned; NA: Not applicable, due to the subjects being healthy individuals; cCMV: Congenital CMV; EP: Ectopic pregnancy; CNS: Central nervous system; FT: Liver function; RF: Rheumatoid factor; CT: Computed tomography; GDM: Gestational diabetes mellitus.
4.4. Laboratory Diagnosis of CMV in the MENA Region
CMV diagnosis was traditionally performed by serologic testing and viral cultures from multiple samples; however, molecular diagnosis is currently the standard [116]. A similar trend can be seen in the MENA region with the diagnosis of CMV. In the 1980s, culture and serological methods were used to detect CMV. The trend moved towards molecular testing, with polymerase chain reaction (PCR) becoming more prominent over time. In the reviewed literature, multiple diagnostic laboratory tools were used, including serology, PCR, antigenemia assays, immunohistochemistry, and culture.
Serological methods indirectly provide evidence of current or prior infection by detection of antibodies in serum. The presence of anti-CMV IgM antibodies can be used to diagnose a recent or acute infection, or at least a fourfold increase in IgG antibody titer in subsequent specimens obtained two to four weeks apart [116]. However, IgM antibodies can persist for several months, leading to false-positive results for acute infection [117]. Thus, a definite diagnosis of CMV infection cannot be obtained from serological tests. Nevertheless, serological antibody detection remains a cost-effective screening tool. Serological methods alone, or in conjunction with other methods, are employed in the MENA region to screen for CMV before and after transplantation [23,47,52,57,63,68,70,118,119,120,121,122,123,124]. Moreover, serology is used in Israel to screen pregnant women for CMV infection. Although no formal regulations exist, still, most hospitals perform routine screening for anti-CMV antibodies using the criteria mentioned earlier to establish a diagnosis [74,125,126]. Once the presence of CMV is established in the mother, congenital CMV is diagnosed either by pre-natal amniocentesis followed by culture or PCR, or by PCR or antigenemia assays in the urine of the infant [73,75,76,78,80,104,108,127].
In recent years, PCR has become prominent in CMV testing in the MENA region. PCR is highly sensitive and specific. However, its main drawback is that it cannot differentiate between latent and active virus [116]. Additionally, there is variability in the viral loads obtained by different laboratories for the same specimen. In response, the WHO developed an international quantitative standard to standardize measurements and facilitate studies to correlate viral load with the development of the disease [128,129]. Nevertheless, the variability among assays continues to exist due to differences in specimen types, DNA extraction methods, as well as the PCR protocol [130,131,132]. Currently, PCR is commonly used in the MENA region for the diagnosis of CMV. It is used to test for congenital CMV in amniotic fluid or urine [73,75,76,78,80,104,108,127], to monitor CMV infection after transplant [41,62,133,134,135,136], as well as end-organ testing when a specific organ is affected, such as HIV associated CMV retinitis [137].
Finally, CMV antigenemia, immunohistochemistry, and culture methods are used in the MENA region as a definite diagnosis of CMV infection. Antigenemia is used in the diagnosis of congenital CMV and the monitoring of viremia in transplant patients. The assay is based on the detection of the CMV pp65 protein in peripheral blood leukocytes. Their advantage over measuring anti-CMV antibodies and PCR is that it can detect the active virus, correlating the result of the assay with viremia [116]. Immunohistochemistry assays use labeled antibodies directed towards CMV antigens (early antigen, immediate early antigens, pp65, and late antigen) and are visualized under the microscope. A positive result in immunohistochemistry indicates the presence of CMV [138]. In the MENA region, this method was only used in studies of tissue biopsies of invasive CMV infections in the case of cancer [139,140,141,142]. This usage is in line with the international consensus for the diagnosis of CMV in invasive tissue disease [138]. As for culture, conventional methods have been replaced by the more rapid shell-vial cultures [116]. Shell-vial culture involves the centrifugation of a patient specimen onto a cell monolayer in a vial, which speeds up the time to the result. The virus is then detected in the monolayer using fluorescent antibodies. The MENA region also sees the same trend of replacing traditional cell culture with shell-vial cultures. In Israel, shell-vial culture is the method in use for the diagnosis of congenital CMV from urine specimens from the infants [72,89,102,118,143,144].
While we summarized in this review the techniques used in CMV diagnosis, they do not necessarily reflect the status of CMV diagnosis in the region. The reviewed studies did not cover all the countries in the MENA region; thus, a more in-depth analysis was not possible. Nevertheless, the information does reflect the availability of the diagnostic tools in the region during the study period. However, even though the diagnostic tools are available and widely used in the region, there is no information on country-wide screening guidelines and policies. Instead, hospitals implement their own practices and guidelines on screening.
5. Conclusions
The literature search yielded a total of 72 primary research articles covering 11 out of the 21 MENA countries over 31 years. The studies on transplant and transfusion recipients reported the incidence of CMV with uncertainty about the disease. Although infection with CMV virus is common among graft recipients in this region (up to 77%), the actual rate of clinically confirmed CMV disease was unclear as most of the studies depend solely on seroprevalence reports. Multiple other studies exist outside of those included in this review; however, they do not measure the incidence of CMV. Although some studies assessed the frequency of transplantation, the CMV incidence as a critical outcome was not reported in many of the studies. Similarly, with blood transfusion, studies report CMV seroprevalence with no clear indication of clinical burden. The same can be observed with cCMV, where substantial variability in cCMV reporting criteria leads to a less-than-accurate picture about the status of cCMV in the MENA region. The low number of published articles, the low number of reporting countries, the inconsistency in measurement and detection protocols, as well as the variability in endpoint evaluation of the clinical illness, are all factors that limited the ability to generalize the information across the region. Thus, there is an incessant need to conduct well-designed studies under the umbrella of the WHO to estimate the burden of CMV disease on transplant recipients in the MENA region and to standardized both the prophylactic and preemptive treatment and predict the subsequent morbidity and mortality.
Acknowledgments
The authors of this research paper would like to acknowledge the Biomedical Research Center in Qatar University for sponsoring the publication fees of this article.
