Abstract
Galium aparine L., family Rubiaceae, is a widely spread species in the Galium genus. The herb of G. aparine is part of folk remedies and dietary supplements. In this study, we analyzed the chemical composition and immunomodulatory activities of G. aparine herb ethanolic extracts obtained from the plant material by maceration with 20%, 60% or 96% ethanol. The contents of hydroxycinnamic acid derivatives, flavonoids and polyphenols were determined spectrophotometrically, with extractives and polysaccharides quantified gravimetrically. The qualitative composition was studied using UHPLC-DAD-MS/MS analysis; isolation not previously described in G. aparine quercetin rhamnoglucoside was carried out through column chromatography, and the immunomodulatory activity of extracts was determined in the reaction of lymphocyte blast transformation. Major constitutes of extracts were iridoids, i.e., monotropein, 10-desacetylasperulosidic acid and asperulosidic acid; p-hydroxybenzoic acid; hydroxycinnamic acid derivatives, i.e., 3-O-caffeoylquinic, 5-O-caffeoylquinic, 3,4-O-dicaffeoylquinic, 3,5-O-dicaffeoylquinic, 4,5-O-dicaffeoylquinic acids and caffeic acid derivatives; flavonoids, i.e., rutin, quercetin 3-O-rhamnoglucoside-7-O-glucoside, and isorhamnetin 3-O-glucorhamnoside. Significantly, quercetin 3-O-rhamnoglucoside-7-O-glucoside was first isolated and identified in Galium species so far investigated. All G. aparine herb ethanolic extracts stimulate the transformational activity of immunocompetent blood cells, with 96% ethanolic extract being the most active. The data obtained necessitate further research into the mechanisms of immunomodulatory activity of extracts from G. aparine herb.
Keywords: Galium aparine L., ethanolic extracts, phenolic compounds, iridoids, immunomodulatory activity, lymphocyte blast transformation
1. Introduction
Disturbances of the immune system lead to the development and complications of chronic diseases. Numerous studies have proved that the restoration of immune system function is a prerequisite for the successful therapy of various illnesses [1,2]. The development of the immune response is the result of the cooperative impact T-, B-lymphocytes and macrophages, associated with activation, proliferation and differentiation of immunocompetent cells.
Specific immunostimulants include thymus preparations, interleukins, interferons, biologically active peptides, polysaccharides of certain fungi and therapeutic vaccines, whose effect is explained through their ability to influence the metabolism of cells and body tissues and activate immunocompetent cells.
Many plant-derived compounds, like sterols, polysaccharides, alkaloids, flavonoids, lectins and glycoprotein, are used for immunomodulation [3]. For example, among polysaccharides, acidic arabinogalactan and ramnogalacturonan have been shown to manifest immunostimulatory effect in vitro and in vivo [4]. Numerous studies look into the immunomodulative activities of saponins [5,6,7,8]. The proven effect of triterpenoid glycosides on the immune system of mammals contributed to the development of a wide range of dietary supplements for the prevention of the immune system disturbances, i.e., human immunity system enhancement [9], and for the prevention and treatment of allergies [10,11].
In our previous studies, the immunomodulative effect of the aqueous and ethanolic extracts of Galium verum L. herb was established [12,13]. Among other species of the genus Galium L., one of the most widely spread is Galium aparine L., also called cleavers or goosegrass, which can be found all over Ukraine, Europe, Northern America and certain parts of Asia; its habitat in the north reaches Alaska and Greenland whereas as introduced species it can be found in Australia, New Zealand and sub-Antarctic islands. Extensive research on the phytochemical composition of G. aparine showed that cleavers herb contains iridoids: asperulosidic acid and 10-deacetylasperulosidic acid [14], monotropein, asperuloside, acumine and aucubin [15]; alkaloids: protopine, harmine, (±)-vasicinone, (−)-l-hydroxypeganine and (−)-8-hydroxy-2,3-dihydrodesoxypeganine [16]; phenolcarbonic and hydroxycinnamic acids: chlorogenic, caffeic, n-coumaric, ferulic, caftaric and gentisic [17,18]; flavonoids: quercetin, dihydroquercetin, rutin, hyperosid, isoquercetrin, kaempferol, kaempferol-3-O-glucoside (astragalin), epicatechin, neohesperidin, luteolin, luteolin-7-O-diglucoside and apigenin [18,19,20,21]; cholesterol, campesterol, stigmasterol, sitosterol, Δ-[5]-avenasterol, Δ-[7]-stigmasterol and Δ-[7]-avenasterol [21,22,23]. According to our previous studies triterpenoids (oleanolic, ursolic, euscaphic and tormentic acids, betulin and lupeol), sesquiterpenoids, squalene, aromatic compounds and higher alkanes, and their derivatives fatty acids, chlorophylls, and carotenoids were identified in extracts of cleavers herb [24,25].
The extract from the herb of G. aparine is one of the ingredients of some galenic remedies and dietary supplement that are recommended as immunomodulatory, anti-inflammatory and for detoxication, as well as for the improvement of the functioning of the lymphatic and blood circulatory systems and a drainage drug, based on the activation of the immune system and normalization of impaired functions.
In the ethnopharmacology of many countries, the herb of G. aparine is used for treatment of skin diseases [26,27,28].
In the literature several biological activities of G. aparine are reported. Previous studies confirmed antimicrobial, antioxidant and anti-cancer effects of different extracts from this plant material [18,25,29]. Although the plant material is used as a potential immunomodulator there are no papers investigating this kind of bioactivity of G. aparine.
The aim of the present article is to investigate the chemical composition and immunomodulatory activity of different ethanolic extracts from G. aparine using the lymphocyte blast transformation model in vitro (RLBT).
2. Results and Discussion
2.1. Phytochemical Screening of G. aparine Herb Ethanolic Extracts
The phytochemical screening of G. aparine herb ethanolic extracts revealed the presence of polysaccharides only in Extract I (20% EtOH, v/v), whereas flavonoids (flavonols and flavones) and phenolic acid derivatives were detected and identified in all samples under study, and the results obtained correspond with previous studies [17,18,19,20,21,22].
