Abstract
The present study aimed to detect and characterize Borrelia spp. in ticks attached to dogs in Korea. Overall, 562 ticks (276 pools) attached to dogs were collected and tested for Borrelia infection by PCR targeting the 5S-23S rRNA intergenic spacer region (rrf-rrl). One tick larva (pool level, 0.4%; individual level, 0.2%) was confirmed by sequencing Borrelia garinii, a zoonotic pathogen. For molecular characterization, the outer surface protein A (ospA) and flagellin genes were analyzed. Phylogenetic ospA analysis distinguished B. garinii from B. bavariensis, which has been recently identified as a novel Borrelia species. On the other hand, phylogenetic analysis showed that single gene analysis involving rrf-rrl or flagellin was not sufficient to differentiate B. garinii from B. bavariensis. In addition, the B. garinii-infected tick was identified as Ixodes nipponensis by sequencing according to mitochondrial 16S rRNA and the second transcribed spacer region. To our knowledge, this is the first study to report the molecular detection of B. garinii in I. nipponensis parasitizing a dog in Korea. Continuous monitoring of tick-borne pathogens in ticks attached to animals is required to avoid disease distribution and possible transmission to humans.
Keywords: Borrelia garinii, dog, Ixodes nipponensis, Lyme borreliosis, differential diagnosis, tick-borne pathogen
1. Introduction
Lyme borreliosis is a tick-borne infectious zoonotic disease caused by Borrelia burgdorferi sensu lato (s.l.), and it involves at least 19 species. Of these species, B. burgdorferi sensu stricto (s.s.), B. afzelii, B. garinii, B. bavariensis, and B. spielmanii are known to be pathogenic to humans [1,2]. B. burgdorferi was first identified in the USA in 1982 [3], and it has been subsequently reported worldwide, including in Europe and Asia [4,5,6,7]. In the USA, 20,000 to 30,000 people are annually diagnosed with Lyme borreliosis, and in 2016, 26,000 cases were confirmed [8].
In Korea, Borrelia sp. was first identified in Ixodes ticks in 1992, and the first human case of Lyme disease was reported in 1993 [4,9]. From 2011, nationwide surveillance for Lyme disease was initiated and two cases were identified. Subsequently, it showed a gradually increasing tendency, and 23 cases were reported in 2018 [10]. Recently, different molecular and serological studies identified the nationwide distribution of the disease in humans and in various domestic animals, such as horses and dogs, and wild animals [11,12,13,14]. According to previous studies, dogs are among the most popular companion animals and are considered as sentinel animals for zoonotic diseases, including Lyme borreliosis [15,16,17,18]. It is worth noting that recent studies in Korea have shown close relationships among humans, ticks, and companion dogs with regard to tick-borne diseases [19,20].
Ticks are responsible for the transmission of various vector-borne pathogens, such as Anaplasma spp., Borrelia spp., Ehrlichia spp., Rickettsia spp., and severe fever with thrombocytopenia syndrome virus [21,22]. It is known that B. burgdorferi s.l. is mainly transmitted by Ixodes spp. [23], and consistently, B. burgdorferi s.l. has been isolated from I. ricinus and I. persulcatus in Korea [24]. B. garinii was first identified in I. persulcatus from vegetation in Korea in 1993, and other Borrelia spp., including B. afzelii, B. yangtzensis, and B. bavariensis, have been reported in ticks [24,25,26]. Because tick-borne diseases are associated with close relationships among humans, ticks, and companion animals, it is important to investigate tick-borne pathogens in ticks, especially those parasitizing animals, in order to avoid disease distribution and possible transmission to humans. However, to the best of our knowledge, molecular studies on Borrelia spp. are limited, especially in ticks parasitizing animals, in Korea.
Recently, B. bavariensis was separated from B. garinii, and it is considered a novel species. Margos et al. [27] suggested that multilocus genotying is required to classify these two Borrelia species. The differential diagnosis of pathogens is essential for disease diagnosis, treatment, vaccine development, and prevention. However, the suggested method is not convenient for this purpose because it requires analysis of 11 genes.
The purposes of the present study were to evaluate the prevalence of Borrelia spp. in ticks attached to dogs in Korea and to assess the molecular characteristics of the 5S-23S rRNA intergenic spacer region (rrf-rrl), outer surface protein A (ospA), and flagellin genes using phylogenetic analysis and determine whether single gene analysis can be used for species identification.
2. Results
2.1. Molecular Identification of Borrelia Spp.
In nested PCR, 1 of the 276 tested tick pools (pool level, 0.4%; individual level, 0.2%) was positive for the rrf-rrl fragment of Borrelia spp. In addition, the ospA and flagellin genes in the rrf-rrl-positive sample were amplified by PCR. All the amplified bands were single and clear.