Author Contributions
Conceptualization, G.K.N. and D.W.A.-S.; methodology, H.A.M.; software, H.A.M.; validation, H.A.M. and H.M.Y.; formal analysis, H.A.M., H.M.Y, N.N.Y., A.A.-M., D.W.A.-S., D.A., E.A.N., G.K.N.; investigation, H.A.M., H.M.Y., N.N.Y., A.A.-M., D.W.A.-S., D.A., E.A.N., G.K.N.; resources, H.A.M.; data curation, H.A.M., H.M.Y., N.N.Y., A.A.-M., D.W.A.-S., D.A., E.A.N., G.K.N.; writing—original draft preparation, H.A.M., H.M.Y., N.N.Y., A.A.-M., D.W.A.-S., D.A., E.A.N., G.K.N.; writing—review and editing, H.A.M., N.N.Y., D.W.A.-S., G.K.N., H.M.Y.; visualization, H.A.M.; supervision, G.K.N., H.M.Y.; project administration, G.K.N.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cannon M.J., Schmid D.S., Hyde T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 2.Schottstedt V., Blümel J., Burger R., Drosten C., Gröner A., Gürtler L., Heiden M., Hildebrandt M., Jansen B., Montag-Lessing T. Human cytomegalovirus (HCMV)–revised. Transfus. Med. Hemotherapy. 2010;37:365–375. doi: 10.1159/000322141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolich-Žugich J., van Lier R.A. Cytomegalovirus (CMV) research in immune senescence comes of age: Overview of the 6th International Workshop on CMV and Immunosenescence. Geroscience. 2017;39:245–249. doi: 10.1007/s11357-017-9984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC Cytomegalovirus (CMV) and Congenital CMV Infection. [(accessed on 30 April 2019)]; Available online: https://www.cdc.gov/cmv/overview.html.
- 5.Sylwester A.W., Mitchell B.L., Edgar J.B., Taormina C., Pelte C., Ruchti F., Sleath P.R., Grabstein K.H., Hosken N.A., Kern F., et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancini D., Faddy H.M., Flower R., Hogan C. Cytomegalovirus disease in immunocompetent adults. Med. J. Aust. 2014;201:578–580. doi: 10.5694/mja14.00183. [DOI] [PubMed] [Google Scholar]
- 7.Miller-Kittrell M., Sparer T.E. Feeling manipulated: Cytomegalovirus immune manipulation. Virol. J. 2009;6:4. doi: 10.1186/1743-422X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poole E., McGregor Dallas S.R., Colston J., Joseph R.S., Sinclair J. Virally induced changes in cellular microRNAs maintain latency of human cytomegalovirus in CD34(+) progenitors. J. Gen. Virol. 2011;92:1539–1549. doi: 10.1099/vir.0.031377-0. [DOI] [PubMed] [Google Scholar]
- 9.Pawelec G., Akbar A., Beverley P., Caruso C., Derhovanessian E., Fulop T., Griffiths P., Grubeck-Loebenstein B., Hamprecht K., Jahn G., et al. Immunosenescence and Cytomegalovirus: Where do we stand after a decade? Immun. Ageing I A. 2010;7:13. doi: 10.1186/1742-4933-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limaye A.P., Bakthavatsalam R., Kim H.W., Randolph S.E., Halldorson J.B., Healey P.J., Kuhr C.S., Levy A.E., Perkins J.D., Reyes J.D., et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645–1652. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 11.Steininger C. Clinical relevance of cytomegalovirus infection in patients with disorders of the immune system. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2007;13:953–963. doi: 10.1111/j.1469-0691.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 12.Limaye A.P., Kirby K.A., Rubenfeld G.D., Leisenring W.M., Bulger E.M., Neff M.J., Gibran N.S., Huang M.L., Santo Hayes T.K., Corey L., et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.2008.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffiths P., Whitley R., Snydman D.R., Singh N., Boeckh M. Contemporary management of cytomegalovirus infection in transplant recipients: Guidelines from an IHMF workshop, 2007. Herpes J. IHMF. 2008;15:4–12. [PubMed] [Google Scholar]
- 14.Revello M.G., Zavattoni M., Furione M., Fabbri E., Gerna G. Preconceptional primary human cytomegalovirus infection and risk of congenital infection. J. Infect. Dis. 2006;193:783–787. doi: 10.1086/500509. [DOI] [PubMed] [Google Scholar]
- 15.Razonable R.R. Epidemiology of cytomegalovirus disease in solid organ and hematopoietic stem cell transplant recipients. Am. J. Health-Syst. Pharm. Ajhp Off. J. Am. Soc. Health-Syst. Pharm. 2005;62:S7–S13. doi: 10.1093/ajhp/62.suppl_1.S7. [DOI] [PubMed] [Google Scholar]
- 16.Manicklal S., Emery V.C., Lazzarotto T., Boppana S.B., Gupta R.K. The “Silent” Global Burden of Congenital Cytomegalovirus. Clin. Microbiol. Rev. 2013;26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azevedo L.S., Pierrotti L.C., Abdala E., Costa S.F., Strabelli T.M.V., Campos S.V., Ramos J.F., Latif A.Z.A., Litvinov N., Maluf N.Z., et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo) 2015;70:515–523. doi: 10.6061/clinics/2015(07)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsohn D.A., Vogelsang G.B. Acute graft versus host disease. Orphanet. J. Rare Dis. 2007;2:35. doi: 10.1186/1750-1172-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madi N., Al-Qaser M., Edan R., Al-Nakib W. Clinical Utility of Viral Load in the Management of Cytomegalovirus Infection in Solid Organ Transplant Patients in Kuwait. Transplant. Proc. 2015;47:1802–1807. doi: 10.1016/j.transproceed.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Nemati E., Taheri S., Pourfarziani V., Einollahi B. Cytomegalovirus disease in renal transplant recipients: An Iranian experience. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2008;6:132–136. [PubMed] [Google Scholar]
- 21.Charfeddine K., Kharrat M., Yaich S., Jarraya F., Mkawar K., Hachicha J. Infection in kidney transplant recipients in Tunisia. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2002;13:195–198. [PubMed] [Google Scholar]