2.2. Quantification of Main Groups of Phytochemicals in Analyzed Extracts
The content of the main groups of phytochemicals in G. aparine herb ethanolic extracts is given in Table 1 below.
Table 1.
The content of the main groups of phytochemicals in Galium aparine herb ethanolic extracts.
| Extract | Extraction Yield (mg/mL) | Group of Phytochemicals (mg/g) | |||
|---|---|---|---|---|---|
| Polysaccharides | Hydroxycinnamic Derivates | Flavonoids | Polyphenols | ||
| Extract I (20% EtOH, v/v) | 252.7 ± 12.6 | 129.4 ± 1.6 | 75.9 ± 0.5 * | 10.7 ± 0.1 # | 66.1 ± 0.5 * |
| Extract II (60% EtOH, v/v) | 246.3 ± 12.3 | n.d. | 77.1 ± 0.6 * | 10.2 ± 0.1 # | 50.8 ± 0.6 # |
| Extract III (96% EtOH, v/v) | 163.4 ± 8.1 | n.d. | 91.2 ± 0.5 # | 15.3 ± 0.1 * | 69.8 ± 0.5 * |
*, # significant differences at p < 0.05, no significant differences observed for values with the same mark; n.d.—not detected.
A comparative study shows differences between the G. aparine herb ethanolic extracts investigated in the present research.
The highest extraction yield (252.7 mg/mL) was obtained when 20% ethanol was used (extract I), and the lowest (163.4 mg/mL) in the case of 96% ethanol extraction (extract III).
Comparable content of hydroxycinnamic acid derivates is marked in the extracts I and II, and the highest content in extract III. Flavonoid content is comparable in extracts I and III whereas it was significantly lower in extract II. Extracts I and III contain similar amounts of polyphenols while in extract II the content of this class of phytochemicals was significantly lower.
The presence of polysaccharides was confirmed only in extract I (129.4 mg/g). This observation is in the agreement with the general expectation that polysaccharides are easily extracted with a solvent mixture with a high water content and generally not present if extracts are prepared with polar organic solvents like ethanol.
The data obtained display some differences from those by other researchers [17,22], which may result from various factors, such as the growth conditions of the plants under study or the methods of extraction and analysis.
2.3. UHPLC-DAD-MS/MS Analysis
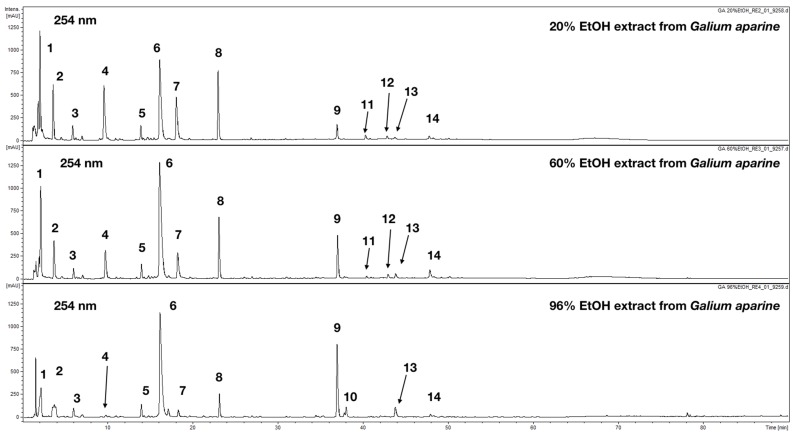
The chromatographic analysis of tested extracts was performed using a hyphenated chromatographic technique in order to characterize the phytochemical content of prepared extracts. In total 14 major constituents in three analyzed extracts (20% EtOH, 60%, EtOH and 96% EtOH) were detected and characterized (Figure 1, Table 2).
Figure 1.
UHPLC-DAD-MS/MS chromatogram of G. aparine extracts recorded at 254 nm.
Table 2.
UHPLC-DAD-MS/MS data of compounds detected in analyzed extracts.
| Peak No. | Compound Name | Retention Time (min) | UV (nm) | (M − H)− m/z | MS2 ions | (M + H)−+ m/z | MS2 ions | Compound Content ug/mg | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Extract I 20% EtOH | Extract II 60% EtOH | Extract III 96% EtOH | ||||||||
| 1 | Monotropeint | 2.1 | 237 | 389 | 369, 227, 209b, 183, 137 | 381 | - | n.q. | n.q. | n.q. |
| 2 | 10-Desacetylasperulosidic acidt | 3.7 | 237 | 389 | 227b, 209, 183 | 381 | - | n.q. | n.q. | n.q. |
| 3 | p-Hyroxybenzoic acids | 5.9 | 259, 294 | 153 | - | 155 | - | n.q. | n.q. | n.q. |
| 4 | 3-O-Caffeoylquinic acids | 9.7 | 217, 241, 300sh, 324 | 353 | 191b, 179, 161 | 355 | 163b | 8.91 ± 0.09 | 3.72 ± 0.12 | 0.53 ± 0.03 |
| 5 | Asperulosidic acidt | 14.0 | 235 | 431 | 371, 269, 251b, 165 | 433 | - | n.q. | n.q. | n.q. |
| 6 | 5-O-Caffeoylquinic acids (Chlorogenic acid) | 16.2 | 219, 241, 299sh, 325 | 353 | 191b, 179 | 355 | 163b | 18.44 ± 0.21 | 31.51 ± 0.19 | 40.61 ± 0.12 |
| 7 | 4-O-Caffeoylquinic acids | 18.2 | 217, 241, 300sh, 325 | 353 | 191, 179, 173b | 355 | 337, 307, 163b | 8.12 ± 0.03 | 4.75 ± 0.08 | 1.16 ± 0.02 |
| 8 | Quercetin 3-O-rhamnoglucoside-7-O-glucosides | 23.1 | 255, 263sh, 353 | 771 | 609b, 301 | 773 | 627, 611, 465b, 303 | 3.35 ± 0.10 | 2.95 ± 0.05 | 1.50 ± 0.02 |
| 9 | Quercetin 3-O-rhamnoglucosides (Rutin) | 36.9 | 255, 262sh, 354 | 609 | 591, 301b, 179 | 611 | 465, 303b | 1.08 ± 0.05 | 2.49 ± 0.03 | 6.33 ± 0.07 |
| 10 | Caffeic acid derivative | 38.0 | 254, 299sh, 327 | 381 | 207, 191, 179b, 135 | 383 | 365, 163b | n.q. | n.q. | n.q. |
| 11 | Isorhamnetin 3-O-glucorhamnosides | 40.4 | 259, 260sh, 350 | 615 | 609b, 542, 461, 315 | 617 | - | n.q. | n.q. | n.q. |
| 12 | 3,4-O-Dicaffeoylquinic acids | 42.9 | 239, 300sh, 324 | 515 | 353b, 255, 173b | 517 | 499b, 317, 163 | n.q. | n.q. | n.q. |
| 13 | 3,5-O-Dicaffeoylquinic acids | 43.8 | 240, 299sh, 324 | 515 | 353, 233, 191b, 179 | 517 | 499b, 147 | 0.23 +/− 0.02 | 0.42 +/− 0.08 | 2.43 +/− 0.11 |
| 14 | 4,5-O-Dicaffeoylquinic acids | 47.9 | 240, 300sh, 325 | 515 | 515, 353b, 299, 255, 203 | 517 | 499, 335b, 278 | n.q. | n.q. | n.q. |
s—comparisons with chemical standard have been made; b—base peak (the most abundant ion in recorded spectrum); sh-shoulder; t—tentative assignment; n.q.—not quantified.