The Borrelia-positive tick was collected from a two-year-old male Alaskan Malamute in Uiseong-gun, Gyeongbuk province. The dog did not show any clinical symptoms at tick collection. Hematological and biochemical evaluations were not performed.
For species identification and molecular characterization, sequencing was performed for the three genes. On sequencing, 255, 313, and 354 bp of the rrf-rrl, ospA, and flagellin gene fragments were obtained, respectively, and all the sequences were found to be associated with B. garinii by the basic local alignment search tool (BLAST) search. The B. garinii sequences obtained in this study were deposited in the GenBank database (accession numbers: KU848760 for rrf-rrl, KU848761 for ospA, and MH716232 for flagellin).
2.2. Identification of Tick Species
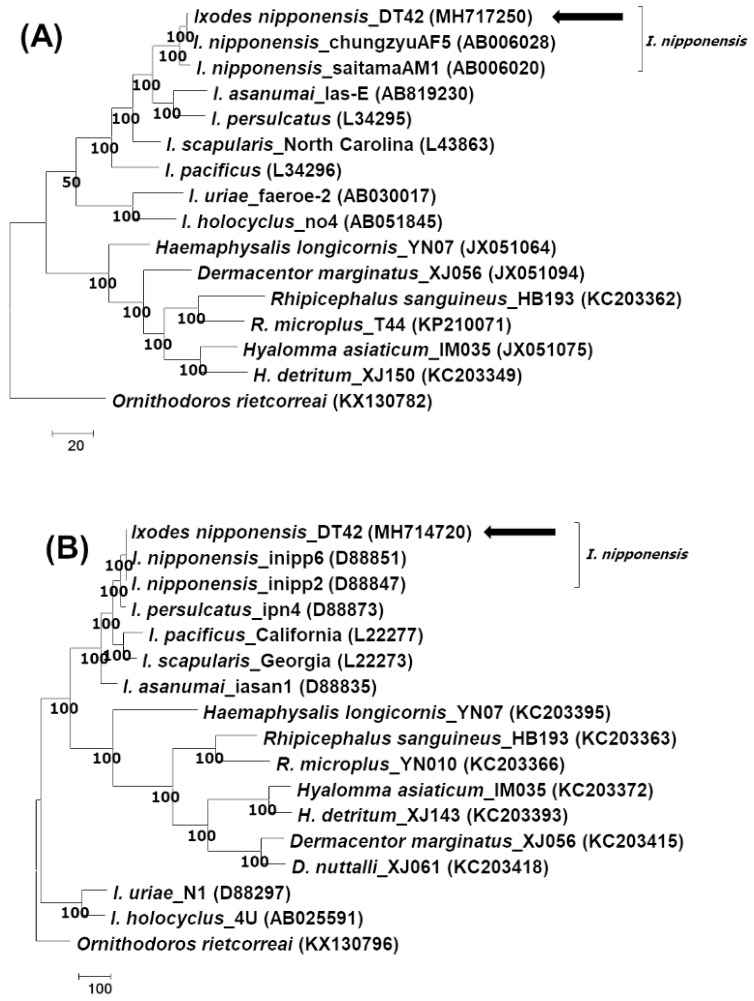
The tick DNA sample positive for B. garinii was sequenced according to mitochondrial 16S rRNA and the second internal transcribed spacer region (ITS-2). On sequencing, 444 and 911 bp of the mitochondrial 16S rRNA and ITS-2 region fragments were obtained, respectively. Using BLAST and phylogenetic analysis, both sequences were found to be associated with the tick species Ixodes nipponensis (Figure 1 and Figure S1). The I. nipponensis sequences obtained in this study were deposited in the GenBank database (accession numbers: MH717250 for 16S rRNA and MH714720 for ITS-2).
Figure 1.
Phylogenetic analysis of the tick species. The trees are analyzed according to (A) mitochondrial 16S rRNA and (B) the second intergenic spacer region, using the maximum parsimony method by MEGA 7.0. The consensus trees inferred from the two most parsimonious trees are shown, and the cut-off value for the consensus tree is 50%. The sequences identified in this study are indicated by arrows.
2.3. Molecular Characterization and Phylogenetic Analysis of B. garinii
The rrf-rrl sequence identified in this study showed 98.8% (DQ150544)–97.3% (AB091797) identity with the rrf-rrl sequence of B. garinii. Additionally, the ospA sequence identified in this study showed 99.7% (AB009862)–90.4% (GU826980) identity with the ospA sequence of B. garinii. Moreover, the flagellin sequence identified in this study showed 99.7% (MG245785)–98.6% (KF894054) identity with the flagellin sequence of B. garinii.