- 22.Cohen L., Yeshurun M., Shpilberg O., Ram R. Risk factors and prognostic scale for cytomegalovirus (CMV) infection in CMV-seropositive patients after allogeneic hematopoietic cell transplantation. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2015;17:510–517. doi: 10.1111/tid.12398. [DOI] [PubMed] [Google Scholar]
- 23.Valadkhani B., Kargar M., Ashouri A., Hadjibabaie M., Gholami K., Ghavamzadeh A. The risk factors for cytomegalovirus reactivation following stem cell transplantation. J. Res. Pharm. Pract. 2016;5:63–69. doi: 10.4103/2279-042X.176554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behzad-Behbahani A., Ehsanipour F., Alborzi A., Nourani H., Ramzi M., Rasoli M. Qualitative detection of human cytomegalovirus DNA in the plasma of bone marrow transplant recipients: Value as a predictor of disease progression. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2004;2:196–200. [PubMed] [Google Scholar]
- 25.Enan K.A., Rennert H., El-Eragi A.M., El Hussein A.R.M., Elkhidir I.M. Comparison of Real-time PCR to ELISA for the detection of human cytomegalovirus infection in renal transplant patients in the Sudan. Virol. J. 2011;8:222. doi: 10.1186/1743-422X-8-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siddiqui W.A., Al Salmi I., Jha A., Pakkyara A., Yasir M., Shaheen F.A.M. Early clinical manifestations and laboratory findings before and after treatment of cytomegalovirus infection in kidney transplant patients. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2017;28:774–781. [PubMed] [Google Scholar]
- 27.Babazadeh A., Javanian M., Oliaei F., Akbari R., Akbarzadepasha A., Bijani A., Sadeghi M. Incidence and risk factors for cytomegalovirus in kidney transplant patients in Babol, northern Iran. Casp. J. Intern. Med. 2017;8:23–29. [PMC free article] [PubMed] [Google Scholar]
- 28.Pour-Reza-Gholi F., Labibi A., Farrokhi F., Nafar M., Firouzan A., Einollahi B. Signs and symptoms of cytomegalovirus disease in kidney transplant recipients. Transplant. Proc. 2005;37:3056–3058. doi: 10.1016/j.transproceed.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 29.Shibolet O., Ilan Y., Kalish Y., Safadi R., Ashur Y., Eid A., Shouval D., Wolf D. Late cytomegalovirus disease following liver transplantation. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2003;16:861–865. doi: 10.1111/j.1432-2277.2003.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 30.Heybar H., Alavi S.M., Farashahi Nejad M., Latifi M. Cytomegalovirus infection and atherosclerosis in candidate of coronary artery bypass graft. Jundishapur J. Microbiol. 2015;8:e15476. doi: 10.5812/jjm.15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boeckh M., Geballe A.P. Cytomegalovirus: Pathogen, paradigm, and puzzle. J. Clin. Investig. 2011;121:1673–1680. doi: 10.1172/JCI45449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Souza M.A., Passos A.M., Treitinger A., Spada C. Seroprevalence of cytomegalovirus antibodies in blood donors in southern, Brazil. Rev. Soc. Bras. Med. Trop. 2010;43:359–361. doi: 10.1590/S0037-86822010000400004. [DOI] [PubMed] [Google Scholar]
- 33.Simon D.M., Levin S. Infectious complications of solid organ transplantations. Infect. Dis. Clin. North Am. 2001;15:521–549. doi: 10.1016/S0891-5520(05)70158-6. [DOI] [PubMed] [Google Scholar]
- 34.Al-Hajjar S., Al Seraihi A., Al Muhsen S., Ayas M., Al Jumaah S., Al Jefri A., Shoukri M., El Solh H. Cytomegalovirus infections in unrelated cord blood transplantation in pediatric patients: Incidence, risk factors, and outcomes. Hematol. Oncol. Stem Cell Ther. 2011;4:67–72. doi: 10.5144/1658-3876.2011.67. [DOI] [PubMed] [Google Scholar]
- 35.Alsheikh R., Gabardi S. Post-Renal Transplantation Outcomes in Elderly Patients Compared to Younger Patients in the Setting of Early Steroid Withdrawal. Prog. Transplant. (Aliso Viejo CA) 2018;28:322–329. doi: 10.1177/1526924818800039. [DOI] [PubMed] [Google Scholar]
- 36.Stadler L.P., Bernstein D.I., Callahan S.T., Turley C.B., Munoz F.M., Ferreira J., Acharya M., Simone G.A.G., Patel S.M., Edwards K.M. Seroprevalence and risk factors for cytomegalovirus infections in adolescent females. J. Pediatric Infect. Dis. Soc. 2012;2:7–14. doi: 10.1093/jpids/pis076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staras S.A., Dollard S.C., Radford K.W., Flanders W.D., Pass R.F., Cannon M.J. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 38.Boeckh M., Nichols W.G., Papanicolaou G., Rubin R., Wingard J.R., Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol. Blood Marrow Transplant. 2003;9:543–558. doi: 10.1016/S1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 39.Sousa H., Boutolleau D., Ribeiro J., Teixeira A.L., Vaz C.P., Campilho F., Branca R., Campos A., Jr., Baldaque I., Medeiros R. Cytomegalovirus infection in patients who underwent allogeneic hematopoietic stem cell transplantation in Portugal: A five-year retrospective review. Biol. Blood Marrow Transplant. 2014;20:1958–1967. doi: 10.1016/j.bbmt.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Jaskula E., Bochenska J., Kocwin E., Tarnowska A., Lange A. CMV serostatus of donor-recipient pairs influences the risk of CMV infection/reactivation in HSCT patients. Bone Marrow Res. 2012;2012 doi: 10.1155/2012/375075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taherimahmoudi M., Ahmadi H., Baradaran N., Montaser-Kouhsari L., Salem S., Mehrsai A., Kalantar E., Jahani Y., Pourmand G. Cytomegalovirus infection and disease following renal transplantation: Preliminary report of incidence and potential risk factors. Transplant. Proc. 2009;41:2841–2844. doi: 10.1016/j.transproceed.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Zekri A.R., Mohamed W.S., Samra M.A., Sherif G.M., El-Shehaby A.M., El-Sayed M.H. Risk factors for cytomegalovirus, hepatitis B and C virus reactivation after bone marrow transplantation. Transpl. Immunol. 2004;13:305–311. doi: 10.1016/j.trim.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Ghods F.J., Solgi G., Amirzargar A.A., Nikbin B., Ghods A.J. High frequency of clinically significant infections and cytomegalovirus disease in kidney transplant recipients with serum mannose-binding lectin deficiency. Iran. J. Kidney Dis. 2009;3:28–33. [PubMed] [Google Scholar]
