Abstract
Streptococcus suis is an important zoonotic pathogen. Serotype 2 and sequence type (ST) 1 are the most frequently reported strains in both infected humans and pigs. ST7 is only endemic to China, and it was responsible for outbreaks in 1998 and 2005 in China. In the present study, 38 sporadic ST7 S. suis strains, which mostly caused sepsis, were collected from patients in the Guangxi Zhuang Autonomous Region (GX) between 2007 and 2018. Of 38 sporadic ST7 strains, serotype 14 was the most frequent (27 strains, 71.1%), followed by serotype 2 (11 strains, 28.9%). The phylogenetic structure of the ST7 population, including epidemic and sporadic ST7 strains, was constructed using mutational single-nucleotide polymorphisms (SNPs). High diversity within the ST7 population was revealed and divided into five lineages. Only one sporadic ST7 strain, GX14, from a Streptococcal toxic-shock-like syndrome (STSLS) patient was clustered into the same lineage as the epidemic strains. GX14 and the epidemic strains diverged in 1974. The sporadic ST7 strains of GX were mainly clustered into lineage 5, which emerged in 1980. Comparing to genome of epidemic strain, the major differences in genome of sporadic ST7 strains of GX was the absence of 89 kb pathogenicity island (PAI) specific to epidemic strain and insertion of 128 kb ICE_phage tandem MGE or ICE portion of the MGE. These mobile elements play a significant role in the horizontal transfer of antibiotic resistance genes in sporadic ST7 strains. Our results enhanced the understanding of the evolution of the ST7 strains and their ability to cause life-threatening infections in humans.
Keywords: Streptococcus suis, sequence type 7, sporadic strain, serotype 14, phylogenetic structure, comparative genomes
1. Introduction
Streptococcus suis is an important zoonotic pathogen, with pigs as the main reservoir. Patients get infected through close contact with infected pigs or pork-derived products. The majority of S. suis infections are reported in Thailand, Vietnam, and China [1]. Moreover, S. suis has been identified as the third leading cause of bacterial meningitis in adults in Vietnam and Thailand [1,2,3,4]. Acute meningitis is the most common clinical feature of sporadic infections. Distinctively, most cases in China were from outbreaks [2]. A large outbreak in the summer of 2005 in Sichuan Province resulted in 215 cases and 39 deaths [5,6], whereas a smaller outbreak, previously overlooked, occurred in Jiangsu Province in 1998 with 25 cases and 14 deaths [5]. These two outbreaks were characterized by high rates of Streptococcal toxic-shock-like syndrome (STSLS), which is rare in sporadic cases. Multilocus sequence typing (MLST) analysis showed that the two outbreaks were caused by sequence type (ST) 7 strains and were referred to as epidemic strains which derived from ST1 by acquiring 5 genomic islands [6,7]. By minimum core genome sequence typing (MCG), epidemic strains were typed into MCG 1 which also contained ST1, ST6, ST11, ST17 and ST81 [8]. The epidemic strains were specifically distinguished from the other STs of MCG1 by the 13 single-nucleotide polymorphisms (SNPs) [8].
High level of genetic heterogeneity was observed among S. suis strains within the same ST [9,10]. Defining the phylogenetic relationships among different S. suis ST7 strains will contribute to a better understanding of their emergence and evolution as important pathogens in humans. However, the population structure and genetic diversity of the ST7 strains remain poorly understood, given that S. suis ST7 strains have been isolated only in China and most strains were derived from outbreaks [11]. In the present study, 38 S. suis ST7 strains were collected from patients occurred sporadically between 2007 and 2018 in GX. These strains were referred to as sporadic ST7 strains. We sequenced the genomes of the 38 sporadic ST7 strains of GX and evaluated the phylogenetic relationships among the ST7 epidemic and sporadic strains using genomic data. The clinical manifestations, virulence gene genotypes, antibiotic resistance (AR) genes, and corresponding antimicrobial susceptibility profiles of these sporadic ST7 strains of GX were also determined.
2. Results
2.1. MCG Analysis and Serotyping of S. suis ST7 Sporadic Strains
By MCG typing, all 38 ST7 strains from GX were typed to MCG 1. Interestingly, the 13 SNPs used to track the epidemic strains could also distinguish all the sporadic ST7 strains from the epidemic strains [8]. By serotyping, the 38 ST7 GX strains were divided into two serotypes with 27 (71.1%) serotype 14 isolates and 11 (28.9%) serotype 2 isolates.
2.2. Phylogenetic Relationships among the S. suis ST7 Sporadic and Epidemic Strains
The sequencing depth of the 38 strains was 652 ± 94 in average. SC84 was used as a reference for mapping and 4735 SNPs were identified among them, ranging from 1 to 1423 high quality SNPs per genome.
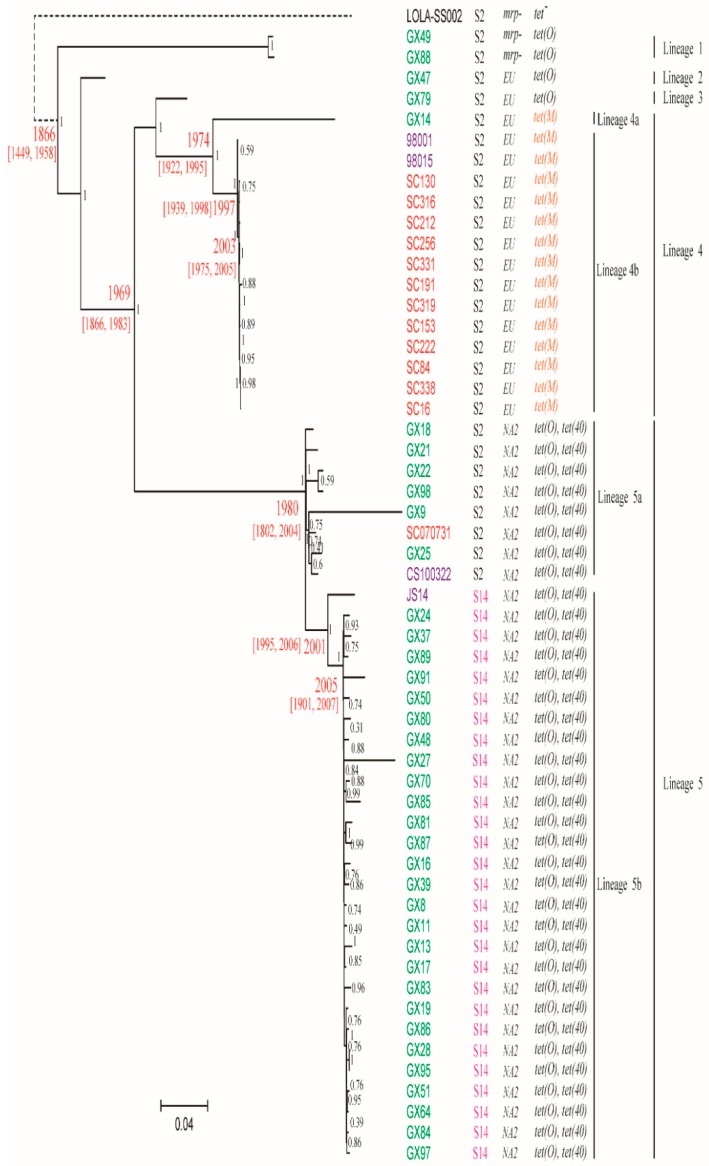
An ST1 strain, LOLA-SS0002, was used as an out-group of the phylogenetic tree. We classified ST7 population into five lineages. A total of 1785 SNPs supported the classification of the lineages (Figure 1).
Figure 1.
A maximum-likelihood phylogenetic tree of ST7 population strains based on mutational SNPs differences across the whole core genome. The ST1 strain LOLA-SS002 was used as an outgroup to root the tree. The strains are colored on based on the regions of the isolation. Red is Sichuan Province, purple is Jiangsu Province and green is GX. Serotype 14 and tet(M) gene were colored in pink and orange red, respectively. The dates shown in red are the median estimates for the indicated nodes and corresponding 95% confidence intervals, taken from the results of the BEAST analysis. The bootstrap values were added in each node in black. The scale is given as the number of substitutions per variable site.