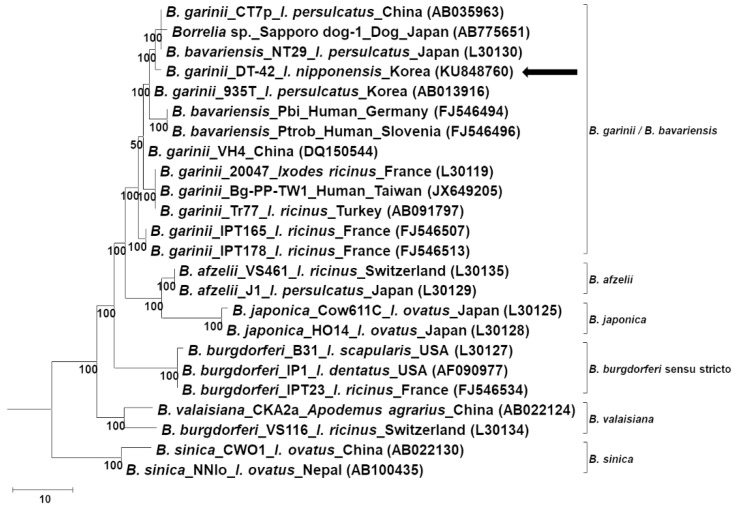
Phylogenetic analysis according to the rrf-rrl and flagellin genes showed that the sequences identified in this study clustered with the ones of B. garinii/B. bavariensis (Figure 2 and Figure 3). On the other hand, phylogenetic analysis according to the ospA gene showed that the sequence identified in this study belonged to B. garinii, which was different from that of B. bavariensis (Figure 4). All phylogenetic trees using the same data with different methods showed consistent results, that is, the major nodes were consistent among the trees, indicating that the trees constructed by the maximum parsimony method were reliable (Figures S2–S4).
Figure 2.
Phylogenetic analysis of Borrelia garinii according to the 5S–23S intergenic spacer region. The tree is constructed using the maximum parsimony method by MEGA 7.0. The consensus trees inferred from the six most parsimonious trees are shown, and the cut-off value for the consensus tree is 50%. The Borrelia spp., isolate, host, identified country, and nucleotide accession number are described in the tree. The sequence identified in this study is indicated by an arrow.
Figure 3.
Phylogenetic analysis of Borrelia garinii according to the flagellin gene. The tree is constructed using the maximum parsimony method by MEGA 7.0. The consensus trees inferred from the eight most parsimonious trees are shown, and the cut-off value for the consensus tree is 50%. The Borrelia spp., isolate, host, identified country, and nucleotide accession number are described in the tree. The sequence identified in this study is indicated by an arrow.
Figure 4.
Phylogenetic analysis of Borrelia garinii according to the outer surface protein A (ospA) gene. The tree is constructed using the maximum parsimony method by MEGA 7.0. The consensus trees inferred from the four most parsimonious trees are shown, and the cut-off value for the consensus tree is 50%. The Borrelia spp., isolate, host, identified country, and nucleotide accession number are described in the tree. The sequence identified in this study is indicated by an arrow.
The phylogenetic trees were constructed considering the isolated host and country; however, no specific associations were noted among the molecular characteristics, host, and country.
3. Discussion
Borrelia spp. have been identified in dogs and ticks parasitizing dogs in different countries. In the UK, 2.0% (94/4737) of ticks removed from dogs were found to be infected with Borrelia spp., and B. burgdorferi s.s., B. garinii, B. afzelii, and B. spielmanii were identified [17]. Additionally, ticks from dogs showed positive rates of 6.2% (13/209) in Poland, including B. afzelii [28], and 5.6% (6/108) in Hungary, including B. afzelii and B. garinii [29]. Serologically, dogs were found to be positive for B. burgdorferi s.l. at rates of 22.0% (122/555) in Australia [16], 0.9% (6/637) in China [30], 17.3% (13/75) in the Netherlands [7], and 4.7% (78/1666) in the USA [18].
B. garinii has been molecularly identified worldwide, including in Europe, North America, and Asia [31,32,33,34]. With regard to the isolation source, B. garinii has been identified in humans, animals, and ticks, including both parasitizing and nonparasitizing [31,32]. It is worth noting that there have been two clinical cases of B. garinii infection in dogs in Japan [33], suggesting the importance of the dog as a sentinel animal for zoonotic transmission, including B. garinii transmission.
In Korea, some studies have serologically identified Borrelia in dogs, and the seroprevalence ranges between 1.1% and 2.2% [12,35,36]; however, Borrelia infection in dogs has not been molecularly proven. Haemaphysalis longicornis is known as a dominant tick species in Korea, and other tick species, including Ixodes, Amblyomma, and Rhipicephalus, have been identified in different environments [37,38]. The fact that I. nipponensis is not a dominant tick species in dogs and in environments in Korea [38] might be the reason for the low seroprevalence of Lyme borreliosis in dogs in this country. Considering the distribution of Borrelia in animals, the existence of vector ticks, and the gradual increase in human clinical cases, continuous monitoring of Borrelia in vector ticks and animals in Korea is required.