- 44.Almehmadi M., Hammad A., Heyworth S., Moberly J., Middleton D., Hopkins M.J., Hart I.J., Christmas S.E. CD56+ T cells are increased in kidney transplant patients following cytomegalovirus infection. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2015;17:518–526. doi: 10.1111/tid.12405. [DOI] [PubMed] [Google Scholar]
- 45.Sedky M., Mekki Y., Mialou V., Bleyzac N., Girard S., Salama E., Abdel Rahman H., Bertrand Y. Cytomegalovirus infection in pediatric allogenic hematopoietic stem cell transplantation. A single center experience. Pediatric Hematol. Oncol. 2014;31:743–753. doi: 10.3109/08880018.2013.859188. [DOI] [PubMed] [Google Scholar]
- 46.Khalifa R., Asaad A., Hussein M. Human leukocyte antigen-A genotype as a predictor of cytomegalovirus-pp65 antigenemia and cytomegalovirus disease in solid-organ transplant recipients. Egypt. J. Med Hum. Genet. 2016;17:345–352. doi: 10.1016/j.ejmhg.2015.12.009. [DOI] [Google Scholar]
- 47.Futohi F., Saber A., Nemati E., Einollahi B., Rostami Z. Human Leukocyte Antigen Alleles and Cytomegalovirus Infection After Renal Transplantation. Nephro Urol. Mon. 2015;7:e31635. doi: 10.5812/numonthly.31635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niknam A., Karimi M.H., Yaghobi R., Geramizadeh B., Roozbeh J., Salehipour M., Iravani M. The Association Between Viral Infections and Co-stimulatory Gene Polymorphisms in Kidney Transplant Outcomes. Jundishapur J. Microbiol. 2016;9:e31338. doi: 10.5812/jjm.31338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotton C.N., Kumar D., Caliendo A.M., Huprikar S., Chou S., Danziger-Isakov L., Humar A. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900–931. doi: 10.1097/TP.0000000000002191. [DOI] [PubMed] [Google Scholar]
- 50.Meijer E., Boland G.J., Verdonck L.F. Prevention of cytomegalovirus disease in recipients of allogeneic stem cell transplants. Clin. Microbiol. Rev. 2003;16:647–657. doi: 10.1128/CMR.16.4.647-657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alsuhaibani O., Pereira W.C., Tareeqanwar M., Khizzi N.E., Bakheswain S., Shaker A., Elyamany G. Infectious disease screening among stem cell transplant donors: An Institutional experience in Saudi Arabia. Ann. Neurosci. 2015;22:81–86. doi: 10.5214/ans.0972.7531.220206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Essa S., Pacsa A., Said T., Nampoory M.R., Raghupathy R., Johny K.V., Al-Nakib W., Al-Mosawy M. Is combined pretransplantation seropositivity of kidney transplant recipients for cytomegalovirus antigens (pp150 and pp28) a predictor for protection against infection? Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2008;17:66–70. doi: 10.1159/000109593. [DOI] [PubMed] [Google Scholar]
- 53.Safabakhsh H., Tehranian F., Tehranian B., Hatami H., Karimi G., Shahabi M. Prevalence of anti-CMV antibodies in blood donors in Mashhad, Iran. Iran. J. Epidemiol. 2013;9:52–57. [Google Scholar]
- 54.Sepehrvand N., Khameneh Z.R., Eslamloo H.R. Survey the seroprevalence of CMV among hemodialysis patients in Urmia, Iran. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2010;21:363–367. [PubMed] [Google Scholar]
- 55.Mahmoud R.A., El-Mazary A.A., Khodeary A. Seroprevalence of Hepatitis C, Hepatitis B, Cytomegalovirus, and Human Immunodeficiency Viruses in Multitransfused Thalassemic Children in Upper Egypt. Adv. Hematol. 2016;2016:9032627. doi: 10.1155/2016/9032627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gawad A.A., Hashish M., Abaza A., El-Kayal A. Cytomegalovirus Immunoglobulin G Avidity Index among Blood Donors in Alexandria, Egypt. Cent. Eur. J. Public Health. 2016;24:314–320. doi: 10.21101/cejph.a4157. [DOI] [PubMed] [Google Scholar]
- 57.Khameneh Z.R., Sepehrvand N., Aghazadeh T. Cytomegalovirus infection among Iranian kidney graft recipients. Transplant. Proc. 2013;45:178–181. doi: 10.1016/j.transproceed.2012.09.115. [DOI] [PubMed] [Google Scholar]
- 58.Yaghobi R., Zamani S., Gramizadeh B., Rahsaz M. Etiology of DNA Virus Infections in Liver Transplant Recipients With Neonatal Hepatitis. Transplant. Proc. 2010;42:837–838. doi: 10.1016/j.transproceed.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 59.Madi N., Al-Nakib W., Pacsa A., Saeed T. Cytomegalovirus genotypes gB1 and gH1 are the most predominant genotypes among renal transplant recipients in Kuwait. Transplant. Proc. 2011;43:1634–1637. doi: 10.1016/j.transproceed.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 60.Basri N., Abdullah K.A., Shaheen F.A. Cytomegalovirus disease in renal transplant recipients: A single-center experience. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2007;5:601–603. [PubMed] [Google Scholar]
- 61.Pourmand G., Salem S., Mehrsai A., Taherimahmoudi M., Ebrahimi R., Pourmand M.R. Infectious complications after kidney transplantation: A single-center experience. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2007;9:302–309. doi: 10.1111/j.1399-3062.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 62.Ramzi M., Yaghobi R., Etminan H. The role of clinical, therapeutic and laboratory findings in monitoring of HCMV infection in bone marrow transplant Recipients. Iran. Red. Crescent Med. J. 2009;11:46–51. [Google Scholar]