Lineages 1, 2 and 3 contained 2, 1 and 1 sporadic serotype 2 GX strains, respectively, which appeared to be early diverged lineages (Figure 1). Lineage 4 contained one sporadic serotype 2 strain GX14 and all epidemic serotype 2 strains, with the latter being clustered together and separated from GX14 by 1582 SNPs. The remaining 33 sporadic GX strains and three complete genomes of sporadic ST7 strains were clustered into lineage 5.
Within the lineage, serotype 2 strains and serotype 14 strains were separated as two sub-lineage 5a and 5b with 70 SNPs separating them. It is clear that serotype 14 strains shared a common origin and were derived from a serotype 2 strain. The serotype 14 strain JS14 from Jiangsu Province appeared to have diverged from serotype 14 strains from GX with 56 SNPs specific to JS14.
We used BEAST to estimate the divergence time of the main lineages. The rate of SNP accumulation in core genome was estimated to be 1.95 SNPs year−1 (95% confidence interval [CI] 0.34–3.1), which was similar to 1.8 SNPs year−1 in epidemic strains [12]. Based on the accumulation rate of 1.95 SNPs year−1, the most recent common ancestor of the epidemic and sporadic ST7 strains dated back to 1866. The serotype 14 ST7 lineage arose in 2001 and likely spread in GX in 2005. Lineage 4 which gave rise to the epidemic lineage (lineage 4b) arose in 1974, while lineage 5 which gave rise to the serotype 14 lineage arose in 1980. The time estimates were consistent with those in our previous study on the divergence of the outbreak strains [12].
2.3. Geographic Distribution of S. suis ST7 Sporadic Strains
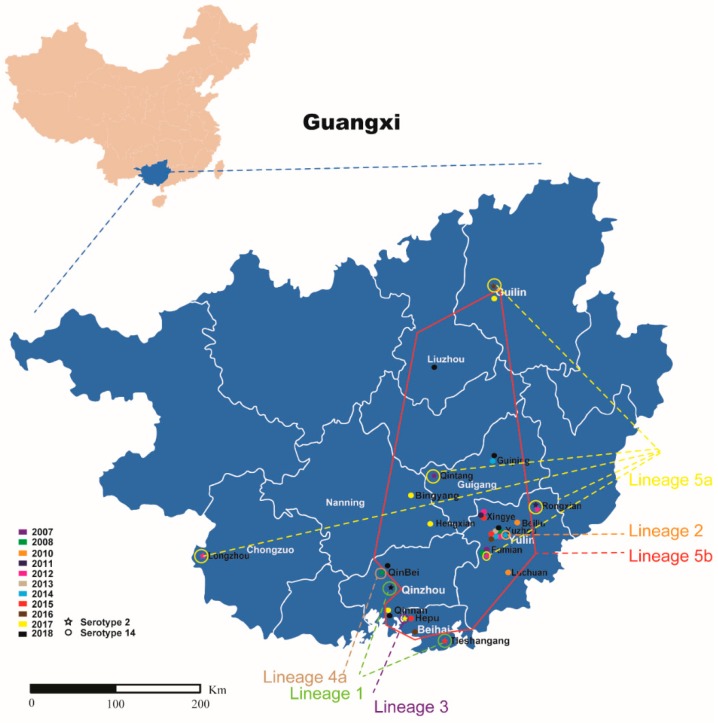
There was a wide geographic distribution of the strains within the same lineage. Two lineage 1 strains were found in two different cities, namely, Beihai City and Qinzhou City. Six lineage 5a strains were distributed in four cities, and 28 lineage 5b strains were distributed in seven cities (Figure 2).
Figure 2.
The geographic spread of the sporadic ST7 strains of GX. Strains of different lineages are indicated in different colors (lineage 1, 2, 3, 4a, 5a and 5b is green, orange, purple, brown, yellow and red, respectively). Stars in different colors on the map represent serotype 2 isolated in different years; Cycles in different colors on the map represent serotype 14 isolated in different years.
2.4. Virulence Genes of S. suis ST7 Sporadic Strains
All the ST7 GX strains were positive for the ef and sly genes. Except for two lineage 1 strains, all were positive for the mrp gene. SSU05_0473, neuB, neuC, SpyM3-0908, rgg, nadR, ofs, sao and revS, present in highly pathogenic strains [11,13], were present in all sporadic ST7 GX strains. Additionally, the 8 regions of difference (RD) [11], which were previously identified to be preferentially present in highly pathogenic strains were also present in sporadic ST7 GX strains (excluding RD17 coding serotype 2 cps locus, normally absent in serotype 14 strains).
2.5. Whole Genome Synteny Analysis
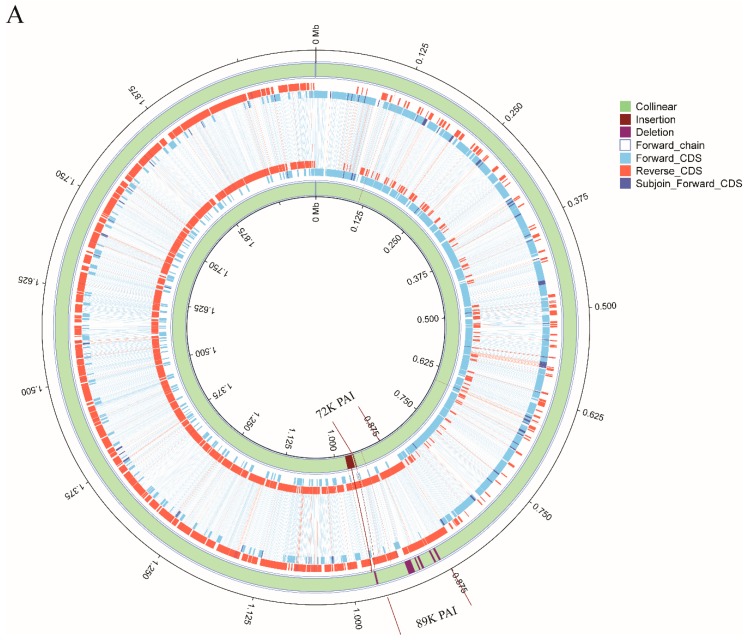
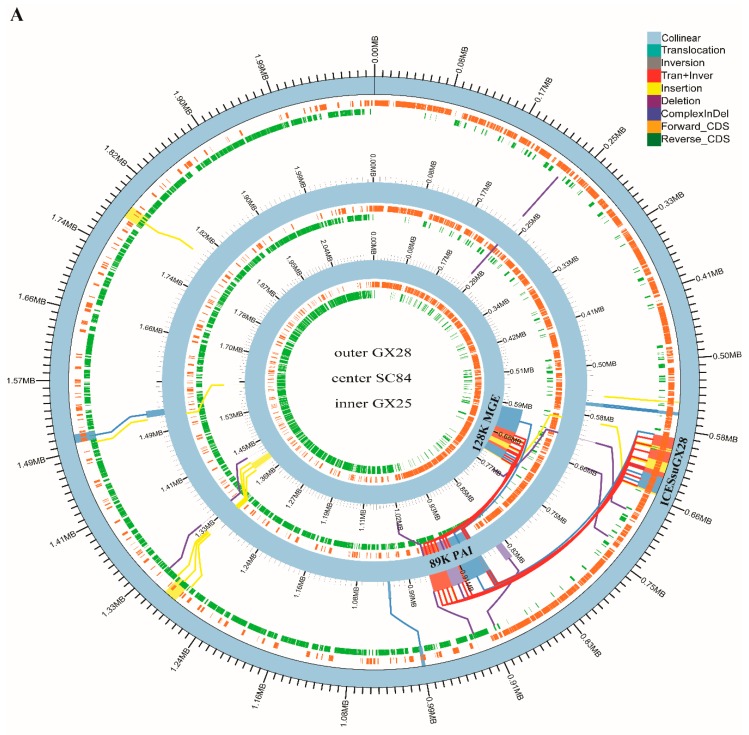
To gain insights into the genomic differences between sporadic ST7 strains and epidemic strains, complete genomes of lineage 4 strain GX14, lineage 5a strain GX25 and lineage 5b strain GX28 were firstly compared to that of SC84. A high degree of conservation was noted in the genome organizations and sequences between epidemic and two sporadic ST7 strains, major differences among them in that: (i) an 89 kb pathogenicity island (PAI) specific to epidemic strain [14] is absent in Lineage 5 strain GX25 and GX28. Only Lineage 4 strain GX14 harbored a similar island with 99.3% identity and 80% coverage with the 89K PAI at the nucleotide level, which we designated as the 72K PAI (Figure 3A,B). The 72K PAI was inserted into the same site as the 89K PAI between rplL and hdy and harbored a 15-bp att sequence 5′-TTATTTAAGAGTAAC-3′ at both ends of the island. The NisK-NisR-like and SalR-SalK-like 2-component signal transduction systems of the 89K PAI, which may contribute to the virulence of the epidemic strain, were also present in the 72K PAI. An intact type IV secretion system (T4SS) containing the VirB1, VirB4, VirB6, and VirD4 genes identified in the 72K PAI was identical to that of the 89K PAI. tet(M) and ant6ia genes were present in both PAIs. The distinct differences between the two PAIs revealed that a large lantibiotic biosynthesis cluster and an ABC transport system carried by the 89K PAI were absent in the 72K PAI.