In this study, B. garinii was identified in I. nipponensis. This result is consistent with the findings of previous studies that showed Ixodes as the main vector tick of Borrelia spp. [31]. Additionally, previous studies have experimentally confirmed H. concinna and Dermacentor silvarum as vectors of Borrelia spp. [39]. Consistently, Borrelia spp. have been identified in Ixodes and Haemaphysalis ticks in Korea and Japan [40,41]. Pal et al. [23] revealed that tick receptor for ospA (TROSPA) acts as a receptor for Borrelia spp. in I. scapularis, and the trospa gene was identified in I. ricinus and I. persulcatus, suggesting that these ticks could be vectors of Borrelia spp. [42]. Additional studies involving experimental infection are required to reveal the vector competence of ticks for Borrelia spp.
The rrf-rrl sequence has been widely used for the detection and differentiation of Borrelia spp. owing to its conserved characteristics among Borrelia spp. [31,40,43]. However, as suggested by De Michelis et al. [6], it is difficult to construct a reliable molecular phylogeny based on only rrf-rrl owing to the fact that the fragment is short and consists of highly conserved and variable regions. Therefore, regarding species identification, it is difficult to reliably confirm the species of Borrelia with single gene analysis of rrf-rrl.
It is known that ospA is not related to the infectivity and cause of Lyme borreliosis; however, ospA-deficient Borrelia spp. failed to colonize and survive in vector ticks [44]. In addition, a recent study found that Borrelia spp. showed different serotypes according to the molecular characteristics of ospA [45]. Another study suggested that single gene analysis is not sufficient to differentiate B. garinii from B. bavariensis, and it suggested multilocus sequence typing using housekeeping genes to differentiate B. garinii from B. bavariensis [27]. However, single phylogenetic analysis of ospA in this study showed the potential of ospA analysis for differentiating B. garinii from B. bavariensis.
Flagellin is a functional gene of Borrelia spp. and is responsible for their invasion of host cells [46]. Phylogenetic analysis of flagellin showed well-conserved characteristics according to species. Park et al. [47] suggested that flagellin could be useful for interspecies Borrelia differentiation, and phylogenetic analysis using the maximum parsimony (MP), maximum-likelihood (ML), and Bayesian inference (BI) methods in this study showed that flagellin was useful for interspecies Borrelia differentiation; however, it has limitations for differentiating between B. garinii and B. bavariensis.
According to the evaluation of the three selected genes using phylogenetic analysis, only the ospA analysis could differentiate B. garinii from B. bavariensis, including other Borrelia spp. On the other hand, rrf-rrl and flagellin gene analysis could successfully identify Borrelia spp., except for B. bavariensis. Considering that the molecular characteristics of ospA could induce serotype differences, which might be related to vaccine development, the differentiation of Borrelia spp. according to ospA is important.
In conclusion, this is the first study to identify B. garinii in I. nipponensis parasitizing a dog in Korea. Phylogenetic analysis of ospA helped differentiate B. garinii from B. bavariensis. According to the phylogenetic analysis of flagellin, the B. garinii identified in this study showed high identity and a close relationship with other B. garinii, including those identified in humans. Considering the findings of previous studies on the relationship of tick-borne pathogens in dogs and humans and the increasing tendency of Lyme borreliosis in humans, continuous monitoring of tick-borne pathogens in ticks attached to animals is required to avoid disease distribution and possible transmission to humans.
4. Materials and Methods
4.1. Tick Collection and Species Identification
In this study, 562 ticks (5 larvae, 507 nymphs, and 50 adults) attached to dogs were collected from 27 regions in Gyeongbuk province, Korea between 2007 and 2015 and were preserved in 70% ethanol. The ticks were collected by practicing veterinarians at local clinics during monitoring, surveillance, and treatment or during regular check-ups after obtaining verbal consent from the dog owners. The tick collection did not require ethical approval from any authority. In addition, removal of ticks from dogs is neither harmful nor against animal welfare. In cases of larvae or nymphs, one to five tick samples were pooled depending on their sizes for DNA extraction. Finally, 276 tick pools were included in this study.
Tick species were identified in some selected samples by sequencing according to mitochondrial 16S rRNA and ITS-2 [48,49]. Mitochondrial 16S rRNA and ITS-2 have been reported to be reliable molecular markers for tick species identification [50].
4.2. DNA Extraction, PCR, and Sequencing
DNA was extracted using the DNeasy® Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The quality of the extracted DNA was assessed using a spectrometer (Infinite® 200 PRO NanoQuant; Tecan, Mannedorf, Switzerland).