Compounds (4, 6, 7, 10 and 12–14) exhibiting characteristic UV-Vis maxima at ca. 240 and 325 nm, with a shoulder at 300 nm, were characterized as caffeic acid derivatives. Based on observation of their fragmentation patterns and after comparison with chemical standards available they were identified as chlorogenic acids (4, 6, 7, M-H at m/z = 353 in MS-) and dicaffeoylquinic acids (12–14 M-H at m/z = 515 in MS−). Compound 10 present only in 96% EtOH extract was a caffeic acid derivative but further identification was not possible due to the lack of more data. Compounds 3 was identified as a simple phenolic acid (p-hydroxybenzoic acid).
Compounds 8, 9 and 11 were identified as flavonoids with typical UV-Vis maxima observed at ca. 255, 260 and 350 nm. Compound 9 was easily elucidated (M-H at m/z 609 fragmented to 301 amu in negative ion mode) as popular quercetin derivative—rutin. This compound was previously described in investigated species and other belonging to the Galium genus [19]. Compound 11 showed a higher molecular mass with a pseudomolecular ion at m/z = 615 in MS−. The fragmentation showed easy cleavage of the methyl group M-H-15 at m/z = 609 and presence of aglycone moiety in fragmentation spectrum at m/z = 315. The comparison made with available chemical standards led to the identification of 11 as isorhamnetin 3-O-rhamnoglucoside. Compound 8 was also assigned as a quercetin derivative due to the presence of aglycone moiety ion at m/z = 301 in MS-. The molecular mass of 772 amu and fragmentation patterns in MS− and MS+ led to the conclusion that this compound consists of quercetin linked to 2 hexose and 1 rhamnose units. The final identification required isolation and structure elucidation by 1H NMR.
Compounds 1, 2 and 5 displayed a single absorption maximum at ca. 235 nm. Based on MS, spectrum molecular masses of 390 amu were confirmed for 1 and 2 and 432 amu for 5. After literature research compounds were characterized as iridoid glycosides. Compound 1 and 2 showed the same molecular masses and they were tentatively assigned as monotropein and 10-desacetylasperulosidic acid, respectively. Both chemicals were previously detected in aerial parts of G. rivale and G. mollugo. Additionally, Compound 2 was reported from G. album. Compound 5 was assigned as asperulosidic acid. This natural product was found in aerial parts of G. rivale, G. album and G. mollugo [30,31,32].
To the best of our knowledge, apart from monotropein, 10-desacetylasperulosidic acid, asperulosidic acid (1, 2, 5), chlorogenic acid (6) and rutin (9), the presence of the rest of the natural products is reported in G. aparine for the first time.
2.4. Structure Elucidation of Isolated Quercetin Derivative
Because the full elucidation of the chemical structure of Compound 8 was not possible based on UHPLC data obtained from performed analysis, this flavonoid derivative was isolated, and its chemical structure had to be confirmed using NMR analysis. The preliminary 1H analysis showed that 8 most probably is a quercetin derivative (it was also confirmed by the observation of its fragmentation pattern, Table 2). The 13C NMR spectrum displayed signals that were in the absolute agreement with previous report on flavonoid glycosides from Ficaria verna [33]. Finally, Compound 8 was identified as quercetin 3-O-rhamnoglucoside-7-O-glucoside. 1H and 13C NMR data of Compound 8 are given below.
Quercetin 3-O-rhamnoglucoside-7-O-glucoside (8)—yellow powder, 1H NMR (300 MHz, DMSO-d6) δ 7.56 (s, 1H), 7.54 (s, 1H), 6.85 (d, J = 8.9 Hz, 1H), 6.72 (d, J = 1.9 Hz, 1H), 6.44 (d, J = 1.9 Hz, 1H), 5.38 (d, J = 7.0 Hz, 1H), 5.07 (d, J = 7.2 Hz, 1H), 4.38 (s, 2H), 3.71 (d, J = 9.8 Hz, 2H), 3.14–3.01 (m, 3H), 1.00 (d, J = 6.1 Hz, 2H), 13C NMR (75 MHz, DMSO-d6) δ 177.54, 162.83, 160.90, 157.18, 156.01, 148.69, 144.81, 133.55, 121.67, 120.97, 116.44, 115.23, 105.62, 101.05, 100.73, 99.86, 94.52, 77.16, 76.39, 76.00, 74.06, 73.14, 71.83, 70.55, 70.36, 69.57, 68.24, 17.76.