- 63.Saghafi H., Qorashi M., Heidari A. Is screening for IgG antibody to cytomegalovirus and Epstein-Barr virus infections mandatory in potential renal transplant recipients and donors in Iran? Transplant. Proc. 2009;41:2761–2763. doi: 10.1016/j.transproceed.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 64.Al-Sweedan S., Al-Seraihy A., Al-Ahmari A., Al-Jefri A., Mohammed V., Jafri R., Siddiqui K., Ayas M. Factors Determining the Outcome of Hematopoietic Stem Cell Transplantation in Patients With Acute Lymphoblastic Leukemia at King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia. J. Pediatric Hematol. Oncol. 2017;39:33–37. doi: 10.1097/MPH.0000000000000679. [DOI] [PubMed] [Google Scholar]
- 65.Hussein A.A., Al-Antary E.T., Najjar R., Al-Hamdan D.S., Al-Zaben A., Frangoul H. Incidence and risk factors for cytomegalovirus (CMV) reactivation following autologous hematopoietic stem cell transplantation in children. Pediatric Blood Cancer. 2015;62:1099–1101. doi: 10.1002/pbc.25292. [DOI] [PubMed] [Google Scholar]
- 66.Davoudi S., Kasraianfard A., Ahmadinejad Z., Najafi A., Salimi J., Makarem J., Sohrabpour A.A., Jafarian A. Cytomegalovirus reactivation and preemptive therapy after liver transplant. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2014;12(Suppl. 1):72–75. [PubMed] [Google Scholar]
- 67.Al-Alousy B.M., Abdul-Razak S.H., Al-Ajeeli K.S., Al-Jashamy K.A. Anti-HCMV IgG positivity rate among renal transplant recipients in Baghdad. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2011;22:1269–1274. [PubMed] [Google Scholar]
- 68.Khameneh Z.R. Occurrence of cytomegalovirus infection and factors causing reactivation of the infection among renal transplant recipients: A single center study. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2008;19:41–45. [PubMed] [Google Scholar]
- 69.Nasiri S., Ahmadi S.F., Lessan-Pezeshki M., Seyfi S., Alatab S. Lack of cytomegalovirus and polyomavirus coexistence in Iranian kidney transplant recipients. Transplant. Proc. 2011;43:536–539. doi: 10.1016/j.transproceed.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 70.Saber A., Fotuhi F., Rostami Z., Einollahi B., Nemati E. Vitamin D Levels After Kidney Transplantation and the Risk of Cytomegalovirus Infection. Nephro Urol. Mon. 2015;7:e29677. doi: 10.5812/numonthly.29677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amir J., Atias J., Linder N., Pardo J. Follow-up of infants with congenital cytomegalovirus and normal fetal imaging. Arch. Dis. Child. Fetal Neonatal Ed. 2016;101:F428–F432. doi: 10.1136/archdischild-2015-308357. [DOI] [PubMed] [Google Scholar]
- 72.Bilavsky E., Schwarz M., Pardo J., Attias J., Levy I., Haimi-Cohen Y., Amir J. Lenticulostriated vasculopathy is a high-risk marker for hearing loss in congenital cytomegalovirus infections. Acta Paediatr. (Oslo Norway 1992) 2015;104:e388–e394. doi: 10.1111/apa.13053. [DOI] [PubMed] [Google Scholar]
- 73.Hadar E., Yogev Y., Melamed N., Chen R., Amir J., Pardo J. Periconceptional cytomegalovirus infection: Pregnancy outcome and rate of vertical transmission. Prenat. Diagn. 2010;30:1213–1216. doi: 10.1002/pd.2654. [DOI] [PubMed] [Google Scholar]
- 74.Hoffmann C., Grossman R., Bokov I., Lipitz S., Biegon A. Effect of cytomegalovirus infection on temporal lobe development in utero: Quantitative MRI studies. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2010;20:848–854. doi: 10.1016/j.euroneuro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Gindes L., Teperberg-Oikawa M., Sherman D., Pardo J., Rahav G. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG Int. J. Obstet. Gynaecol. 2008;115:830–835. doi: 10.1111/j.1471-0528.2007.01651.x. [DOI] [PubMed] [Google Scholar]
- 76.Lipitz S., Yagel S., Shalev E., Achiron R., Mashiach S., Schiff E. Prenatal diagnosis of fetal primary cytomegalovirus infection. Obstet. Gynecol. 1997;89:763–767. doi: 10.1016/S0029-7844(97)00084-7. [DOI] [PubMed] [Google Scholar]
- 77.Lipitz S., Yinon Y., Malinger G., Yagel S., Levit L., Hoffman C., Rantzer R., Weisz B. Risk of cytomegalovirus-associated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2013;41:508–514. doi: 10.1002/uog.12377. [DOI] [PubMed] [Google Scholar]
- 78.Farkas N., Hoffmann C., Ben-Sira L., Lev D., Schweiger A., Kidron D., Lerman-Sagie T., Malinger G. Does normal fetal brain ultrasound predict normal neurodevelopmental outcome in congenital cytomegalovirus infection? Prenat. Diagn. 2011;31:360–366. doi: 10.1002/pd.2694. [DOI] [PubMed] [Google Scholar]
- 79.Ari-Even Roth D., Lubin D., Kuint J., Teperberg-Oikawa M., Mendelson E., Strauss T., Barkai G. Contribution of targeted saliva screening for congenital CMV-related hearing loss in newborns who fail hearing screening. Arch. Dis. Child. Fetal Neonatal Ed. 2017;102:F519–F524. doi: 10.1136/archdischild-2016-311859. [DOI] [PubMed] [Google Scholar]
- 80.Amir J., Schwarz M., Levy I., Haimi-Cohen Y., Pardo J. Is lenticulostriated vasculopathy a sign of central nervous system insult in infants with congenital CMV infection? Arch. Dis. Child. 2011;96:846–850. doi: 10.1136/adc.2010.208405. [DOI] [PubMed] [Google Scholar]
- 81.Barkai G., Ari-Even Roth D., Barzilai A., Tepperberg-Oikawa M., Mendelson E., Hildesheimer M., Kuint J. Universal neonatal cytomegalovirus screening using saliva—Report of clinical experience. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2014;60:361–366. doi: 10.1016/j.jcv.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 82.Barkai G., Barzilai A., Mendelson E., Tepperberg-Oikawa M., Roth A.E.D., Kuint J. Newborn screening for congenital cytomegalovirus using real-time polymerase chain reaction in umbilical cord blood. Israel Med. Assoc. J. 2013;15:279–283. [PubMed] [Google Scholar]
- 83.Arabpour M., Kaviyanee K., Jankhah A., Yaghobi R. Human cytomegalovirus infection in women of childbearing age, Fars Province: A population-based cohort study. Iran. Red Crescent Med. J. 2008;10:100–106. doi: 10.21161/mjm.01107. [DOI] [Google Scholar]