For the detection of Borrelia spp., nested PCR assays were performed using the AccuPower HotStart PCR Premix Kit (Bioneer, Daejeon, Korea). The primer sets for the detection of Borrelia spp. were Bb23S/Bb23Sa and Bb23SnF/Bb23SanR targeting the rrf-rrl, as previously described [40]. B. afzelii, which was previously confirmed in our laboratory, and distilled water were used as positive and negative controls, respectively [40].
For molecular characterization, rrf-rrl-positive sample was submitted to amplify the ospA and flagellin genes, as previously described [40,51]. All the amplicons were directly sequenced by Solgent (Daejeon, Korea) bidirectionally. The obtained sequences were aligned by MUSCLE in MEGA 7.0 [52].
4.3. Phylogenetic Analysis
The obtained sequences were compared with those deposited in the GenBank database using the BLAST. Moreover, phylogenetic analysis was performed according to the rrf-rrl, ospA, and flagellin genes for molecular characterization. The trees were constructed using MEGA 7.0 according to the MP method with the Tree Bisection–Reconnection method for the MP search method [52].
In addition, phylogenetic trees were constructed according to the ML method (Tamura 3-parameter, gamma distributed rate) using MEGA 7.0 and the BI method (Tamura 3-parameter, gamma distributed rate) using MrBayes v3.2.6 [53]. The constructed trees were compared to confirm the absence of significant differences [54]. The best-fit model for phylogeny was selected according to the ML value, and the reliability of topology was supported by 500 bootstrap replications [52]. The sequences analyzed in this study were included with consideration of the isolated host and country.
Ticks and Borrelia species included in the phylogenetic analysis are summarized according to species, strain or isolate, length (bp), and GenBank accession number (Tables S1–S5).
Acknowledgments
This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of South Korea (NRF).
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/8/4/289/s1, Figure S1: Phylogenetic analysis of tick species based on (A and B) mitochondrial 16S rRNA and (C and D) the second intergenic spacer region, Figure S2: Phylogenetic analysis of Borrelia garinii based on the 5S–23S intergenic spacer region, Figure S3: Phylogenetic analysis of Borrelia garinii based on the flagellin gene, Figure S4. Phylogenetic analysis of Borrelia garinii based on the outer surface protein A (ospA) gene. Table S1: Mitochondrial 16S rRNA sequences from ticks included in the phylogenetic analysis summarized according to species, strain or isolate, length (bp), and GenBank accession number, Table S2: The second intergenic spacer region sequences from ticks included in the phylogenetic analysis summarized according to species, strain or isolate, length (bp), and GenBank accession number, Table S3: The 5S-23S intergenic spacer region of Borrelia species included in the phylogenetic analysis summarized according to species, strain or isolate, length (bp), and GenBank accession number, Table S4: Flagellin gene of Borrelia species included in the phylogenetic analysis summarized according to species, strain or isolate, length (bp), and GenBank accession number, Table S5: Outer surface protein A gene of Borrelia species included in the phylogenetic analysis summarized according to species, strain or isolate, length (bp), and GenBank accession number.
Author Contributions
Conceptualization, S.-H.L. and D.K.; methodology, S.-H.L.; writing—original draft preparation, S.-H.L.; validation, formal analysis, and review, Y.-K.G., P.J.L.G., O.-D.K. and D.K.; supervision, D.K.
Funding
This research was funded by the Ministry of Education (Grant No. NRF-2016R1D1A1B02015366).
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Stanek G., Reiter M. The expanding Lyme Borrelia complex-clinical significance of genomic species? Clin. Microbiol. Infect. 2011;17:487–493. doi: 10.1111/j.1469-0691.2011.03492.x. [DOI] [PubMed] [Google Scholar]
- 2.Takano A., Nakao M., Masuzawa T., Takada N., Yano Y., Ishiguro F., Fujita H., Ito T., Ma X., Oikawa Y., et al. Multilocus sequence typing implicates rodents as the main reservoir host of human-pathogenic Borrelia garinii in Japan. J. Clin. Microbiol. 2011;49:2035–2039. doi: 10.1128/JCM.02544-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgdorfer W., Barbour A.G., Hayes S.F., Benach J.L., Grunwaldt E., Davis J.P. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 4.Park K.H., Lee S.H., Won W.J., Jang W.J., Chang W.H. Isolation of Borrelia burgdorferi, the causative agent of Lyme disease, from Ixodes ticks in Korea. J. Korean Soc. Microbiol. 1992;27:307–312. [Google Scholar]
- 5.Shih C.M., Chang H.M., Chen S.L., Chao L.L. Genospecies identification and characterization of Lyme disease spirochetes of genospecies Borrelia burgdorferi sensu lato isolated from rodents in Taiwan. J. Clin. Microbiol. 1998;36:3127–3132. doi: 10.1128/jcm.36.11.3127-3132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Michelis S., Sewell H.S., Collares-Pereira M., Santos-Reis M., Schouls L.M., Benes V., Holmes E.C., Kurtenbach K. Genetic diversity of Borrelia burgdorferi sensu lato in ticks from mainland Portugal. J. Clin. Microbiol. 2000;38:2128–2133. doi: 10.1128/jcm.38.6.2128-2133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goossens H.A., van den Bogaard A.E., Nohlmans M.K. Dogs as sentinels for human Lyme borreliosis in The Netherlands. J. Clin. Microbiol. 2001;39:844–848. doi: 10.1128/JCM.39.3.844-848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Lyme Disease Data Tables: Historical Data. [(accessed on 23 July 2018)];2018 Available online: https://www.cdc.gov/lyme/stats/tables.html.