Figure 3.
(A). Mauve alignment and structure variation of the genome of sporadic ST7 GX14 and epidemic strain SC84. The syntenic regions and unique regions in the genomes are shown as corresponding colored areas. The inner and outer circle is GX14 and SC84, respectively. (B). Schematic comparison of 72K PAI in the study and 89K PAI. The direction of the arrow indicates the direction of transcription. Regions of >70% identity were marked by blue shading. The AR genes, 2-component signal-transduction systems, type IV secretion system, lantibiotic biosynthesis cluster and ABC transport system were indicated by different colors. Tn916 was highlighted in a black box. The att sites are located in the flanking region of PAIs.
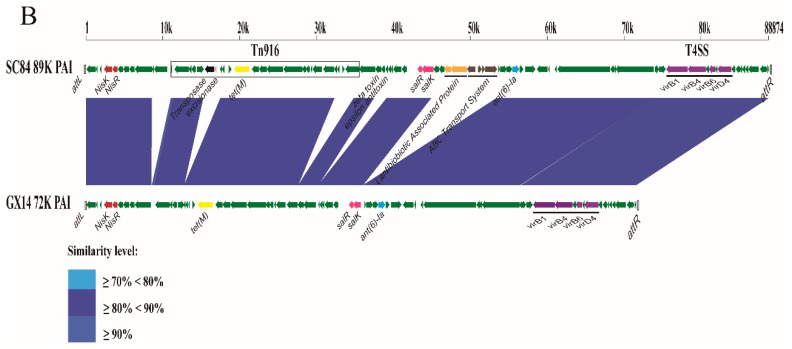
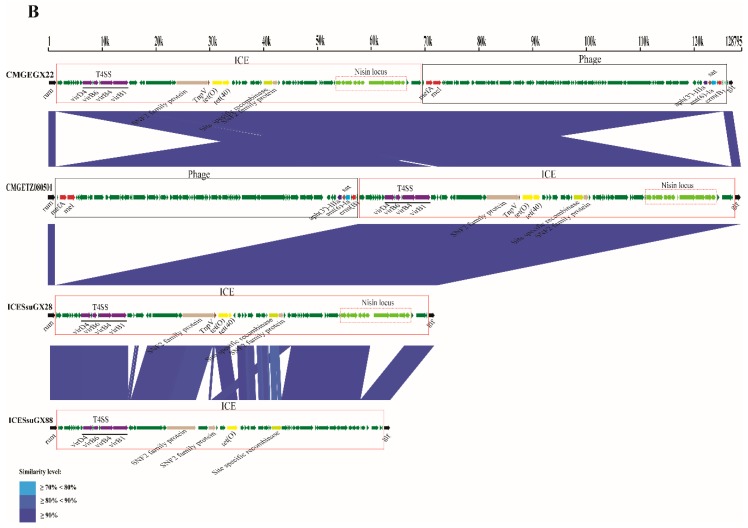
(ii) a 128 kb ICE_phage tandem MGE identical to CMGETZ080501 [15] exists in GX25 but is absent in the epidemic strain SC84 (Figure 4A). Multiple tandem AR genes were present in these MGEs, including tet(O)- tet(40), mefA - mel and aph(3’)-IIIa-sat4-erm(B)-ant6ia (Figure 4B). The tet(O)and tet(40) genes encoded for tetracyclines resistance. The ermb, mef(A) and mel genes were responsible for the resistance to macrolides and lincosamide. The aminoglycoside O-nucleotidylyltransferase ant6ia gene conferred resistance to streptomycin, while the aminoglycoside O-phosphotransferase aph(3’)-IIIa gene primarily inactivated kanamycin. The sat4 gene conferred resistance to streptothricin. In addition, only ICE portion of CMGETZ080501 was present in GX28, named ICESsuGX28 (Figure 4A). ICESsuGX28 harbored tandem tet(O)- tet(40) genes (Figure 4B). Both of elements were inserted into same site between rum and glf genes. All of them harbored a 14 bp att sequence 5′-CACGTGGAGTGCGT-3′ and 5′-CACATAGAAGTTGT-3′ in 5′ and 3′ side of region, respectively.
Figure 4.
(A). Mauve alignment and structure variation of the genome of sporadic ST7 GX25, GX28 and epidemic strain SC84. The syntenic regions and unique regions in the genomes are shown as corresponding colored areas. (B). Schematic comparison of ICEs and ICE-phage tandem MGE in the study. The direction of the arrow indicates the direction of transcription. Regions of >70% identity were marked by blue shading. The AR genes were indicated by different colors. ICE and phage were highlighted in a red and black box, respectively.
2.6. Identification of Lineage-related Genetic Characteristics
In the study, GX strains from same lineage possessed similar genetic elements and features:
(i) 128 kb MGE harbored in lineage 5a strains GX25 were also present in lineage 5a strains GX9, GX21, GX24 and GX87. Interestingly, both GX22 and GX98 harbored similar 128K MGE, which was named CMGEGX22. Compared to that of CMGETZ080501, the arrangement of the ICE and phage portions was reversed in the CMGEGX22 (Table 1, Figure 4B).
Table 1.
The information of strains used in the study.