It was detected for the first time in G. aparine and there are no reports on its occurrence in other Galium species.
2.5. In Vitro Reaction of Lymphocyte Blast Transformation
The research presented is the first known study of the immunomodulatory activity of G. aparine herb ethanolic extracts.
It was established that all samples used in the current study considerably stimulate the transformational activity of peripheral blood mononuclear cells. Under the influence of the extracts used, 32.4–45.2% of mononuclear cells were involved in the proliferation process, which indicates the stimulating effect of the substances on T- and B-lymphocytes (Table 3), which is slightly lower than the level of PHA (phytohemagglutinin) preparation (Table 3).
Table 3.
The effect of ethanolic extracts of G. aparine on the indices of lymphocyte blast transformation (X ± m), n = 5.
| Extract | Extract Concentration (mg/mL) | RLBT, % |
|---|---|---|
| Extract I (20% EtOH, v/v) | 0.10 | 32.4 ± 2.3 * |
| 0.74 | 35.2 ± 2.5 * | |
| 1.47 | 34.7 ± 2.2 * | |
| Extract II (60% EtOH, v/v) | 0.10 | 34.6 ± 2.5 * |
| 0.74 | 38.5 ± 2.7 * | |
| 1.47 | 36.8 ± 2.5 * | |
| Extract III (96% EtOH, v/v) | 0.10 | 36.9 ± 2.3 * |
| 0.74 | 45.2 ± 3,0 # | |
| 1.47 | 45.1 ± 3.1 # | |
| PHA | 2.5 | 48.1 ± 2.1 # |
| Spontaneous RLBT | - | 8.5 ± 0.7 |
PHA = phytohemagglutinin, RLBT = the reaction of lymphocyte blast transformation. *, #—p < 0.05 in comparison with PHA, different markers show statistically significant differences.
Table 3 shows the summary of obtained results in the lymphocyte blast transformation model. The highest immunostimulatory activity was observed for 96% EtOH at concentrations of 0.74 and 1.47 mg/mL. The rest of the investigated samples displayed weaker activity than the positive control PHA.
The data obtained show that ethanolic extracts from G. aparine have an immunomodulatory potential. The highest activity in the investigated model was observed for 96% EtOH preparation (III). Based on phytochemical analysis it was shown that most probably polysaccharides that usually are suspected as the group of phytochemicals with immunostimulatory activity are not responsible for the observed results in the present study. Polysaccharides were detected and quantified only in 20% EtOH extracts, which did not display the best immunostimulatory potential (Table 1 and Table 3) as it would be expected, taking into account the general biological properties of plant polysaccharides. Interestingly the highest content of major phenolics detected, namely flavonoids and hydroxycinnamic derivates (Table 1), was observed for the 96% ethanolic extract. The UHPLC analysis revealed that the content of chlorogenic acid (6) increased (Table 2, from 18.44 to 40.61 µg/mg of dry extract) together with the ethanol percentage in the solvent used for extract’s preparation. The same pattern was observed for Compound 13 namely 3,5-O-dicaffeoylquinic acid (Table 2, 0.23 to 2.43 µg/mg). At the same time the content of other chlorogenic acid isomers (4 and 7) was decreasing (Table 2). In the case of flavonoids, the content in Compound 8 was lower in 96% EtOH extract (1.50 µg/mg) compared to 3.35 µg/mg in 20% EtOH extract. On the other hand, the rutin (9) content was raising with the EtOH percentage in the extraction medium (Table 1, from 1.08 to 6.33 µg/mg). The content of iridoids, which were not quantified in the present study seem to be decreasing based on the observation of peak, highs and areas in chromatograms acquired at 254 nm (Figure 1). The increasing content of phenolics (caffeic acid derivatives and flavonoids) in investigated extracts and lower the content of iridoids in 96% and 60% EtOH compared to 20% EtOH suggest that polyphenols contained in G. aparine may be responsible for the observed immunomodulatory activity. It is also possible that other more lipophilic compounds are present in analyzed extracts which were not detected with available methodology. The obtained results suggest significantly weaker activity of investigated extracts as immunostimulatory agents compared to previously reported results for extracts from G. verum [12].
3. Materials and Methods
3.1. Plant Material
G. aparine herb was harvested at full flowering stage in the botanical garden of the National University of Pharmacy, Kharkiv, Ukraine, (geographic coordinates, latitude: 50°01′08.6″ N, longitude: 36°19′12.5″ E) in May, 2017. Voucher specimens no. 20052017–23052017 were deposited at the Department of Pharmacognosy (National University of Pharmacy, Ukraine). The identity of plants was established with the consulting assistance of T. Gontova, D.Sc. (Pharmacy), the Head of Department of Botany National University of Pharmacy [34].
3.2. Equipment
Spectrophotometer EvolutionTM 60S UV-Visible (Thermo Fisher Scientific, Waltham, MA, USA), Dionex Ultimate 3000RS system (Dionex, San Jose, CA, USA) coupled with an Amazon SL spectrometer, prep-HPLC—Shimadzu LC-20AP equipped with UV-Vis detector, sampler SIL-10A and fraction collector FRC-10 (all, Shimadzu, Kioto, Japan), Varian VNMRS 300 MHz spectrometer, electronic analytical scales AN 100 “Axis” (AXIS, Warszawa, Poland), electrical temperature chamber TC80M-3 (Medlabortekhnika, Ukraine), centrifuge OPN-3 (Phizpribor, Russia), microscope ZEISS Primo Star (ZEISS, Oberkochen, Germany), pipette Thermo Scientific, Lait series 1–200 μL (Thermo Fisher Scientific, Waltham, USA), pipette Thermo Scientific, Lait series 1–50 μL (Thermo Fisher Scientific, Waltham, USA), pipette Thermo Scientific, Lait series 1–1000 μL (Thermo Fisher Scientific, Waltham, MA, USA), pipette Thermo Scientific, Lait series 1–20 μL (Thermo Fisher Scientific, Waltham, MA, USA), CO2 incubator (Binder, Tuttlingen, Germany), bioanalyzer Agilent 2100 (Agilent, Santa Clara, CA, USA).