- 9.Lee M.G., Chung K.Y., Choi Y.S., Cho S.N. Lyme disease. Korean J. Dermatol. 1993;31:601–605. doi: 10.5124/jkma.2004.47.11.1063. (In Korean) [DOI] [Google Scholar]
- 10.KCDC . Infectious Diseases Surveillance Yearbook 2018. Korea Centers for Disease Control and Prevention; Chungbuk, Korea: 2019. [Google Scholar]
- 11.Lee S.H., Yun S.H., Choi E., Park Y.S., Lee S.E., Cho G.J., Kwon O.D., Kwak D. Serological detection of Borrelia burgdorferi among horses in Korea. Korean J. Parasitol. 2016;54:97–101. doi: 10.3347/kjp.2016.54.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh G.H., Ahn K.S., Ahn J.H., Kim H.J., Leutenegger C., Shin S.S. Serological and molecular prevalence of canine vector-borne diseases (CVBDs) in Korea. Parasites Vectors. 2017;10:146. doi: 10.1186/s13071-017-2076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S.H., Hwang K.J., Chu H., Park M.Y. Serological detection of Lyme borreliosis agents in patients from Korea, 2005–2009. Osong Public Health Res. Perspect. 2011;2:29–33. doi: 10.1016/j.phrp.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang J.G., Chae J.B., Cho Y.K., Jo Y.S., Shin N.S., Lee H., Choi K.S., Yu D.H., Park J., Park B.K. Molecular detection of Anaplasma, Bartonella, and Borrelia theileri in raccoon dogs (Nyctereutes procyonoides) in Korea. Am. J. Trop. Med. Hyg. 2018;98:1061–1068. doi: 10.4269/ajtmh.17-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith F.D., Ballantyne R., Morgan E.R., Wall R. Estimating Lyme disease risk using pet dogs as sentinels. Comp. Immunol. Microbiol. Infect. Dis. 2012;35:163–167. doi: 10.1016/j.cimid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Irwin P.J., Robertson I.D., Westman M.E., Perkins M., Straubinger R.K. Searching for Lyme borreliosis in Australia: Results of a canine sentinel study. Parasites Vectors. 2017;10:114. doi: 10.1186/s13071-017-2058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdullah S., Helps C., Tasker S., Newbury H., Wall R. Prevalence and distribution of Borrelia and Babesia species in ticks feeding on dogs in the UK. Med. Vet. Entomol. 2018;32:14–22. doi: 10.1111/mve.12257. [DOI] [PubMed] [Google Scholar]
- 18.Duncan A.W., Correa M.T., Levine J.F., Breitschwerdt E.B. The dog as a sentinel for human infection: Prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic states. Vector Borne Zoonotic Dis. 2004;4:221–229. doi: 10.1089/vbz.2004.4.221. [DOI] [PubMed] [Google Scholar]
- 19.Song T.Y., Yang E.M., Kim C.J. A pediatric case of severe fever with thrombocytopenia syndrome in Korea. J. Korean Med. Sci. 2017;32:704–707. doi: 10.3346/jkms.2017.32.4.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim U.J., Kim D.M., Kim S.E., Kang S.J., Jang H.C., Park K.H., Jung S.I. Case report: Detection of the identical virus in a patient presenting with severe fever with thrombocytopenia syndrome encephalopathy and the tick that bit her. BMC Infect. Dis. 2018;18:181. doi: 10.1186/s12879-018-3092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S.H., Kim H.J., Byun J.W., Lee M.J., Kim N.H., Kim D.H., Kang H.E., Nam H.M. Molecular detection and phylogenetic analysis of severe fever with thrombocytopenia syndrome virus in shelter dogs and cats in the Republic of Korea. Ticks Tick Borne Dis. 2017;8:626–630. doi: 10.1016/j.ttbdis.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Lee S.H., Mossaad E., Ibrahim A.M., Ismail A.A., Moumouni P.F.A., Liu M., Ringo A.E., Gao Y., Guo H., Li J., et al. Detection and molecular characterization of tick-borne pathogens infecting sheep and goats in Blue Nile and West Kordofan states in Sudan. Ticks Tick Borne Dis. 2018;9:598–604. doi: 10.1016/j.ttbdis.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Pal U., Li X., Wang T., Montgomery R.R., Ramamoorthi N., Bao F., Yang X., Pypaert M., Pradhan D., Kantor F.S. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Kee S., Hwang K.J., Oh H.B., Kim M.B., Shim J.C., Ree H.I., Park K.S. Isolation and identification of Borrelia burgdorferi in Korea. J. Korean Soc. Microbiol. 1994;29:301–310. [Google Scholar]