| Lineage | Strain Name | Accession No. | City | Year | Symptom | PAI/GI | PEN G (BP: > 4 μg/mL) | CEF (BP: > 4 μg/mL) | TET (BP: > 8 μg/mL) | ERY (BP: > 1 μg/mL) | AZI (BP: >1 μg/mL) | CLI (BP: > 1 μg/mL) | STR (BP: > 250 μg/mL) | KAN (BP: > 250 μg/mL) | SPE (BP: > 256 μg/mL) | GEN (BP: > 250 μg/mL) | TRI (BP *: > 4 μg/mL) | Antibiotic Resistance Genes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage 1 | GX49 | SRR8523142 | Beihai | 2015 | Sepsis | ICESsuGX88 | 0.064 | 0.047 | 16 | 0.19 | 0.38 | 0.5 | 2 | 6 | 32 | 6 | 1 * | tet(O) |
| GX88 | SRR8523131 | Qinzhou | 2018 | Meningitis | ICESsuGX88 | 0.064 | 0.064 | 24 | 48 | >256 | >256 | 2 | 4 | 32 | 4 | 0.75 * | tet(O) | |
| Lineage 2 | GX47 | SRR8523138 | Yulin | 2014 | Sepsis | ICESsuGX88 | 0.064 | 0.064 | 24 | >256 | >256 | >256 | 4 | 3 | 32 | 6 | 3 * | erm(B), tet(O) |
| Lineage 3 | GX79 | SRR8523125 | Beihai | 2017 | Meningitis | / | 0.047 | 0.047 | 24 | >256 | >256 | >256 | >1024 | >256 | 16 | 12 | 0.5 * | aph(3’)-IIIa, erm(B), tet(O), sat4, ant(6)-Ia |
| Lineage 4a | GX14 | SRR8523149 | Qinzhou | 2008 | STSLS | 72K | 0.094 | 0.047 | 24 | 0.19 | 0.38 | 0.38 | >1024 | 8 | 24 | 8 | 0.75 * | ant(6)-Ia, tet(M) |
| Lineage 5a | GX18 | SRR8523148 | Yulin | 2011 | Sepsis | ICESsuGX81 | 0.094 | 0.125 | 24 | 0.75 | 0.75 | 0.75 | 3 | 6 | 24 | 8 | 0.5 * | tet(O), tet(40) |
| GX21 | SRR8523152 | Yulin | 2012 | Sepsis | CMGETZ080501 | 0.032 | 0.064 | 24 | >256 | >256 | >256 | 512 | >256 | 16 | 8 | 0.75 * | aph(3’)-IIIa, erm(B), tet(O), tet(40), sat4, mefA, mel,ant(6)-Ia | |
| GX22 | SRR8523135 | Chongzuo | 2012 | Sepsis | CMGEGX22 | 0.047 | 0.064 | 32 | >256 | >256 | >256 | >1024 | >256 | 24 | 4 | 0.25 * | aph(3’)-IIIa, erm(B), tet(O), tet(40), sat4, mefA, mel,ant(6)-Ia | |
| GX98 | SRR8523127 | Guilin | 2016 | / | CMGEGX22 | 0.047 | 0.047 | 24 | >256 | >256 | >256 | >1024 | >256 | 16 | 6 | 0.38 * | aph(3’)-IIIa, erm(B), tet(O), tet(40), sat4, mefA, mel,ant(6)-Ia | |
| GX9 | SRR8523146 | Guigang | 2007 | Meningitis | CMGETZ080501 | 1.5 | 2 | 16 | >256 | >256 | >256 | >1024 | >256 | 24 | 3 | >32 * | aph(3’)-IIIa, erm(B), tet(O), tet(40), sat4, mefA, mel | |
| GX25 | SRR8523133 | Yulin | 2012 | Sepsis | CMGETZ080501 | 0.047 | 0.064 | 16 | >256 | >256 | >256 | >1024 | >256 | 24 | 6 | 3 * | aph(3’)-IIIa, erm(B), tet(O), tet(40), sat4, mefA, mel,ant(6)-Ia | |
| Lineage 5b | GX24 | SRR8523136 | Yulin | 2012 | Meningitis | ICESsuGX81 | 0.064 | 0.094 | 16 | >256 | >256 | >256 | 2 | 3 | 24 | 6 | 0.75 * | erm(B), tet(O), tet(40) |
| GX37 | SRR8523140 | Guigang | 2014 | Sepsis | ICESsuGX81 | 0.047 | 0.094 | 24 | 0.75 | 0.75 | 0.5 | 6 | 4 | 48 | 8 | 0.5 * | tet(O), tet(40) | |
| GX89 | SRR8523132 | Guigang | 2018 | Meningitis | ICESsuGX81 | 0.064 | 0.064 | 24 | 0.5 | 0.5 | 0.75 | 2 | 3 | 24 | 2 | 0.38 * | tet(O), tet(40) | |
| GX91 | SRR8523117 | Liuzhou | 2018 | / | ICESsuGX81 | 0.047 | 0.064 | 16 | >256 | >256 | >256 | >1024 | 12 | >1024 | 4 | 0.38 * | spw, erm(B), tet(O), ant(6)-Ia, tet(40) | |
| GX50 | SRR8523121 | Beihai | 2015 | Sepsis | ICESsuGX81 | 0.064 | 0.38 | 12 | 0.125 | 0.25 | 0.5 | 2 | 4 | 24 | 6 | 0.75 * | tet(O), tet(40) | |
| GX80 | SRR8523124 | Qinzhou | 2017 | Meningitis | ICESsuGX81 | 0.047 | 0.125 | 12 | >256 | >256 | >256 | 2 | 4 | 24 | 4 | 0.75 * | tet(O), tet(40) | |
| GX48 | SRR8523141 | Yulin | 2015 | Sepsis | ICESsuGX81 | 0.064 | 0.064 | 16 | 0.5 | 0.75 | 0.75 | 6 | 8 | 24 | 8 | 1 * | tet(O), tet(40) | |
| GX27 | SRR8523134 | Yulin | 2012 | Sepsis | ICESsuGX81 | 0.064 | 0.125 | 16 | 0.38 | 0.38 | 0.75 | 2 | 2 | 48 | 4 | 0.5 * | tet(O), tet(40) | |
| GX70 | SRR8523118 | Beihai | 2016 | Sepsis | ICESsuGX81 | 0.047 | 0.19 | 16 | 0.38 | 0.5 | 0.75 | 2 | 2 | 32 | 4 | 0.19 * | tet(O), tet(40) | |
| GX85 | SRR8523115 | Qinzhou | 2018 | Meningitis | ICESsuGX81 | 0.064 | 0.064 | 16 | 0.75 | 0.75 | 0.75 | 2 | 3 | 48 | 4 | 1 * | tet(O), tet(40) | |
| GX81 | SRR8523123 | Nanning | 2017 | Sepsis | ICESsuGX81 | 0.094 | 0.094 | 12 | 48 | >256 | >256 | 8 | 12 | 24 | 6 | 2 * | tet(O), tet(40) | |
| GX87 | SRR8523130 | Qinzhou | 2018 | Sepsis | ICESsuGX81 | 0.047 | 0.094 | 24 | >256 | >256 | >256 | >1024 | >256 | 32 | >256 | 0.25 * | AAC(6′)-Ie, APH(2″)-Ia, erm(B), tet(O), ant(6)-Ia, tet(40) | |
| GX16 | SRR8523150 | Yulin | 2010 | Sepsis | ICESsuGX81 | 0.094 | 0.064 | 16 | 0.75 | 0.75 | 0.75 | 2 | 2 | 32 | 4 | 0.38 * | tet(O), tet(40) | |
| GX39 | SRR8523137 | Yulin | 2014 | / | ICESsuGX81 | 0.064 | 0.064 | 16 | >256 | >256 | >256 | >1024 | >256 | 16 | >256 | >32 * | dfrG, erm(B), tet(O), ant(6)-Ia, tet(40), AAC(6′)-Ie-APH(2″)-Ia | |
| GX8 | SRR8523145 | Yulin | 2007 | Sepsis | ICESsuGX81 | 0.094 | 0.094 | 12 | >256 | >256 | >256 | 3 | 8 | 24 | 6 | 0.25 * | erm(B), tet(O), tet(40) | |
| GX11 | SRR8523143 | Yulin | 2008 | Sepsis | ICESsuGX81 | 0.094 | 0.094 | 24 | 0.75 | 0.75 | 0.5 | 3 | 8 | 32 | 6 | 1 * | tet(O), tet(40) | |
| GX13 | SRR8523144 | Yulin | 2008 | Meningitis | ICESsuGX81 | 0.064 | 0.094 | 24 | >256 | >256 | >256 | 4 | 6 | 32 | 4 | 0.25 * | erm(B), tet(O), tet(40) | |
| GX17 | SRR8523147 | Yulin | 2010 | Sepsis | ICESsuGX81 | 0.064 | 0.094 | 16 | 0.5 | 0.5 | 0.75 | 8 | 6 | 32 | 3 | 0.094 * | tet(O), tet(40) | |
| GX83 | SRR8523122 | Nanning | 2017 | Sepsis | ICESsuGX81 | 0.094 | 0.094 | 12 | 0.75 | 0.75 | 0.38 | 4 | 6 | 24 | 8 | 3 * | tet(O), tet(40) | |
| GX19 | SRR8523151 | Yulin | 2011 | Meningitis | ICESsuGX81 | 0.047 | 0.094 | 16 | 0.75 | 0.75 | 0.5 | 12 | 8 | 32 | 3 | 0.125 * | tet(O), tet(40) | |
| GX86 | SRR8523129 | Yulin | 2018 | Sepsis | ICESsuGX81 | 0.064 | 0.064 | 16 | >256 | >256 | >256 | >1024 | 8 | >1024 | 3 | 0.25 * | spw, erm(B), tet(O), ant(6)-Ia, tet(40) | |
| GX28 | SRR8523139 | Yulin | 2013 | Sepsis | ICESsuGX81 | 0.047 | 0.064 | 16 | >256 | >256 | >256 | >1024 | 8 | >1024 | 4 | 0.75 * | spw, erm(B), tet(O), ant(6)-Ia, tet(40) | |
| GX95 | SRR8523128 | / | 2013 | / | ICESsuGX81 | 0.047 | 0.064 | 24 | >256 | >256 | >256 | >1024 | 6 | >1024 | 2 | 0.19 * | spw, erm(B), tet(O), ant(6)-Ia, tet(40) | |
| GX51 | SRR8523120 | Yulin | 2015 | Sepsis | ICESsuGX81 | 0.047 | 0.047 | 16 | >256 | >256 | >256 | >1024 | 2 | >1024 | 8 | 0.75 * | spw, erm(B), tet(O), ant(6)-Ia, tet(40) | |