- 84.Karimian P., Yaghini O., Nasr Azadani H., Mohammadizadeh M., Arabzadeh S.A., Adibi A., Rahimi H. Prevalence, Characteristics, and One-Year Follow-Up of Congenital Cytomegalovirus Infection in Isfahan City, Iran. Interdiscip. Perspect. Infect. Dis. 2016;2016:7812106. doi: 10.1155/2016/7812106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rasti S., Ghasemi F.S., Abdoli A., Piroozmand A., Mousavi S.G., Fakhrie-Kashan Z. ToRCH “co-infections” are associated with increased risk of abortion in pregnant women. Congenit. Anom. 2016;56:73–78. doi: 10.1111/cga.12138. [DOI] [PubMed] [Google Scholar]
- 86.Al-Ali H.Y., Yasseen S.A., Raof T.Y. Follow-up of pregnant women with active cytomegalovirus infection. East. Mediterr. Health J. 1999;5:949–954. [PubMed] [Google Scholar]
- 87.Fahimzad A., Afgeh S.A., Eghbali E., Abdinia B., Shiva F., Rahbar M. Screening of congenital CMV infection in saliva of neonates by PCR: Report of a pilot screening study in Iran. Clin. Lab. 2013;59:1171–1174. doi: 10.7754/Clin.Lab.2013.120910. [DOI] [PubMed] [Google Scholar]
- 88.Bilavsky E., Schwarz M., Bar-Sever Z., Pardo J., Amir J. Hepatic involvement in congenital cytomegalovirus infection—Infrequent yet significant. J. Viral Hepat. 2015;22:763–768. doi: 10.1111/jvh.12374. [DOI] [PubMed] [Google Scholar]
- 89.Hadar E., Dorfman E., Bardin R., Gabbay-Benziv R., Amir J., Pardo J. Symptomatic congenital cytomegalovirus disease following non-primary maternal infection: A retrospective cohort study. BMC Infect. Dis. 2017;17:31. doi: 10.1186/s12879-016-2161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abdelmogheth A.A., Al-Nair A.M., Balkhair A.A., Mahmoud A.M., El-Naggari M. Pattern of Viral Infections among Infants and Children Admitted to the Paediatric Intensive Care Unit at Sultan Qaboos University Hospital, Oman. Sultan Qaboos Univ. Med. J. 2014;14:e546–e550. [PMC free article] [PubMed] [Google Scholar]
- 91.Jahromi Cytomegalovirus Immunity in Pregnancy in South of Iran. Am. J. Infect. Dis. 2010;6:8–12. doi: 10.3844/ajidsp.2010.8.12. [DOI] [Google Scholar]
- 92.Van Zuylen W.J., Hamilton S.T., Naing Z., Hall B., Shand A., Rawlinson W.D. Congenital cytomegalovirus infection: Clinical presentation, epidemiology, diagnosis and prevention. Obstet. Med. 2014;7:140–146. doi: 10.1177/1753495X14552719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dollard S.C., Grosse S.D., Ross D.S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007;17:355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 94.Istas A.S., Demmler G.J., Dobbins J.G., Stewart J.A. Surveillance for congenital cytomegalovirus disease: A report from the National Congenital Cytomegalovirus Disease Registry. Clin. Infect. Dis. 1995;20:665–670. doi: 10.1093/clinids/20.3.665. [DOI] [PubMed] [Google Scholar]
- 95.Ludwig A., Hengel H. Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Euro. Surveill. 2009;14:19140. doi: 10.2807/ese.14.09.19140-en. [DOI] [PubMed] [Google Scholar]
- 96.Ebrahimi-Rad M., Shakeri T.S., Shirvani F., Shahrokhi K., Shahrokhi N. Prevalence of congenital cytomegalovirus infection in symptomatic newborns under 3 weeks in Tehran, Iran. BMC Infect. Dis. 2017;17:688. doi: 10.1186/s12879-017-2799-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schlesinger Y., Reich D., Eidelman A.I., Schimmel M.S., Hassanin J., Miron D. Congenital cytomegalovirus infection in Israel: Screening in different subpopulations. Isr. Med Assoc. J. IMAJ. 2005;7:237–240. [PubMed] [Google Scholar]
- 98.Dietrich M.L., Schieffelin J.S. Congenital Cytomegalovirus Infection. Ochsner J. 2019;19:123–130. doi: 10.31486/toj.18.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Townsend C.L., Forsgren M., Ahlfors K., Ivarsson S.A., Tookey P.A., Peckham C.S. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin. Infect. Dis. 2013;56:1232–1239. doi: 10.1093/cid/cit018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vestergaard H.T., Thomsen M.K., Nielsen L., Panum I. Diagnostics of congenital cytomegalovirus in Denmark. Ugeskr. Laeger. 2018;180:pii:V03180221. [PubMed] [Google Scholar]
- 101.El-Mekki A., Deverajan L.V., Soufi S., Strannegard O., al-Nakib W. Specific and non-specific serological markers in the screening for congenital CMV infection. Epidemiol. Infect. 1988;101:495–501. doi: 10.1017/S0950268800029381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pasternak Y., Ziv L., Attias J., Amir J., Bilavsky E. Valganciclovir Is Beneficial in Children with Congenital Cytomegalovirus and Isolated Hearing Loss. J. Pediatrics. 2018;199:166–170. doi: 10.1016/j.jpeds.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 103.Miron D., Brosilow S., Felszer K., Reich D., Halle D., Wachtel D., Eidelman A.I., Schlesinger Y. Incidence and clinical manifestations of breast milk-acquired Cytomegalovirus infection in low birth weight infants. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2005;25:299–303. doi: 10.1038/sj.jp.7211255. [DOI] [PubMed] [Google Scholar]
- 104.Bilavsky E., Shahar-Nissan K., Pardo J., Attias J., Amir J. Hearing outcome of infants with congenital cytomegalovirus and hearing impairment. Arch. Dis. Child. 2016;101:433–438. doi: 10.1136/archdischild-2015-309154. [DOI] [PubMed] [Google Scholar]
- 105.Neirukh T., Qaisi A., Saleh N., Rmaileh A.A., Zahriyeh E.A., Qurei L., Dajani F., Nusseibeh T., Khamash H., Baraghithi S., et al. Seroprevalence of Cytomegalovirus among pregnant women and hospitalized children in Palestine. BMC Infect. Dis. 2013;13:528. doi: 10.1186/1471-2334-13-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bilavsky E., Pardo J., Attias J., Levy I., Magny J.F., Ville Y., Leruez-Ville M., Amir J. Clinical Implications for Children Born With Congenital Cytomegalovirus Infection Following a Negative Amniocentesis. Clin. Infect. Dis. 2016;63:33–38. doi: 10.1093/cid/ciw237. [DOI] [PubMed] [Google Scholar]