- 25.Park K.H., Chang W.H., Schwan T.G. Identification and characterization of lyme disease spirochetes, Borrelia burgdorferi sensu lato, isolated in Korea. J. Clin. Microbiol. 1993;31:1831–1837. doi: 10.1128/jcm.31.7.1831-1837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park K.H., Choi Y.J., Kim J., Park H.J., Song D., Jang W.J. Reclassification of Borrelia spp. isolated in South Korea using multilocus sequence typing. Jpn. J. Infect. Dis. 2018;71:350–353. doi: 10.7883/yoken.JJID.2018.139. [DOI] [PubMed] [Google Scholar]
- 27.Margos G., Vollmer S.A., Cornet M., Garnier M., Fingerle V., Wilske B., Bormane A., Vitorino L., Collares-Pereira M., Drancourt M., et al. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl. Environ. Microbiol. 2009;75:5410–5416. doi: 10.1128/AEM.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zygner W., Jaros S., Wędrychowicz H. Prevalence of Babesia canis, Borrelia afzelii, and Anaplasma phagocytophilum infection in hard ticks removed from dogs in Warsaw (central Poland) Vet. Parasitol. 2008;153:139–142. doi: 10.1016/j.vetpar.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 29.Földvári G., Márialigeti M., Solymosi N., Lukács Z., Majoros G., Kósa J.P., Farkas R. Hard ticks infesting dogs in Hungary and their infection with Babesia and Borrelia species. Parasitol. Res. 2007;101:25–34. doi: 10.1007/s00436-007-0608-6. [DOI] [Google Scholar]
- 30.Wang J., Kelly P., Zhang J., Shi Z., Song C., Zheng X., Zhang Y., Hao Y., Dong H., El-Mahallawy H.S., et al. Detection of Dirofilaria immitis antigen and antibodies against Anaplasma phagocytophilum, Borrelia burgdorferi and Ehrlichia canis in dogs from ten provinces of China. Acta Parasitol. 2018;63:412–415. doi: 10.1515/ap-2018-0047. [DOI] [PubMed] [Google Scholar]
- 31.Chao L.L., Liu L.L., Ho T.Y., Shih C.M. First detection and molecular identification of Borrelia garinii spirochete from Ixodes ovatus tick ectoparasitized on stray cat in Taiwan. PLoS ONE. 2014;9:e110599. doi: 10.1371/journal.pone.0110599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fomenko N.V., Livanova N.N., Chernousova N.Y. Diversity of Borrelia burgdorferi sensu lato in natural foci of Novosibirsk region. Int. J. Med. Microbiol. 2008;298:139–148. doi: 10.1016/j.ijmm.2007.11.008. [DOI] [Google Scholar]
- 33.Inokuma H., Maetani S., Fujitsuka J., Takano A., Sato K., Fukui T., Masuzawa T., Kawabata H. Astasia and pyrexia related to Borrelia garinii infection in two dogs in Hokkaido, Japan. J. Vet. Med. Sci. 2013;75:975–978. doi: 10.1292/jvms.13-0027. [DOI] [PubMed] [Google Scholar]
- 34.Smith R.P., Jr., Muzaffar S.B., Lavers J., Lacombe E.H., Cahill B.K., Lubelczyk C.B., Kinsler A., Mathers A.J., Rand P.W. Borrelia garinii in seabird ticks (Ixodes uriae), Atlantic Coast, North America. Emerg. Infect. Dis. 2006;12:1909–1912. doi: 10.3201/eid1212.060448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim S., Irwin P.J., Lee S., Oh M., Ahn K., Myung B., Shin S. Comparison of selected canine vector-borne diseases between urban animal shelter and rural hunting dogs in Korea. Parasites Vectors. 2010;3:32. doi: 10.1186/1756-3305-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung B.Y., Gebeyehu E.B., Seo M.G., Byun J.W., Kim H.Y., Kwak D. Prevalence of vector-borne diseases in shelter dogs in Korea. Vet. Rec. 2012;171:249. doi: 10.1136/vr.100650. [DOI] [PubMed] [Google Scholar]
- 37.Kim B.J., Kim H., Won S., Kim H.C., Chong S.T., Klein T.A., Kim K.G., Seo H.Y., Chae J.S. Ticks collected from wild and domestic animals and natural habitats in the Republic of Korea. Korean J. Parasitol. 2014;52:281–285. doi: 10.3347/kjp.2014.52.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choe H.C., Fudge M., Sames W.J., Robbins R.G., Lee I.Y., Chevalier N.A., Chilcoat C.D., Lee S.H. Tick surveillance of dogs in the Republic of Korea. Syst. Appl. Acarol. 2011;16:215–222. doi: 10.11158/saa.16.3.5. [DOI] [Google Scholar]