| GX64 | SRR8523119 | Yulin | 2016 | / | ICESsuGX81 | 0.094 | 0.064 | 16 | >256 | >256 | >256 | >1024 | 12 | >1024 | 6 | 0.75 * | spw, erm(B), tet(O), ant(6)-Ia, tet(40) | |
| GX84 | SRR8523116 | Guilin | 2017 | / | ICESsuGX81 | 0.064 | 0.064 | 16 | >256 | >256 | >256 | >1024 | 8 | >1024 | 3 | 0.5 * | spw, erm(B), tet(O), ant(6)-Ia, tet(40) | |
| GX97 | SRR8523126 | / | 2018 | / | ICESsuGX81 | 0.047 | 0.064 | 16 | >256 | >256 | >256 | >1024 | 4 | >1024 | 1.5 | 0.38 * | spw, erm(B), tet(O), ant(6)-Ia, tet(40) |
Abbreviations: PEN G = Penicillin G; CEF = Cefaclor; TET = Tetracycline; ERY = Erythromycin; AZI = Azithromycin; CLI = Clindamycin; STR = Streptomycin; KAN = Kanamycin; SPE = Spectinomycin; GEN = Gentamicin; TRI = Trimethoprim*-sulfamethoxazole (1/19) *: MIC value of Trimethoprim.
(ii) ICESsuGX28 were present in all GX strains of lineage 5b (Table 1). Different from GX strains of lineage 5b, three GX strains of lineage 1 and 2 harbored a similar ICE to ICESsuGX28, named ICESsuGX88 (Table 1, Figure 4B). The tet(40) gene was absent in ICESsuGX88. A nonsense mutation, an insertion of an A base at position 390, was found in the tet(40) gene of all the GX strains in lineage 5b.
(iii) Except for two lineage 1 GX strains, all were positive for the mrp gene. Based on the sequence variation in the central portion of the mrp gene, three subtypes, namely, EU, NA1, and NA2 have been reported [16]. All the GX strains of lineages 2, 3, and 4 harbored the EU subtype, whereas all GX strains of lineage 5 harbored the NA2 subtype (Figure 1).
(iv) All strains from STSLS patients were clustered into lineage 4. All 28 lineage 4 specific genes were located in 89K and 72K PAIs
2.7. Antimicrobial Susceptibility Profiles
In addition to tandem AR genes in tandem MGEs and ICE, multiple tandem AR genes were also present in the chromosome of 10 GX strains from lineage 5b, including erm(B)-ant6ia- spw (GX28, GX51, GX64, GX84, GX86, GX91, GX95 and GX97), ant6ia - aac(6′)Ie-aph(2″)Ia - dfrG (GX39) and ant6ia- aac(6′)Ie-aph(2″)Ia - erm(B) (GX87). In additional, aph(3″)IIIa -ant6ia- sat4 was present in the chromosome of GX79 from lineage 3.
Based on the detection of the AR genes, we tested all the strains for susceptibility to penicillin G, cefaclor, tetracycline, erythromycin, azithromycin, clindamycin, streptomycin, kanamycin, spectinomycin, gentamicin, and trimethoprim–sulfamethoxazole. All the strains were susceptible to penicillin G and cefaclor. By contrast, all the strains were resistant to tetracycline with MICs between 16 and 32 μg/mL. Concomitant resistance to erythromycin and clindamycin was observed in strains carrying erm(B) as reported in previous studies [17,18]. The MICs of both antibiotics were >256 μg/mL.
High degrees of kanamycin (MIC >256 μg/mL), spectinomycin (MIC >1024 μg/mL), and gentamicin (MIC >256 μg/mL) resistance were found in strains carrying aph3-iiia, spw, and aac(6′)Ie-aph(2″)Ia genes, consistent with previous studies [19,20,21], respectively. The MIC of streptomycin was >1024 μg/mL in 17 strains carrying the ant6ia gene. GX39 carrying the dfrG gene was resistant to trimethoprim–sulfamethoxazole with an MIC >32 μg/mL.
Two strains, GX81 and GX87, were resistant to erythromycin, azithromycin and clindamycin with MICs of 48 μg/mL, >256 μg/mL, and >256 μg/mL, respectively, but they did not harbor known AR genes. Similarly, strain GX9 had an MIC of >32 μg/mL against trimethoprim–sulfamethoxazole. It also did not harbor known AR genes (Table 1).
3. Discussion
MLST has been wildly used to genetically classify S. suis strains. To date, ST1 and ST7 have been predominately reported to be responsible for human infections in China. Different from ST1 as most common culprit of the human cases worldwide, the ST7 strains were endemic to China and responsible for two deadly outbreaks in 1998 and 2005 [6]. However, few sporadic cases caused by ST7 strains have been studied, even though ST7 strains have been usually isolated from diseased pigs in China [22,23]. In China, sporadic ST7 strains from patients were mainly reported in GX and Guangdong province [24]. In this study, 38 sporadic cases infected with ST7 strains were collected in GX between 2007 and 2018. Our study revealed that the majority of the sporadic cases were caused by serotype 14, derived from serotype 2 within the ST7 strain. Worldwide, serotype 2 is the most frequently reported serotype at 74.7%, followed by serotype 14 at only 2.0% [2]. Among the 177 S. suis strains isolated from patients in Thailand, 165 (93.2%) and 12 (6.8%) were identified as serotype 2 and 14, respectively [25].
Our phylogenetic analysis of the epidemic and sporadic ST7 strains revealed a high level of genomic heterogeneity among the strains, which were divided into five lineages. All the sporadic ST7 strains could be clearly separated from the epidemic strains by 13 SNPs specific to the epidemic strains as found in our previous study [8].
Sporadic GX strains were mainly clustered into lineage 5 which contained two serotypes. Interestingly, three sporadic strains from Jiangsu and Sichuan Provinces were also clustered with GX sporadic strains. Our data suggested that lineage 5 emerged in 1980, and then spread across China. The epidemic strains were previously found to have likely spread from Jiangsu to Sichuan Province via an inter-provincial spread [12]. It is likely that the trans-provincial transportation of breeder pigs played a key role in the spread of ST7 strains across China.
Serotype 2 and 14 strains in lineage 5 were clustered into separate sub-lineages. In particular serotype 14 strains were closely related and belonged to the same sub-lineage. It is most likely that the horizontal transfer of cps genes resulted in the replacement of the serotype 2 cps gene cluster by the serotype 14 cps gene cluster [26,27]. Since all serotype 14 strains shared a common origin and were very closely related, the replacement occurred only once and was estimated to have occurred in 2005. The exchange of serotypes has further diversified the ST7 population.
