Abstract
Accumulating evidence suggests that perinatal air pollutant exposures are associated with increased risk of autism spectrum disorder (ASD), but evidence for traffic pollutants outside the United States is inconclusive. We assessed the association between nitrogen dioxide, a traffic pollution tracer, and risk of ASD. We conducted a nested case-control study among the entire population of children born during 2005–2009 in the central coastal area of Israel. Cases were identified through the National Insurance Institute of Israel (n = 2,098). Controls were a 20% random sample of the remaining children (n = 54,191). Exposure was based on an optimized dispersion model. We estimated adjusted odds ratios and 95% confidence intervals using logistic regression and a distributed-lag model. In models mutually adjusted for the 2 periods, the odds ratio per 5.85-parts per billion (ppb) increment of nitrogen dioxide exposure during pregnancy (median, 16.8 ppb; range, 7.5–31.2 ppb) was 0.77 (95% confidence interval: 0.59, 1.00), and the odds ratio for exposure during the 9 months after birth was 1.40 (95% confidence interval: 1.09, 1.80). A distributed-lag model revealed reduced risk around week 13 of pregnancy and elevated risk around week 26 after birth. These findings suggest that postnatal exposure to nitrogen dioxide in Israel is associated with increased odds of ASD, and prenatal exposure with lower odds. The latter may relate to selection effects.
Keywords: air pollution, autism spectrum disorder, traffic
Autism spectrum disorder (ASD) is a developmental condition characterized by persistent deficits in social communication and social interaction across multiple contexts, accompanied by restricted, repetitive patterns of behavior, interests, or activities (1). ASD is a multifactorial condition with unknown etiology, although many genetic and environmental risk factors have been implicated (2–4).
Several epidemiologic studies have explored associations between air pollutants and risk of ASD (5, 6). Traffic-related pollution, in particular, has been implicated in several studies (7–9); however, only 1 of 3 research groups outside of the United States has seen this association: In a population-based study from Taiwan, Jung et al. (10) found associations between risk of ASD and exposure to traffic-related pollution, such as nitrogen dioxide and carbon monoxide, during the 4 years preceding the diagnosis. In contrast, studies carried out in Sweden (11, 12) and one that analyzed autistic traits in 4 prospectively selected European cohorts (13) did not see associations between traffic-related pollutants and ASD.
Levels of traffic-related pollutants in Sweden are relatively low (12), and associations with symptom traits in the general population may be different from those with clinically defined ASD cases. Therefore, we sought to explore this association in Israel—a different geographical context in which we had population-based data on ASD cases and traffic-related exposures that were on the higher end of those in studies that have explored this question.
METHODS
Study design
We used national Israeli data to identify all children born during the years 2005–2009 in the central coastal area of Israel (the area covered by our exposure model (approximately 70 × 25 km) included approximately 40% of the total Israeli population and approximately 45% of the births occurring in the country during that period) who were still alive and residing in Israel at age 4 years and had an Israeli mother and father. ASD cases were identified by confirmed claims from the National Insurance Institute of Israel (NII), a welfare governmental organization responsible for the social security of all Israeli residents, at the time of data abstraction (July 2014). Controls were a 20% random sample of the remaining children. After exclusion of 4 cases and 14 controls for whom nitrogen dioxide estimates could not be obtained and 3 cases and 1 control with mixed parental ethnicity, the sample included 2,098 ASD cases and 54,191 controls. Ethical permission for the study was granted by the ethics committee of the Hebrew University of Jerusalem (Jerusalem, Israel) and by the Institutional Review Board of the Harvard T.H. Chan School of Public Health (Boston, Massachusetts). Participants’ data were deidentified.