- 39.Sun Y., Xu R. Ability of Ixodes persulcatus, Haemaphysalis concinna and Dermacentor silvarum ticks to acquire and transstadially transmit Borrelia garinii. Exp. Appl. Acarol. 2003;31:151. doi: 10.1023/B:APPA.0000005119.30172.43. [DOI] [PubMed] [Google Scholar]
- 40.VanBik D., Lee S.H., Seo M.G., Jeon B.R., Goo Y.K., Park S.J., Rhee M.H., Kwon O.D., Kim T.H., Geraldino P.J.L., et al. Borrelia species detected in ticks feeding on wild Korean water deer (Hydropotes inermis) using molecular and genotypic analyses. J. Med. Entomol. 2017;54:1397–1402. doi: 10.1093/jme/tjx106. [DOI] [PubMed] [Google Scholar]
- 41.Lee K., Takano A., Taylor K., Sashika M., Shimozuru M., Konnai S., Kawabata H., Tsubota T. A relapsing fever group Borrelia sp. similar to Borrelia lonestari found among wild sika deer (Cervus nippon yesoensis) and Haemaphysalis spp. ticks in Hokkaido, Japan. Ticks Tick Borne Dis. 2014;5:841–847. doi: 10.1016/j.ttbdis.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Figlerowicz M., Urbanowicz A., Lewandowski D., Jodynis-Liebert J., Sadowski C. Functional insights into recombinant TROSPA protein from Ixodes ricinus. PLoS ONE. 2013;8:e76848. doi: 10.1371/annotation/5c0143e4-65c5-4feb-90a6-2824125f9cad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz J.J., Gazumyan A., Schwartz I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J. Bacteriol. 1992;174:3757–3765. doi: 10.1128/jb.174.11.3757-3765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X.F., Pal U., Alani S.M., Fikrig E., Norgard M.V. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 2004;199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Comstedt P., Hanner M., Schüler W., Meinke A., Schlegl R., Lundberg U. Characterization and optimization of a novel vaccine for protection against Lyme borreliosis. Vaccine. 2015;33:5982–5988. doi: 10.1016/j.vaccine.2015.07.095. [DOI] [PubMed] [Google Scholar]
- 46.Sadziene A., Thomas D.D., Bundoc V.G., Holt S.C., Barbour A.G. A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization. J. Clin. Investig. 1991;88:82–92. doi: 10.1172/JCI115308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park H.S., Lee J.H., Jeong E.J., Koh S.E., Park T.K., Jang W.J., Park K.H., Kim B.J., Kook Y.H., Lee S.H. Evaluation of groEL gene analysis for identification of Borrelia burgdorferi sensu lato. J. Clin. Microbiol. 2004;42:1270–1273. doi: 10.1128/JCM.42.3.1270-1273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chao L.L., Shih C.M. Molecular analysis of Rhipicephalus sanguineus (Acari: Ixodidae), an incriminated vector tick for Babesia vogeli in Taiwan. Exp. Appl. Acarol. 2016;70:469–481. doi: 10.1007/s10493-016-0094-6. [DOI] [PubMed] [Google Scholar]
- 49.Lu X., Lin X.D., Wang J.B., Qin X.C., Tian J.H., Guo W.P., Fan F.N., Shao R., Xu J., Zhang Y.Z. Molecular survey of hard ticks in endemic areas of tick-borne diseases in China. Ticks Tick Borne Dis. 2013;4:288–296. doi: 10.1016/j.ttbdis.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Lv J., Wu S., Zhang Y., Chen Y., Feng C., Yuan X., Jia G., Deng J., Wang C., Wang Q. Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida) Parasites Vectors. 2014;7:93. doi: 10.1186/1756-3305-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barbieri A.M., Venzal J.M., Marcili A., Almeida A.P., González E.M., Labruna M.B. Borrelia burgdorferi sensu lato infecting ticks of the Ixodes ricinus complex in Uruguay: First report for the Southern Hemisphere. Vector Borne Zoonotic Dis. 2013;13:147–153. doi: 10.1089/vbz.2012.1102. [DOI] [PubMed] [Google Scholar]
- 52.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The Editors Editorial. Cladistics. 2016;32:1. doi: 10.1111/cla.12148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.