3.3. Chemicals
96% Ethanol (POCH, Gliwice, Poland) and purified water (produced by Merck Millipore, Simplicity UV system) used during extraction complied with the requirements of the State Pharmacopoeia of Ukraine [35]; chemicals for phytochemical screening: ethanol (POCH, Gliwice, Poland), DMSO, p.a. (POCH, Gliwice, Poland), hydrochloric acid, p.a. (Sobstar, Zaporizhia, Ukraine), acetic acid, puriss. (PJSC AZOT, Cherkasy, Ukraine), lead (II) acetate, p.a. (Unikhim Ltd., Novbosibirsk, Russia), aluminum chloride, p.a., granulated zinc, p.a. (PC Uralskiy zavod khimicheskih reaktivov, Moscow, Russia), ferric (III) chloride, puriss (Sigma-Aldrich, St. Louise, USA), gallic acid (Carl Roth, Karlsruhe, Germany) chlorogenic acid and rutin were of analytical grade (Merck Millipore, Burlington, MA USA); acetonitrile and formic acid for UHPLC was purchased from Sigma-Aldrich, St. Louise, USA.
3.4. Preparation of the Extracts
As a solvent, ethanol at various concentrations (20%, 60% and 96%, v/v) was used; the extraction was carried out at a general ratio of the plant material solvent of 1:10 on heating with reflux. The extraction was repeated thrice under the same conditions (30 min each). The extracts obtained were combined and concentrated on a vacuum rotary evaporator to dryness at 45 °C. For all bioassays stock solutions of extracts (20 mg/mL) were prepared in DMSO-water 1:1 and diluted properly.
3.5. Preliminary Phytochemical Screening of G. aparine Herb Ethanolic Extracts
The preliminary phytochemical screening was performed using generally accepted methods and techniques of phytochemical analysis [36,37]. Polysaccharides were precipitated with three volumes of 96% ethanol. Glycosides and aglycons of flavonoids were determined in extracts from G. aparine herb in the reactions of identification: cyanidin reaction by Bryant (yellow-red colouring of the aqueous phase and yellow-hot colouring of the octal phase), the reaction with 3% solution of iron (III) chloride (dark green colour of flavonols, flavones); the reaction with an alkaline solution (bright yellow colour); the reaction with 5% solution of aluminium chloride (yellow-green colouring); the boric-acid reaction (yellow colouring on detection of 3- and 5-hydroxyflavones and 5-hydroxyflavanones); the reaction with ammonia (flavones, flavonols, flavanones and flavanonols dissolve with formation of yellow colour, which, when heated, changes to orange or brown colour).
To determine tannins, the reactions of the sediment were carried out with a 1% gelatine solution, a 1% solution of quinine hydrochloride and 10% solution of basic acetate of lead. The group of tannins was detected by the reaction with a solution of iron ammonium alum.
3.6. Quantification of the Main Groups of Phytochemicals
In all the extracts from G. aparine herb the extractive substances were determined gravimetrically [38]; polysaccharides were quantified gravimetrically after complete drying at room temperature taking into account the loss on drying; the sum of the hydroxycinnamic acid derivates was determined by direct spectrophotometry (as chlorogenic acid, λ = 325 nm) according to Yezerska et al. [39], Spagnol et al. [40]; flavonoids were quantified by the method of differential spectrophotometry with aluminium chloride (as rutin, λ = 410 nm) [41]; polyphenols were quantified by direct spectrophotometry (as gallic acid, λ = 270 nm) according to Koshovyi et al. [42]. All assays were performed in triplicate.
3.7. UHPLC-DAD-MS/MS Analysis
Extracts prepared from aerial parts of G. aparine were dissolved in methanol:water or water to obtain a final concentration 10 mg/mL. Samples were filtered through a 0.45 µm syringe filter and subjected to UHPLC-DAD-MS analysis using a Dionex Ultimate 3000RS system coupled with an Amazon SL spectrometer. The separation was carried out with a Kinetex XB-C18 column (150 mm × 2.1 mm × 1.7 µm, Phenomenex, USA) maintained at 25 °C. The flow rate was set at 0.3 mL/min. The mobile phase A was aqueous solution of formic acid (0.1%) and B was 0.1% HCOOH in acetonitrile. The following gradient elution was used: 0 min—4%B, 60 min—26%B and 90 min—95%B. A 3 µ extract solution was injected into the UHPLC column. UV-Vis spectra of detected compounds were recorded over the range from 190 to 450 nm. The chromatogram was acquired at 254 nm, 325 nm and 350 nm. Mass spectra were recorded in the negative and positive ion mode. Compounds were identified basing on their UV-Vis and MS spectra. Comparisons with the available chemical standards and suitable literature were performed [43,44,45,46]. Compounds were quantified using calibration curves for chlorogenic acid at 325 nm for all caffeic acid derivatives found in the extracts or using calibration curve for isoquercitrin for all flavonoids. Calibration curves were generated based on the amounts of compounds injected into UHPLC vs. peak areas. A linear fit was used for all calculations. Stock solution of chlorogenic acid and isoquecitrin was prepared in ethanol (final concentration was 50 µg/mL.
3.8. Isolation of Major Constituents of 96% EtOH Extract (Compounds 6, 8 and 9)
The isolation of major compounds detected in 96% EtOH extract was performed using prep-HPLC—Shimadzu LC-20AP equipped with UV-Vis detector, sampler SIL-10A and fraction collector FRC-10 (all, Shimadzu, Kioto, Japan). One gram of 96% EtOH extract was dissolved in DMSO and filtered through PVDF 5 µm syringe filter. Compounds were separated on Kinetex XB-C18 column (Phenomenex, USA, 150 mm × 22.1 mm × 5 µm) maintained at 25 °C. The flow rate was 20 mL/min. The mobile phase A was aqueous solution of formic acid (0.1%) and B was 0.1% HCOOH in acetonitrile. The following gradient elution was used: 0 min—2%B, 60 min—26%B and 90 min—95%B. Four hundred µL of dissolved raw extract was injected into the HPLC system ten times. Chromatogram was recorded at 254 nm. Compounds were collected at 18.6–19.5 min (6), 22.6–22.9 min (8) and 34.6–35.2 min (9). Obtained eluates were freeze dried to obtain 11 mg of 6, 4 mg of 8 and 7 mg of 9. The NMR spectra were recorded with Varian VNMRS 300 MHz spectrometer in DMSO-d6.