All epidemic strains were clustered into lineage 4b. The ancestor of lineage 4 strains was estimated to have emerged in 1974. In our previous study, the estimated time of the most recent common ancestor for the epidemic strains in China was May 1996 [12], indicating that it took over 20 years for ST7 to evolve into an epidemic strain capable of causing severe outbreaks. One sporadic ST7 strain, GX14, was clustered in the same lineage as the epidemic strains that caused STSLS, which was characteristic of the epidemic cases. GX14 differs from the epidemic strains by the presence of a 72K PAI, whereas the epidemic strains harbor an 89K PAI [14]. GX14 appears to be ancestral to the epidemic strains. The 89K PAI may contribute to the development of STSLS by the T4SS-like system and two-component signal transduction systems (TCSTS) [28,29,30]. Both PAIs contained an identical T4SS-like system and two-component signal transduction system but differ in that a large lantibiotic biosynthesis cluster and ABC transporter system carried by the 89K PAI. However, the cluster cannot produce a functional antibiotic due to mutation [31]. It is likely that the most recent common ancestor of GX14 and the epidemic strains (lineage 4) acquired the 72K PAI, and the epidemic lineage further acquired other genes to become the 89K PAI. There are at least five insertions in the 89K PAI compared to the 72 K PAI, suggesting the recent multiple acquisitions of genes. All 28 lineage 4 specific genes were located in 89K and 72K PAIs. These findings further emphasize the fact that these PAIs have contributed to their increased virulence and the capacity of causing more severe outbreaks.
The most prevalent genotype of the mrp gene in the sporadic ST7 GX strains was NA2, whereas the genotype of the epidemic strains was EU. The difference in the mrp genotype has further confirmed the different origins of the epidemic and major sporadic ST7 strains. It is noteworthy that the NA2 subtype was also common in the ST7 strains from diseased pigs [22] and non-ST7 strains of MCG2 [32] in China.
The differences in genome organizations between epidemic and sporadic ST7 strains were caused by acquiring and deletion of MGEs. MGEs also play a significant role in the horizontal transfer of AR genes in Streptococcus [15]. ICE_phage tandem MGE carrying multiple tandem AR genes were found in GX strains from lineage 5a. The ICE portion carried tet(O) in tandem with tet(40), whereas the phage portion carried an erm(B)-containing MAS-like fragment and the mel/mef(A) cassette. They conferred the tetracycline–macrolide–lincosamide–aminoglycoside antibiotics resistance to host strains. The phage portion was absent in remaining GX strains. Our data indicated that the integration of the phage enhanced MGE diversity and played a key role in the dissemination of AR genes in the sporadic ST7 strains.
Different from the sporadic ST7 strains of lineage 1, 2, 3 and 5, only the lineage 4 strains carried the tetracycline resistance gene tet(M) by transposon Tn916 (Figure 3). It is noteworthy that tet(M) is a prevalent tetracycline resistance gene in S. suis strains from sporadic meningitis patients in Vietnam, which is also associated with the presence of Tn916-like elements [33].
Two sporadic ST7 GX strains were resistant to trimethoprim–sulfamethoxazole. To our knowledge, this is the first case of S. suis strains from patients that were resistant to trimethoprim–sulfamethoxazole. The dfrG gene, which confers resistance to trimethoprim [34], contributed to the resistant phenotype of GX39. No known trimethoprim–sulfamethoxazole resistant determinants were identified in GX9. A similar phenomenon was also found in GX81 and GX88 of lineage 5b. Despite the lack of known macrolide and lincosamide resistant determinants, they were resistant to erythromycin, azithromycin and clindamycin. Moreover, mutations in the genes coding for L4 and L22 ribosomal proteins and for 23S rRNA were not identified in their genomes. Novel macrolide, lincosamide and sulfonamide resistance determinants may be present in the three strains, and further studies are needed to address this.
In conclusion, sporadic ST7 S suis infections in GX were predominantly due to serotype 14 strains, in contrast to outbreaks in China, which were caused by serotype 2 strains. The major symptoms also differed, with sepsis for sporadic cases. The ST7 serotype 14 was derived from a ST7 serotype 2 strain about 18 years ago (2001). The sporadic strains could be divided into five lineages. Only one sporadic GX strain fell into lineage 4, and it had the potential to cause an outbreak. These results have enhanced our understanding of the evolution of the ST7 strains and their ability to cause life-threatening infections in humans. In addition, multiple antibiotic resistance is becoming more common in sporadic ST7 GX strains and remains a threat to local public health.
4. Materials and Methods
4.1. Bacterial Strains, Chromosomal DNA Sequencing and Bioinformatic Analysis
A total of 38 human clinical cases from eight regions of GX between 2007 and 2018 were reported. Among the 38 cases, 31 with epidemiological and clinical information were investigated. A total of 19 cases (61.3%) had been exposed to pigs or pork within one week of the appearance of the initial symptoms (Table 1). Most patients (67.7%, 21/31) had sepsis characterized by the acute onset of fever, chills, headaches, dizziness, malaise, abdominal pain, and diarrhea. One sepsis patient also had a coma. Nearly one-third of cases (29%, 9/31) had meningitis characterized by fever, headache, and neck stiffness. Five patients had a coma, two of which also had petechia and purpura. Only one case (a host of GX14) suffered STSLS with fever, hypotension, jaundice, pneumonia, petechia, purpura, coma, and multi-organ failure, which included liver, heart, and renal impairments. A total of 38 strains from human clinical cases from eight regions of GX between 2007 and 2018 were collected. Except for two strains, all had geographic information based on the place of each patient’s residence. Thirty cases were from four cities in the southeast of GX, including Yulin City (n = 18), Qinzhou City (n = 5), Beihai City (n = 4), and Guigang City (n = 3). The remaining six strains were from another four cities, with two each from Nanning City and Guilin City, and one each from Liuzhou City and Chongzuo City (Table 1). All strains were confirmed to belong to S. suis using 16S rRNA sequencing. The serotype was determined by an agglutination test using serum purchased from Statens Serum Institute (Copenhagen, Denmark). MLST and MCG typing were performed according to previously described methods [27,32]. The strains were sequenced by Illumina sequencing and sequences were assembled using SOAPdenovo (release1.04). Complete genomes of GX14, GX25 and GX28 were sequenced using PacBio and Illumina as representatives of sporadic ST7 strains, respectively. The information of sequences obtained in the study was provided in Table S1. Genes were predicted using Glimmer and gene orthologs were determined using OrthoMCL [8]. Sequence comparisons were performed using the blastN program within BLAST with an e-value cutoff e−10 and visualized using an in-house perl script.
4.2. Phylogenetic Analysis
In our previous study, 94 serotype 2 epidemic strains were clustered into six clades with clade 1 and clades 2 to 6 responsible for the outbreaks in 1998 and 2005, respectively [12]. Thirteen genomic sequencing read datasets (accession no. SRP064815) and one complete genome SC84 (accession no. FM252031) selected from each clade (2-3 genomes from each clade) as representatives of epidemic strains were included in the phylogenetic analysis. For comparison purposes, three complete genomes of the sporadic ST7 strains from diseased pigs from the two provinces with outbreaks were also included; these were the JS14 strain (accession no. CP002465, serotype 14, Jiangsu Province) [35], CS100322 strain (accession no. CP024050, serotype 2, Jiangsu province) and SC070731 strain (accession no. CP003922, serotype 2, Sichuan province) [36]. ST1 strain LOLA-SS002 (accession no. FIFC00000000.1, serotype 2, UK), available in GenBank, was used as outgroup to root the tree.
Single-nucleotide polymorphisms (SNPs) were detected using Bowtie2 and MUMmer v3.23 for sequencing reads and complete genomes, respectively, and the genome sequence of SC84 was used as a reference. SNPs were named by using our automatic pipeline described previously [8]. The high quality SNPs supported by more than 5 reads were then concatenated together according to the reference. The adjacent mutations within 5-bp were filtered, and the remained sequences were further checked by clonalFrameML to avoid recombination. The mutational SNP sites were then selected to construct a phylogenetic tree using the maximum likelihood method by FastTree v2.1.10. The Generalized Time-Reversible (GTR) was used to construct the Maximum Likelihood tree. The tree was presented using FigTree v1.4.0.