- 107.Ziyaeyan M., Alborzi A., Abbasian A., Kalani M., Moravej A., Nasiri J., Amiri A., Hashemi N., Sefiddashti F. Detection of HCMV DNA in placenta, amniotic fluid and fetuses of seropositive women by nested PCR. Euro. J. Pediatrics. 2007;166:723–726. doi: 10.1007/s00431-006-0314-x. [DOI] [PubMed] [Google Scholar]
- 108.Kanengisser-Pines B., Hazan Y., Pines G., Appelman Z. High cytomegalovirus IgG avidity is a reliable indicator of past infection in patients with positive IgM detected during the first trimester of pregnancy. J. Perinatal Med. 2009;37:15–18. doi: 10.1515/JPM.2009.012. [DOI] [PubMed] [Google Scholar]
- 109.Abu Faddan N., Eltayeb A., Refaiy A. Cytomegalo virus as a possible risk factor for neonatal gastrointestinal surgical conditions. Fetal Pediatric Pathol. 2011;30:124–129. doi: 10.3109/15513815.2010.524691. [DOI] [PubMed] [Google Scholar]
- 110.Al-Awadhi R., Al-Harmi J., Alfadhli S. Prevalence of cytomegalovirus DNA in cord blood and voided urine obtained from pregnant women at the end of pregnancy. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2013;22:194–199. doi: 10.1159/000343167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kamel N., Metwally L., Gomaa N., Sayed Ahmed W.A., Lotfi M., Younis S. Primary cytomegalovirus infection in pregnant Egyptian women confirmed by cytomegalovirus IgG avidity testing. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2014;23:29–33. doi: 10.1159/000354758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Erfanianahmadpoor M., Nasiri R., Vakili R., Hassannia T. Seroprevalence, transmission, and associated factors of specific antibodies against cytomegalovirus among pregnant women and their infants in a regional study. Saudi Med. J. 2014;35:360–364. [PubMed] [Google Scholar]
- 113.Ebrahim M.G., Ali A.S., Mustafa M.O., Musa D.F., El Hussein A.R., Elkhidir I.M., Enan K.A. Molecular Detection of Human Cytomegalovirus (HCMV) Among Infants with Congenital Anomalies in Khartoum State, Sudan. Open Virol. J. 2015;9:38–41. doi: 10.2174/1874357901509010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Altayeb M.A., Mokhtar S.I., Adam M.E., Mohammed S.I., Musa H.H. Detection of primary CMV infection in Sudanese pregnant women by IgG avidity test. Asian Pac. J. Trop. Dis. 2016;6:816–818. doi: 10.1016/S2222-1808(16)61137-4. [DOI] [Google Scholar]
- 115.Alwan S.N., Shamran H.A., Ghaib A.H., Al-Mayah Q.S., Al-Saffar A.J., Bayati A.H., Arif H.S., Fu J., Wickes B.L. Genotyping of Cytomegalovirus from Symptomatic Infected Neonates in Iraq. Am. J. Trop. Med. Hyg. 2019 doi: 10.4269/ajtmh.18-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caliendo A.M. Overview of Diagnostic Tests for Cytomegalovirus Infection. [(accessed on 17 April 2019)]; Available online: https://www.uptodate.com/contents/overview-of-diagnostic-tests-for-cytomegalovirus-infection.
- 117.Chou S. Newer methods for diagnosis of cytomegalovirus infection. Rev. Infect. Dis. 1990;12(Suppl. 7):S727–S736. doi: 10.1093/clinids/12.Supplement_7.S727. [DOI] [PubMed] [Google Scholar]
- 118.Engelhard D., Weinberg M., Or R., Shaked O., Naparstek E., Haikin H., Slavin S., Sarov I. Immunoglobulins A, G, and M to cytomegalovirus during recurrent infection in recipients of allogeneic bone marrow transplantation. J. Infect. Dis. 1991;163:628–630. doi: 10.1093/infdis/163.3.628. [DOI] [PubMed] [Google Scholar]
- 119.Abou-Jaoude M.M., Ghantous I., Almawi W.Y. Tacrolimus (FK506) versus cyclosporin A microemulsion (Neoral) maintenance immunosuppression: Effects on graft survival and function, infection, and metabolic profile following kidney transplantation (KT) Mol. Immunol. 2003;39:1095–1100. doi: 10.1016/S0161-5890(03)00070-1. [DOI] [PubMed] [Google Scholar]
- 120.Essa S., Pacsa A., Raghupathy R., Said T., Nampoory M.R., Johny K.V., Al-Nakib W. Low levels of Th1-type cytokines and increased levels of Th2-type cytokines in kidney transplant recipients with active cytomegalovirus infection. Transplant. Proc. 2009;41:1643–1647. doi: 10.1016/j.transproceed.2008.10.098. [DOI] [PubMed] [Google Scholar]
- 121.Pourmand G., Saraji A., Salem S., Mehrsai A., Nikoobakht M.R., Taherimahmoudi M., Rezaeidanesh M., Asadpour A. Could prophylactic monoclonal antibody improve kidney graft survival? Transplant. Proc. 2009;41:2794–2796. doi: 10.1016/j.transproceed.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 122.Kamali K., Abbasi M.A., Behzadi A.H., Mortazavi A., Bastani B. Incidence and risk factors of transplant renal artery stenosis in living unrelated donor renal transplantation. J. Renal Care. 2010;36:149–152. doi: 10.1111/j.1755-6686.2010.00188.x. [DOI] [PubMed] [Google Scholar]
- 123.Vaezi M., Kasaeian A., Souri M., Soufiyan F., Shokri Boosjin A., Setarehdan S.A., Alimoghaddam K., Ghavamzadeh A. How Do Donor-Recipient CMV Serostatus and Post-Hematopoietic Stem Cell Transplantation CMV Reactivation Affect Outcomes in Acute Leukemia Patients? Int. J. Hematol. Oncol. Stem Cell Res. 2017;11:199–208. [PMC free article] [PubMed] [Google Scholar]
- 124.Goldstein G., Rutenberg T.F., Mendelovich S.L., Hutt D., Oikawa M.T., Toren A., Bielorai B. The role of immunoglobulin prophylaxis for prevention of cytomegalovirus infection in pediatric hematopoietic stem cell transplantation recipients. Pediatric Blood Cancer. 2017;64 doi: 10.1002/pbc.26420. [DOI] [PubMed] [Google Scholar]