3.9. Study of Immunomodulatory Activity
To assess the immunomodulatory activity of the extracts obtained, in vitro RLBT with an adequate resolution was used [47,48].
As a sample for substance testing, the mononuclear cells (lymphocytes) removed from venous heparinized blood (donated blood, Kharkiv Regional Blood Banking Centre, UA) by Ficoll-verographine gradient density centrifugation (density 1.077 g/mL) (Research and Production Enterprise “PanEco”, Russia) by the standard technique [49], were used (Protocol of Committee on Biomedical Ethics of SO “Mechnikov Institute of Microbiology and Immunology” No. 8 of December 5, 2018). The study conformed to the principles of the Declaration of Helsinki.
The cells obtained were cultured in medium 199 with addition of 10% bovine fetal serum (Thermo Fisher Scientific, Waltham, MA, USA) and 2 mM L-glutamine (Altera Holding, RU), 100 μg/mL gentamicin (LEK (CZ). A suspension of 106 per 1 mL of the culture medium with the addition of substances was incubated for 15–18 h in a thermostat at 37 °C, in a 5% CO2 atmosphere with saturated water vapor.
The intensity of the proliferative reaction was evaluated by the indices of DNA (deoxyribonucleic acid) synthesis activation recorded by the treatment of samples with anti-BrdU (5-bromo-2′-deoxyuridine) Antibody (3H579) monoclonal antibodies (Santa Cruz Biotechnology, CA, USA) at the concentration of 100 mg/mL. After the final sample preparation, numerical data on the total number of cells and the percentage of blast forms in the samples were established for the flow cytometric analysis with fluorescence detection.
Before the RLBT, the stock solutions of analysed extracts were prepared at concentrations of 0.20, 1.48 and 3.48 mg/mL. One hundred μL of substances were added to 100 μL of primary cultures of immunocompetent cells to obtain final concentrations of 0.10, 0.74 and 1.74 mg/mL. The mitogenic stimulation of lymphocytes by PHA (Research and Production Enterprise “PanEco”, RU) at the concentration of 2.5 μg/mL was performed as a control. RLBT without the addition of the substances under study (spontaneous blast transformation) was also evaluated.
3.10. Statistical Analysis
All statistical analyses were carried out in accordance with the requirements of the State Pharmacopoeia of Ukraine using Microsoft Office Excel 2007 [35,50]. Differences between groups were statistically analysed using one-way analysis of variance (ANOVA). The results were expressed as mean ± standard deviation (SD). p values less than 0.05 were considered statistically significant.
4. Conclusions
Different ethanolic extracts from G. aparine herb were studied for its chemical composition and immunomodulatory activity. All ethanolic extracts from G. aparine herb significantly stimulated the transformational activity of immunocompetent blood cells, with 96% ethanolic extract being most active. The percentage of lymphocytes proliferating in RLBT under the influence of 96% ethanolic extract increased by 4.34–5.32 times compared with the spontaneous transformation. The results justify the traditional use of extracts from G. aparine as immunomodulatory agents.
The UHPLC-DAD-MS/MS analysis allowed comprehensive characterization of investigated extracts. Major phytochemicals from groups of polyphenols and iridoids were detected during the analysis. One flavonoid derivative namely quercetin 3-O-rhamnoglucoside-7-O-glucoside was isolated and identified for the first time in any Galium species investigated so far.
The data obtained give grounds for further research into the mechanisms of immunomodulatory activity of extracts from G. aparine herb.
Acknowledgments
This study was supported by the Laboratory of Immunorehabilitology of the Mechnikov Institute of Microbiology and Immunology of National Academy of Sciences of Ukraine. Authors wish to thank Igor V. Ilyin for professional language editing service. We also thank Dominika Pilśnik for her help in identification of flavonoid from G. aparine (Medical University of Warsaw).
Author Contributions
Conceptualization and methodology, A.K. and O.K.; validation, O.G.; investigation, T.I., I.S., N.K., S.G. and A.B.; writing—original draft preparation, T.I. and S.G.; writing—review and editing, O.G.
Funding
This project was carried out with the use of CePT infrastructure financed by the European Regional Development Found within the Operational Programme “Innovative economy” for 2007–2013.