The BEAST program v2.4.7 was used to estimate the divergence time of the lineages. Four clock models (strict clock, relaxed clock lognormal, relaxed clock exponential, and random local clock) and three population models (constant, exponential, and Bayesian skylines) were tested. The relaxed clock lognormal model in combination with the Bayesian skyline population model was used in accord with our previous study [12]. The chain length was set to 100,000,000 states with resampling every 10,000 states, and the first 1000 trees were ignored. All other parameters were set to default values.
4.3. Whole Genome Synteny Analysis
MUMmer (version 3.22) and Lastz (version 1.02.00) were used to align reference genome SC84 and three complete genomes of GX14, GX25 and GX28. Genomic synteny was performed to identify insertions, deletions, translocations and inversions between genomes of epidemic and sporadic strains.
4.4. Detection of Antibiotic Resistance Determinants and Antimicrobial Susceptibility Profiles
Antibiotic resistance genes were analyzed by searching the Comprehensive Antibiotic Resistance Database (CARD) and the Antibiotic Resistance Genes Database (ARDB). A resistance gene was only regarded as a homolog in tested strains if it showed at least 80% identity in protein sequence across 80% of the length of the protein [37]. Antimicrobial susceptibility testing was performed by assessing the minimum inhibitory concentration (MIC) for all isolates using MIC-test strip (Liofilchem, Roseto degli Abruzzi, Italy). MIC-test strips contained a gradient of concentrations of penicillin G (0.002–32 μg/mL), cefaclor (0.016–256 μg/mL), tetracycline (0.016–256 μg/mL), erythromycin (0.016–256 μg/mL), azithromycin (0.016–256 μg/mL), clindamycin (0.016–256 μg/mL), streptomycin (0.064–1024 μg/mL), kanamycin (0.016–256 μg/mL), spectinomycin (0.064–1024 μg/mL), gentamicin (0.016–256 μg/mL) and trimethoprim-sulfamethoxazole (0.002–32 μg/mL). S. pneumoniae ATCC 49619 was used for quality control. For penicillin G, cefaclor, tetracycline, azithromycin, erythromycin, clindamycin and trimethoprim–sulfamethoxazole, breakpoints were used as recommended in the 2016 Clinical and Laboratory Standard Institute (CLSI) Guidelines (M100-S26) for Streptococcus spp. Viridans Group and Streptococcus pneumoniae. No breakpoint values for streptomycin, kanamycin, gentamicin, and spectinomycin were available for Streptococci, and their breakpoints were taken from previous studies [38,39].
4.5. Nucleotide Sequence Accession Numbers
The sequences of the 38 S. suis strains sequenced in this study were deposited into GenBank under accession numbers ranging from SRR8523115 to SRR8523152.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/8/4/187/s1, Table S1: The information of genomes sequenced in the study.
Author Contributions
Conceptualization, H.Z.; Methodology, H.Z., J.W. and P.D.; Validation, R.L.; Formal Analysis, H.Z. and P.D.; Investigation, M.W.; Resources, M.W., J.H., M.L., Y.J., J.Z., Y.Q. and Z.S.; Data Curation, M.W.; Writing–Original Draft Preparation, H.Z.; Writing–Review & Editing, R.L.; Supervision, H.Z. and M.W. Funding Acquisition, H.Z.
Funding
This work was supported by the National Natural Science Foundation of China under grant [81572044]; the Ministry of Science and Technology of China under grant [2017ZX10303405-002].
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Approval
Clinical strains and related information used in the study were collected with written informed consent from each patient. No information associated with the identity of patient was used. This study was reviewed and approved by the ethics committee of the institution at which the studies were conducted.
References
- 1.Huong V.T., Ha N., Huy N.T., Horby P., Nghia H.D., Thiem V.D., Zhu X., Hoa N.T., Hien T.T., Zamora J., et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg. Infect. Dis. 2014;20:1105–1114. doi: 10.3201/eid2007.131594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyette-Desjardins G., Auger J.P., Xu J., Segura M., Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent—An update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014;3:e45. doi: 10.1038/emi.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wertheim H.F., Nguyen H.N., Taylor W., Lien T.T., Ngo H.T., Nguyen T.Q., Nguyen B.N., Nguyen H.H., Nguyen H.M., Nguyen C.T., et al. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS ONE. 2009;4:e5973. doi: 10.1371/journal.pone.0005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mai N.T., Hoa N.T., Nga T.V., Linh le D., Chau T.T., Sinh D.X., Phu N.H., Chuong L.V., Diep T.S., Campbell J., et al. Streptococcus suis meningitis in adults in Vietnam. Clin. Infect. Dis. 2008;46:659–667. doi: 10.1086/527385. [DOI] [PubMed] [Google Scholar]
- 5.Yu H., Jing H., Chen Z., Zheng H., Zhu X., Wang H., Wang S., Liu L., Zu R., Luo L., et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg. Infect. Dis. 2006;12:914–920. doi: 10.3201/eid1206.051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye C., Zhu X., Jing H., Du H., Segura M., Zheng H., Kan B., Wang L., Bai X., Zhou Y., et al. Streptococcus suis sequence type 7 outbreak, Sichuan, China. Emerg. Infect. Dis. 2006;12:1203–1208. doi: 10.3201/eid1708.060232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye C., Zheng H., Zhang J., Jing H., Wang L., Xiong Y., Wang W., Zhou Z., Sun Q., Luo X., et al. Clinical, experimental, and genomic differences between intermediately pathogenic, highly pathogenic, and epidemic Streptococcus suis. J. Infect. Dis. 2009;199:97–107. doi: 10.1086/594370. [DOI] [PubMed] [Google Scholar]
- 8.Chen C., Zhang W., Zheng H., Lan R., Wang H., Du P., Bai X., Ji S., Meng Q., Jin D., et al. Minimum core genome sequence typing of bacterial pathogens: A unified approach for clinical and public health microbiology. J. Clin. Microbiol. 2013;51:2582–2591. doi: 10.1128/JCM.00535-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Athey T.B., Teatero S., Takamatsu D., Wasserscheid J., Dewar K., Gottschalk M., Fittipaldi N. Population Structure and Antimicrobial Resistance Profiles of Streptococcus suis Serotype 2 Sequence Type 25 Strains. PLoS ONE. 2016;11:e0150908. doi: 10.1371/journal.pone.0150908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Athey T.B., Auger J.P., Teatero S., Dumesnil A., Takamatsu D., Wasserscheid J., Dewar K., Gottschalk M., Fittipaldi N. Complex Population Structure and Virulence Differences among Serotype 2 Streptococcus suis Strains Belonging to Sequence Type 28. PLoS ONE. 2015;10:e0137760. doi: 10.1371/journal.pone.0137760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng X., Zheng H., Lan R., Ye C., Wang Y., Zhang J., Jing H., Chen C., Segura M., Gottschalk M., et al. Identification of genes and genomic islands correlated with high pathogenicity in Streptococcus suis using whole genome tiling microarrays. PLoS ONE. 2011;6:e17987. doi: 10.1371/journal.pone.0017987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du P., Zheng H., Zhou J., Lan R., Ye C., Jing H., Jin D., Cui Z., Bai X., Liang J., et al. Detection of Multiple Parallel Transmission Outbreak of Streptococcus suis Human Infection by Use of Genome Epidemiology, China, 2005. Emerg. Infect. Dis. 2017;23:204. doi: 10.3201/eid2302.160297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang W., Wang M., Hao H., Yang R., Xie J., Su J., Lin M., Cui Y., Jiang Y. Genomic epidemiological investigation of a Streptococcus suis outbreak in Guangxi, China, 2016. Infect. Genet. Evol. 2019;68:249–252. doi: 10.1016/j.meegid.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Chen C., Tang J., Dong W., Wang C., Feng Y., Wang J., Zheng F., Pan X., Liu D., Li M., et al. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS ONE. 2007;2:e315. doi: 10.1371/journal.pone.0000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J., Ma J., Shang K., Hu X., Liang Y., Li D., Wu Z., Dai L., Chen L., Wang L. Evolution and Diversity of the Antimicrobial Resistance Associated Mobilome in Streptococcus suis: A Probable Mobile Genetic Elements Reservoir for Other Streptococci. Front. Cell. Infect. Microbiol. 2016;6:118. doi: 10.3389/fcimb.2016.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fittipaldi N., Fuller T.E., Teel J.F., Wilson T.L., Wolfram T.J., Lowery D.E., Gottschalk M. Serotype distribution and production of muramidase-released protein, extracellular factor and suilysin by field strains of Streptococcus suis isolated in the United States. Vet. Microbiol. 2009;139:310–317. doi: 10.1016/j.vetmic.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Fokas S., Fokas S., Tsironi M., Kalkani M., Dionysopouloy M. Prevalence of inducible clindamycin resistance in macrolide-resistant Staphylococcus spp. Clin. Microbiol. Infect. 2005;11:337–340. doi: 10.1111/j.1469-0691.2005.01101.x. [DOI] [PubMed] [Google Scholar]