- 125.Lipitz S., Hoffmann C., Feldman B., Tepperberg-Dikawa M., Schiff E., Weisz B. Value of prenatal ultrasound and magnetic resonance imaging in assessment of congenital primary cytomegalovirus infection. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2010;36:709–717. doi: 10.1002/uog.7657. [DOI] [PubMed] [Google Scholar]
- 126.Gabbay-Benziv R., Yogev Y., Peled Y., Amir J., Pardo J. Congenital cytomegalovirus infection following antenatal negative diagnostic amniotic fluid analysis—A single center experience. J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2012;25:1787–1790. doi: 10.3109/14767058.2012.663832. [DOI] [PubMed] [Google Scholar]
- 127.Rahav G., Gabbay R., Ornoy A., Shechtman S., Arnon J., Diav-Citrin O. Primary versus nonprimary cytomegalovirus infection during pregnancy, Israel. Emerg. Infect. Dis. 2007;13:1791–1793. doi: 10.3201/eid1311.061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kraft C.S., Armstrong W.S., Caliendo A.M. Interpreting quantitative cytomegalovirus DNA testing: Understanding the laboratory perspective. Clin. Infect. Dis. 2012;54:1793–1797. doi: 10.1093/cid/cis212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Caliendo A.M. The long road toward standardization of viral load testing for cytomegalovirus. Clin. Infect. Dis. 2013;56:374–375. doi: 10.1093/cid/cis905. [DOI] [PubMed] [Google Scholar]
- 130.Tong Y., Pang X.L., Mabilangan C., Preiksaitis J.K. Determination of the Biological Form of Human Cytomegalovirus DNA in the Plasma of Solid-Organ Transplant Recipients. J. Infect. Dis. 2017;215:1094–1101. doi: 10.1093/infdis/jix069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Preiksaitis J.K., Hayden R.T., Tong Y., Pang X.L., Fryer J.F., Heath A.B., Cook L., Petrich A.K., Yu B., Caliendo A.M. Are We There Yet? Impact of the First International Standard for Cytomegalovirus DNA on the Harmonization of Results Reported on Plasma Samples. Clin. Infect. Dis. 2016;63:583–589. doi: 10.1093/cid/ciw370. [DOI] [PubMed] [Google Scholar]
- 132.Cook L., Starr K., Boonyaratanakornkit J., Hayden R., Sam S.S., Caliendo A.M. Does Size Matter? Comparison of Extraction Yields for Different-Sized DNA Fragments by Seven Different Routine and Four New Circulating Cell-Free Extraction Methods. J. Clin. Microbiol. 2018;56 doi: 10.1128/JCM.01061-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Al-Amro A.A., al-Jafari A.A., al-Fagih M.R., Tajeldin M., Qavi H.B. Frequency of occurrence of cytomegalovirus and Chlamydia pneumoniae in lymphocytes of atherosclerotic patients. Cent. Eur. J. Public Health. 2001;9:106–108. [PubMed] [Google Scholar]
- 134.Behzad-Behbahani A., Entezam M., Mojiri A., Pouransari R., Rahsaz M., Banihashemi M., Heidari T., Farhadi A., Azarpira N., Yaghobi R., et al. Incidence of human herpes virus-6 and human cytomegalovirus infections in donated bone marrow and umbilical cord blood hematopoietic stem cells. Indian J. Med. Microbiol. 2008;26:252–255. doi: 10.4103/0255-0857.42038. [DOI] [PubMed] [Google Scholar]
- 135.Peled O., Berkovitch M., Rom E., Bilavsky E., Bernfeld Y., Dorfman L., Pappo A., Ziv-Baran T., Brandriss N., Bar-Haim A., et al. Valganciclovir Dosing for Cytomegalovirus Prophylaxis in Pediatric Solid-organ Transplant Recipients: A Prospective Pharmacokinetic Study. Pediatric Infect. Dis. J. 2017;36:745–750. doi: 10.1097/INF.0000000000001595. [DOI] [PubMed] [Google Scholar]
- 136.Madi N., Al-Nakib W., Pacsa A. Does Cytomegalovirus Develop Resistance following Antiviral Prophylaxis and Treatment in Renal Transplant Patients in Kuwait? Adv. Virol. 2011;2011:260561. doi: 10.1155/2011/260561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shoeibi N., Abrishami M., Mohammad Esmaeil E., Hosseini S.M. Visual prognosis, clinical features, and predisposing factors in non-HIV patients with cytomegalovirus retinitis. Int. Ophthalmol. 2018 doi: 10.1007/s10792-018-0991-2. [DOI] [PubMed] [Google Scholar]
- 138.Kotton C.N., Kumar D., Caliendo A.M., Asberg A., Chou S., Danziger-Isakov L., Humar A. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 139.Shamran H.A., Kadhim H.S., Hussain A.R., Kareem A., Taub D.D., Price R.L., Nagarkatti M., Nagarkatti P.S., Singh U.P. Detection of human cytomegalovirus in different histopathological types of glioma in Iraqi patients. BioMed Res. Int. 2015;2015:642652. doi: 10.1155/2015/642652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taha M.S., Abdalhamid B.A., El-Badawy S.A., Sorour Y.M., Almsned F.M., Al-Abbadi M.A. Expression of cytomegalovirus in glioblastoma multiforme: Myth or reality? Br. J. Neurosurg. 2016;30:307–312. doi: 10.3109/02688697.2015.1119241. [DOI] [PubMed] [Google Scholar]
- 141.Mohammadizadeh F., Mahmudi F. Evaluation of human cytomegalovirus antigen expression in invasive breast carcinoma in a population of Iranian patients. Infect. Agents Cancer. 2017;12:39. doi: 10.1186/s13027-017-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.El Shazly D.F., Bahnassey A.A., Omar O.S., Elsayed E.T., Al-Hindawi A., El-Desouky E., Youssef H., Zekri A.N. Detection of Human Cytomegalovirus in Malignant and Benign Breast Tumors in Egyptian Women. Clin. Breast Cancer. 2018;18:e629–e642. doi: 10.1016/j.clbc.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 143.Shmueli E., Hadar E., Pardo J., Attias J., Amir J., Bilavsky E. Congenital Cytomegalovirus Infection After a Multiple Birth Pregnancy. Pediatric Infect. Dis. J. 2017;36:e298–e302. doi: 10.1097/INF.0000000000001725. [DOI] [PubMed] [Google Scholar]
- 144.Ziv L., Yacobovich J., Pardo J., Yarden-Bilavsky H., Amir J., Osovsky M., Bilavsky E. Hematologic Adverse Events Associated With Prolonged Valganciclovir Treatment in Congenital Cytomegalovirus Infection. Pediatric Infect. Dis. J. 2019;38:127–130. doi: 10.1097/INF.0000000000002079. [DOI] [PubMed] [Google Scholar]