Conflicts of Interest
The authors have declared no financial relationships with any organizations that might have an interest in the submitted work; nor any other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Vorobiov A.A. Principles of classification and strategies for the use of immunomodulators in medicine. J. Microbiol. Biotechn. 2002;4:93–98. (In Russian) [Google Scholar]
- 2.Drannik G.N. Clinical Immunology and Allergology. 2nd ed. MIA; Moscow, Russia: 2006. pp. 193–202. (In Russian) [Google Scholar]
- 3.Walaa N.A. Immunomodulatory and natural immunomodulators. J. Allergy Inflamm. 2017;1:e101. [Google Scholar]
- 4.Wagner H. Immunostimulants of Plant Origin. [(accessed on 13 July 2019)];Croatica Chemica Acta. 1995 68:615–626. Available online: https://hrcak.srce.hr/136721. [Google Scholar]
- 5.Baltina L.A., Ryzhova S.A., Ismailova A.F. Glycyrrhizic acid and its derivatives as new immunomodulators; Proceedings of the 4th Russian National Congress; Moscow, Russia. 8–12 April 1997; p. 247. (In Russian) [Google Scholar]
- 6.Sun H.X., Xie Y., Ye Y.P. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787–1796. doi: 10.1016/j.vaccine.2009.01.091. [DOI] [PubMed] [Google Scholar]
- 7.Sergeev A.V., Kabatskaya G.I., Karaseva L.I., Labourdova E.V., Pavlova S.I., Prosalkova I.R., Shashkina M.Y. Immunopharmacology of drugs “Cascarutol” and “Licorice”. Russ. Biother. J. 2004;3:8–10. (In Russian) [Google Scholar]
- 8.Katayama S., Mine Y. Quillaja saponin can modulate ovalbumin-induced IgE allergic responses through regulation of Th1/Th2 balance in a murine model. J. Agric. Food Chem. 2006;54:3271–3276. doi: 10.1021/jf060169h. [DOI] [PubMed] [Google Scholar]
- 9.Deng B., Zhang Z., Wang T. Functional Food Capable of Reinforcing Human Immunity. CN101361568A. Patent. 2009 Feb 11;
- 10.Health Supplement Food Containing Saponin Derivatives Isolated from Ginseng radix for Preventing and Treating Allergy-Mediated. KR 20050045980 (A) A23L1/29. Patent. 2005 May 17;
- 11.Edelev D.A., Kuznetsova T.A., Ivanushko L.A., Yudina T.P., Frolova G.M., Cherevach E.I., Novak S.A. Immunostimulating activity of triterpene glycosides of the roots of Saponaria officinalis L. Tradit. Med. 2012;2:44–47. (In Russian) [Google Scholar]
- 12.Shinkovenko I.L., Kashpur N.V., Ilyina T.V., Kovalyova A.M., Goryacha O.V., Koshovyi O.M., Kryvoruchko O.V., Komissarenko A.M. The immunomodulatory activity of ethanolic extracts from Galium verum L. herb. Ceska Slov. Farm. 2018;67:101–106. [PubMed] [Google Scholar]
- 13.Shinkovenko I.L., Kashpur N.V., Ilyina T.V., Kovalyova A.M., Goryacha O.V., Koshovyi O.M., Toryanyk E.L., Kryvoruchko O.V. The immunomodulatory activity of the aqueous extract and complexes of biologically active compounds of Galium verum L. herb. Ceska Slov. Pharm. 2018;67:25–29. [PubMed] [Google Scholar]
- 14.Deliorman D., Çalıs I., Ergun F. Iridoids from Galium aparine. Pharm. Biol. 2001;39:234–235. doi: 10.1076/phbi.39.3.234.5928. [DOI] [Google Scholar]
- 15.Mitova M.I., Anchev M.E., Handjieva N.V., Popov S.S. Iridoid patterns in Galium L. and some phylogenetic considerations. Z. Nat. C. 2002;57:226–234. doi: 10.1515/znc-2002-3-405. [DOI] [PubMed] [Google Scholar]
- 16.Sener B., Ergun F. Isolation and structural studies on the alkaloids of Galium aparine L. Guede J. Fac. Pharm. Gazi. 1988;5:33–40. [Google Scholar]
- 17.Vlase L., Mocan A., Hanganu D., Benedec D., Gheldiu A., Crișan G. Comparative study of polyphenolic content, antioxidant and antimicrobial activity of four Galium species (Rubiaceae) Dig. J. Nanomater. Biostruct. 2014;9:1085–1094. [Google Scholar]
- 18.Al-Snafi A.E. Chemical constituents and medical importance of Galium aparine—A review. IAJPS. 2018;5:1739–1744. [Google Scholar]
- 19.Shynkovenko I.L., Ilyina T.V., Goryacha O.V., Kovalyova A.M., Komissarenko A.M., Shemchuk N.S., Golembiovska O.I. Phenolic compounds of the liquid extract from cleavers herb (Galium aparine L.) Visn. Farm. 2018;3:19–24. doi: 10.24959/nphj.18.2213. [DOI] [Google Scholar]
- 20.Moubasher H., El-Ghani M.A., Al-Wakeel S., Bahoor A. Chemotaxonomic significance of flavonoids in some species of Galium (Rubiaceae) from Libya. Austin J. Plant Biol. 2016;2:1014–1021. [Google Scholar]
- 21.Mocan A., Crişan G., Vlase L., Ivănescu B., Bădărău A.S., Arsene A.L. Phytochemical investigations on four Galium species (Rubiaceae) from Romania. Farmacia. 2016;64:95–99. [Google Scholar]
- 22.Aslantürk Ö., Çelik T., Karabey B., Karabey F. Active phytochemical detecting, antioxidant, cytotoxic, apoptotic activities of ethyl acetate and methanol extracts of Galium aparine L. Br. J. Pharm. Res. 2017;15:1–16. doi: 10.9734/BJPR/2017/32762. [DOI] [Google Scholar]
- 23.Tzakou O., Couladi M.M., Philianos S. Fatty acids and sterols in spring and winter samples of Galium aparine. Fitoterapia. 1990;61:93. [Google Scholar]
- 24.Shynkovenko I.L., Ilyina T.V., Kovalyova A.M., Goryacha O.V., Golembiovska O.I., Koshovyi O.M. Saponins of the extracts of Galium aparine and Galium verum. Visn. Farm. 2018;4:16–21. doi: 10.24959/nphj.18.2225. [DOI] [Google Scholar]
- 25.Goryacha O.V., Ilyina T.V., Kovalyova A.M., Kashpur N.V. Phytochemical research of Galium aparine L. lipophilic complex and study of its antibacterial activity. Pharma Innov. J. 2014;3:7–10. [Google Scholar]
- 26.Galium aparine L. [(accessed on 13 July 2019)]; Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:30007294-2.
- 27. [(accessed on 13 July 2019)];Clivers. Available online: https://botanical.com/botanical/mgmh/c/cliver74.html.