- 18.Syrogiannopoulos G.A., Grivea I.N., Ednie L.M., Bozdogan B., Katopodis G.D., Beratis N.G., Davies T.A., Appelbaum P.C. Antimicrobial susceptibility and macrolide resistance inducibility of Streptococcus pneumoniae carrying erm(A), erm(B), or mef(A) Antimicrob. Agents Chemother. 2003;47:2699–2702. doi: 10.1128/AAC.47.8.2699-2702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparo M., Delpech G., Garcia Allende N. Impact on Public Health of the Spread of High-Level Resistance to Gentamicin and Vancomycin in Enterococci. Front. Microbiol. 2018;9:3073. doi: 10.3389/fmicb.2018.03073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurung M., Tamang M.D., Moon D.C., Kim S.R., Jeong J.H., Jang G.C., Jung S.C., Park Y.H., Lim S.K. Molecular Basis of Resistance to Selected Antimicrobial Agents in the Emerging Zoonotic Pathogen Streptococcus suis. J. Clin. Microbiol. 2015;53:2332–2336. doi: 10.1128/JCM.00123-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez M.S., Tolmasky M.E. Aminoglycoside modifying enzymes. Drug Resist. Updates. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng H., Lan R., Zheng X., Cui Z., Liu Z., Bai X., Ji S., Gottschalk M., Xu J. Comparative genomic hybridization identifies virulence differences in Streptococcus suis. PLoS ONE. 2014;9:e87866. doi: 10.1371/journal.pone.0087866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L., Song Y., Wei Z., He H., Zhang A., Jin M. Antimicrobial susceptibility, tetracycline and erythromycin resistance genes, and multilocus sequence typing of Streptococcus suis isolates from diseased pigs in China. J. Vet. Med Sci. 2013;75:583–587. doi: 10.1292/jvms.12-0279. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y., Dong X., Li Z., Zou G., Lin L., Wang X., Chen H., Gasser R.B., Li J. Predominance of Streptococcus suis ST1 and ST7 in human cases in China, and detection of a novel sequence type, ST658. Virulence. 2017;8:1031–1035. doi: 10.1080/21505594.2016.1243193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerdsin A., Oishi K., Sripakdee S., Boonkerd N., Polwichai P., Nakamura S., Uchida R., Sawanpanyalert P., Dejsirilert S. Clonal dissemination of human isolates of Streptococcus suis serotype 14 in Thailand. J. Med. Microbiol. 2009;58:1508–1513. doi: 10.1099/jmm.0.013656-0. [DOI] [PubMed] [Google Scholar]
- 26.Okura M., Takamatsu D., Maruyama F., Nozawa T., Nakagawa I., Osaki M., Sekizaki T., Gottschalk M., Kumagai Y., Hamada S. Genetic analysis of capsular polysaccharide synthesis gene clusters from all serotypes of Streptococcus suis: Potential mechanisms for generation of capsular variation. Appl. Environ. Microbiol. 2013;79:2796–2806. doi: 10.1128/AEM.03742-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King S.J., Leigh J.A., Heath P.J., Luque I., Tarradas C., Dowson C.G., Whatmore A.M. Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: Identification of virulent clones and potential capsular serotype exchange. J. Clin. Microbiol. 2002;40:3671–3680. doi: 10.1128/JCM.40.10.3671-3680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J., Fu S., Liu M., Xu Q., Bei W., Chen H., Tan C. The two-component system NisK/NisR contributes to the virulence of Streptococcus suis serotype 2. Microbiol. Res. 2014;169:541–546. doi: 10.1016/j.micres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y., Liu G., Li S., Wang M., Song J., Wang J., Tang J., Li M., Hu F. Role of a type IV-like secretion system of Streptococcus suis 2 in the development of streptococcal toxic shock syndrome. J. Infect. Dis. 2011;204:274–281. doi: 10.1093/infdis/jir261. [DOI] [PubMed] [Google Scholar]
- 30.Li M., Wang C., Feng Y., Pan X., Cheng G., Wang J., Ge J., Zheng F., Cao M., Dong Y., et al. SalK/SalR, a two-component signal transduction system, is essential for full virulence of highly invasive Streptococcus suis serotype 2. PLoS ONE. 2008;3:e2080. doi: 10.1371/journal.pone.0002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J., Gao Y., Teng K., Zhang J., Sun S., Zhong J. Restoration of bioactive lantibiotic suicin from a remnant lan locus of pathogenic Streptococcus suis serotype 2. Appl. Environ. Microbiol. 2014;80:1062–1071. doi: 10.1128/AEM.03213-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng H., Ji S., Lan R., Liu Z., Bai X., Zhang W., Gottschalk M., Xu J. Population analysis of Streptococcus suis isolates from slaughtered swine by use of minimum core genome sequence typing. J. Clin. Microbiol. 2014;52:3568–3572. doi: 10.1128/JCM.00536-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoa N.T., Chieu T.T., Nghia H.D., Mai N.T., Anh P.H., Wolbers M., Baker S., Campbell J.I., Chau N.V., Hien T.T., et al. The antimicrobial resistance patterns and associated determinants in Streptococcus suis isolated from humans in southern Vietnam, 1997–2008. BMC Infect. Dis. 2011;11:6. doi: 10.1186/1471-2334-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nurjadi D., Olalekan A.O., Layer F., Shittu A.O., Alabi A., Ghebremedhin B., Schaumburg F., Hofmann-Eifler J., Van Genderen P.J., Caumes E., et al. Emergence of trimethoprim resistance gene dfrG in Staphylococcus aureus causing human infection and colonization in sub-Saharan Africa and its import to Europe. J. Antimicrob. Chemother. 2014;69:2361–2368. doi: 10.1093/jac/dku174. [DOI] [PubMed] [Google Scholar]
- 35.Hu P., Yang M., Zhang A., Wu J., Chen B., Hua Y., Yu J., Xiao J., Jin M. Complete genome sequence of Streptococcus suis serotype 14 strain JS14. J. Bacteriol. 2011;193:2375–2376. doi: 10.1128/JB.00083-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z., Wang W., Tang M., Shao J., Dai C., Zhang W., Fan H., Yao H., Zong J., Chen D., et al. Comparative genomic analysis shows that Streptococcus suis meningitis isolate SC070731 contains a unique 105K genomic island. Gene. 2014;535:156–164. doi: 10.1016/j.gene.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y., Yang X., Qin J., Lu N., Cheng G., Wu N., Pan Y., Li J., Zhu L., Wang X., et al. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat. Commun. 2013;4:2151. doi: 10.1038/ncomms3151. [DOI] [PubMed] [Google Scholar]
- 38.Huang K., Zhang Q., Song Y., Zhang Z., Zhang A., Xiao J., Jin M. Characterization of Spectinomycin Resistance in Streptococcus suis Leads to Two Novel Insights into Drug Resistance Formation and Dissemination Mechanism. Antimicrob. Agents Chemother. 2016;60:6390–6392. doi: 10.1128/AAC.01157-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marie J., Morvan H., Berthelot-Herault F., Sanders P., Kempf I., Gautier-Bouchardon A.V., Jouy E., Kobisch M. Antimicrobial susceptibility of Streptococcus suis isolated from swine in France and from humans in different countries between 1996 and 2000. J. Antimicrob. Chemother. 2002;50:201–209. doi: 10.1093/jac/dkf099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.