Case ascertainment
Confirmation of ASD cases by the NII has been described previously (14). Briefly, a claim for NII benefits is accompanied by relevant medical details (from diagnoses typically made at a certified child development center by a multidisciplinary team based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria and reviewed by an NII professional committee headed by a physician (typically a pediatric psychiatrist or pediatric neurologist)) (14, 15). Claims are only confirmed if: 1) the diagnosis is made by a pediatric psychiatrist, a pediatric neurologist, or a pediatrician with at least 3 years of experience in a certified child development center; 2) the child was assessed by a developmental or clinical psychologist, using a functional age-appropriate psychological test; and 3) the tool and results of the assessment are reported. The committee may ask for additional information, and in rare cases may also examine the child.
The ASD disability benefit is independent of clinical severity, income, work, population group, or any other characteristics of the child or his/her family. Importantly, the NII does not provide any services to children with ASD, nor is the submission or granting of claims for disability benefits related to the service needs of the child or services given by other authorities. In addition, all Israeli residents are covered by universal health-care insurance from birth to death. Thus, this case definition is not biased towards ASD cases who are insured or requiring services.
Study area and exposure assessment
The study area—the central coastal area of Israel—is shown in Web Figure 1 (available at https://academic.oup.com/aje). Air pollution monitoring data and meteorological records used for estimating exposure were obtained from the air pollution monitoring database of the Technion Center of Excellence in Exposure Science and Environmental Health (Haifa, Israel). The optimized dispersion model (16), improved by residual interpolation (17), was used to produce a spatiotemporal concentration map at a 500- × 500-m grid resolution using proxies for traffic emissions (traffic volumes obtained from an independent traffic assignment model) and meteorological data as inputs. Further details of the exposure modeling are provided in the Web Appendix.
Exposure estimates were assigned for the addresses of the mothers at birth (as documented by the Ministry of Interior and sent to the NII), which were geocoded to the level of small statistical areas as defined by the Central Bureau of Statistics of Israel, or to the city level when geocoding of the birth address to the small statistical area level was not possible (9% of the controls and 6% of the cases). Each newborn was assigned weekly nitrogen dioxide exposure estimates relative to the date of conception (determined by birth date and gestational age at birth) for exposures incurred before birth and relative to birth date for exposures incurred after birth. The estimates were calculated using an area-weighted average of the nitrogen dioxide estimates of the exposure model in the relevant grid points that comprise the small statistical areas or the city boundaries.
Statistical methods
Average nitrogen dioxide concentrations for each child were calculated for different time periods around the pregnancy. We used generalized additive models with a logit link and penalized splines to test for nonlinear associations between nitrogen dioxide exposure and ASD. Since the associations were found to be linear (estimated degrees of freedom approximately 1), we report results from multivariable logistic regression models with nitrogen dioxide as a continuous linear variable.
We adjusted for year of birth (categorical) and calendar month of birth (categorical) to account for secular and seasonal trends. We also adjusted for population group (Arabs, ultraorthodox Jews, or the general population) because we previously found different groups to have very different likelihoods of ASD diagnosis (14) and because population group could also relate to residential location. Adjustments were also made for paternal age (years; continuous) and socioeconomic status (a composite poverty index based on standardized census-level education, income, employment, transportation, and other variables) of the 2008 census tract (continuous), each of which could also be related to ASD diagnosis.
Adjustments for gestational age at birth and birth weight were not included in the main analyses, because these could be intermediates of exposures incurred prior to birth. However, we did conduct sensitivity analyses adjusting for these factors, as well as for the additional socioeconomic status variables, such as maternal and paternal wages and census tract education variables. In sensitivity analyses, we additionally considered multiple births, parents’ immigration year, and month of conception (instead of month of birth). We performed tests of interaction in separate models by adding a multiplicative term for interaction between the exposure and the interaction variable.
A nonlinear distributed-lag model (R package dlnm; R Foundation for Statistical Computing, Vienna, Austria) (18) was used to fit all weekly exposure estimates from conception to 9 months after birth into 1 model. The association with nitrogen dioxide concentrations was modeled as a function of the lag by means of a natural cubic spline with 7 degrees of freedom. R statistical software was used for all data processing and analysis. All odds ratios and 95 confidence intervals are reported for a unit of 5.85 parts per billion (ppb), which was the interquartile range of the average nitrogen dioxide exposure over the entire course of pregnancy in the study population.