- 28.Friščić M., Baglama M.Š., Milović M., Pilepić K.H., Maleš Ž. Tradicionalna upotreba, kemijski sastav i biološki učinci vrsta roda Galium L. Farm. Glas. 2018;74:343–350. [Google Scholar]
- 29.Ilyina T.V., Goracha O.V., Toryanik E.L., Kulish I.A., Kovaleva A.M. Antimicrobial activity of the genus Galium L. Pharmacogn. Commun. 2016;6:42–47. [Google Scholar]
- 30.de Rosa S., Iodice C., Mitova M., Handjieva N., Popov S., Anchev M. Triterpene saponins and iridoid glucosides from Galium rivale. Phytochemistry. 2000;54:751–756. doi: 10.1016/S0031-9422(00)00149-7. [DOI] [PubMed] [Google Scholar]
- 31.Handjieva N., Mitova M., Ancev M., Popov S. Iridoid glucosides from Galium album and G. lovcense. Phytochemistry. 1996;43:625–628. doi: 10.1016/0031-9422(96)00328-7. [DOI] [PubMed] [Google Scholar]
- 32.Bianco A., Guiso M., Iavarone C., Marini-Bettolo R., Trogolo C. New iridoid glucosides from Rubiaceae. Gazz. Chim. Ital. 1978;108:13–16. [Google Scholar]
- 33.Tomczyk M. Quercetin and kaempferol glycosides from Ficaria verna flowers and their structure studied by 2D NMR spetroscopy. Pol. J. Chem. 2002;76:1601–1605. [Google Scholar]
- 34.Dobrochaeva D.N., Kotov M.I., Prokudin Y.N., Barbarich A.I. Key to Higher Plants of Ukraine. 2nd ed. Science Dumka; Kiev, Ukraine: 1999. p. 546. (In Russian) [Google Scholar]
- 35.The State Pharmacopoeia of Ukraine/State enterprise “Scientific and Expert Pharmacopoeial Centre”. 1st ed. RIREG; Kharkiv, Ukraine: 2011. p. 538. (In Ukrainian) [Google Scholar]
- 36.Korulkin D.Y., Abilov D.A., Muzychkina R.A., Tolstikov G.A. Natural Flavonoids. Geo; Novosibirsk, Russia: 2007. [Google Scholar]
- 37.Koshovyi O.N., Vovk G.V., Akhmedov E.Y., Komissarenko A.N. The study of the chemical composition and pharmacological activity of Salvia officinalis leaves extracts getting by complex processing. Azerbaijan Pharm. Pharmacother. J. 2015;15:30–34. [Google Scholar]
- 38.State Enterprise Scientific and Expert Pharmacopoeial Centre . 3 Vol./State Enterprise “Scientific and Expert Pharmacopoeial Centre”. 2nd ed. Volume 1. State Enterprise “Scientific and Expert Pharmacopoeial Centre”; Kharkiv, Ukraine: 2015. The state pharmacopoeia of Ukraine; p. 1128. (In Ukrainian) [Google Scholar]
- 39.Yezerska O., Kalynyuk T., Vronska L. Quantitative determination of hydroxycinnamic acids in chicory root. Chem. Chem. Technol. 2013;7:247–250. doi: 10.23939/chcht07.03.247. [DOI] [Google Scholar]
- 40.Spagnol C.M., Oliveira T.S., Borges V.I.L., Corrêa M.A., Salgado R.H.N. Validation of caffeic acid in emulsion by UV-Spectrophotometric method. Phys. Chem. 2015;5:16–22. [Google Scholar]
- 41.The State Pharmacopoeia of Ukraine . State Enterprise “Scientific and Expert Pharmacopoeial Centre”. 1st ed. RІREG; Kharkiv, Ukraine: 2008. p. 617. (In Ukrainian) [Google Scholar]
- 42.Koshovyi O.M., Zagayko A.L., Kolychev I.O., Akhmedov E.Y., Komissarenko A.N. Phytochemical study of the dry extract from bilberry leaves. Azerbaijan Pharm. Pharmacother. J. 2016;16:18–23. [Google Scholar]
- 43.Clifford M.N., Knight S., Johnston K.L., Kuhnert N. Hierarchical scheme for LC-MS(n) identification of chlorogenic acids. J. Agric. Food Chem. 2003;51:2900–2911. doi: 10.1021/jf026187q. [DOI] [PubMed] [Google Scholar]
- 44.Clifford M.N., Knight S., Kuhnert N. Discriminating between the six isomers of dicaffeoylquinic acid by LC–MS(n) J. Agric. Food Chem. 2005;53:3821–3832. doi: 10.1021/jf050046h. [DOI] [PubMed] [Google Scholar]
- 45.Granica S., Czerwinska M.E., Piwowarski J.P., Ziaja M., Kiss A.K. Chemical composition, antioxidative and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera paradoxa Hudziok obtained after seeds cultivation. J. Agric. Food Chem. 2013;61:801–810. doi: 10.1021/jf304002h. [DOI] [PubMed] [Google Scholar]
- 46.Granica S., Piwowarski J.P., Kiss A.K. Ellagitannins modulate the inflammatory response of human neutrophils ex vivo. Phytomedicine. 2015;22:1215–1222. doi: 10.1016/j.phymed.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Korneeva M.N., Novokhatskii A.S., Grebenyuk V.N., Kerimov S.G. Use of the lymphocyte blast transformation reaction to assess the state of cellular immunity. Bull. Exp. Biol. Med. 1989;107:533–535. doi: 10.1007/BF00842400. [DOI] [PubMed] [Google Scholar]
- 48.Bashirova D.K., Kochnev O.S., Davletkil’deev F.A., Lagutina M.V. Immunologic activity of human lymph cells in the lymphocyte blast transformation reaction. Bull. Exp. Biol. Med. 1980;89:33–35. doi: 10.1007/BF00835497. [DOI] [PubMed] [Google Scholar]
- 49.Bulanova E.G., Budagyan V.M., Yarilin A.A., Mazurenko N.N. Expression of protooncogenes during lymphocyte activation by growth factors. Biochemistry. 1997;62:1021–1025. [PubMed] [Google Scholar]
- 50.Zulfiqar A., Bhaskar S.B. Basic statistical tools in research and data analysis. Indian J. Anaesth. 2016;60:662–669. doi: 10.4103/0019-5049.190623. [DOI] [PMC free article] [PubMed] [Google Scholar]