RESULTS
Demographic and birth characteristics of the study population are shown by case status in Table 1. As expected, when compared with the general population, cases were more frequently males. Table 2 shows the characteristics of the control population according to quartile of entire-pregnancy nitrogen dioxide level. The mean entire-pregnancy nitrogen dioxide exposure in the study population was 16.7 ppb (median, 16.8 ppb; range, 7.5–31.2 ppb). Nitrogen dioxide exposure in the study area varied widely in both time and space (Web Figure 2) and included a strong seasonal pattern, with winter levels being almost twice the summer levels. Correlations among weekly nitrogen dioxide exposures in the study population had a strong seasonal pattern: Correlations varied between 0.8 (for pairs of 2 following weeks) and almost zero (for pairs of 2 different weeks in a 6-month lag), as demonstrated with data for the first year of life in Web Figure 3. The correlations among various 9-month exposure periods are given in Web Table 1.
Table 1.
Characteristics of Cases and Controls in a Study of Traffic-Related Air Pollution and Autism Spectrum Disorder, Israel, 2005–2009
| Characteristic | ASD Cases (n = 2,098) |
Controls (n = 54,191) |
||||
|---|---|---|---|---|---|---|
| Median (IQR) | No. of Persons | % | Median (IQR) | No. of Persons | % | |
| Birth weight, g | 3,200 (740) | 3,230 (630) | ||||
| Gestational age at birth, weeks | 39 (2) | 39 (2) | ||||
| Maternal age at birth, years | 31.7 (6.9) | 30.5 (7.3) | ||||
| Paternal age at birth, years | 34.0 (7.3) | 33.0 (7.6) | ||||
| Census poverty index (z score)a | 0.49 (1.3) | 0.21 (1.7) | ||||
| Male sex | 1,760 | 83.9 | 27,667 | 51.1 | ||
| Year of birth | ||||||
| 2005 | 144 | 6.9 | 3,267 | 6.0 | ||
| 2006 | 493 | 23.5 | 12,121 | 22.4 | ||
| 2007 | 450 | 21.4 | 12,220 | 22.5 | ||
| 2008 | 473 | 22.5 | 13,078 | 24.1 | ||
| 2009 | 538 | 25.6 | 13,505 | 24.9 | ||
| Maternal income, NIS/month | ||||||
| Not working | 718 | 34.2 | 17,096 | 31.5 | ||
| <4,000 | 365 | 17.4 | 11,169 | 20.6 | ||
| 4,000–5,999 | 320 | 15.3 | 9,024 | 16.7 | ||
| 6,000–9,999 | 358 | 17.1 | 9,595 | 17.7 | ||
| ≥10,000 | 337 | 16.0 | 7,307 | 13.4 | ||
| Paternal income, NIS/month | ||||||
| Not working | 436 | 20.8 | 15,075 | 27.8 | ||
| <4,000 | 168 | 8.0 | 4,894 | 9.0 | ||
| 4,000–5,999 | 210 | 10.0 | 5,559 | 10.3 | ||
| 6,000–7,999 | 237 | 11.3 | 5,619 | 10.4 | ||
| 8,000–9,999 | 220 | 10.5 | 4,820 | 8.9 | ||
| 10,000–13,999 | 285 | 13.6 | 6,454 | 11.9 | ||
| 14,000–19,999 | 234 | 11.2 | 5,239 | 9.7 | ||
| ≥20,000 | 308 | 14.7 | 6,531 | 12.1 | ||
| Population group | ||||||
| General population | 1,828 | 87.1 | 39,126 | 72.2 | ||
| Ultraorthodox Jews | 200 | 9.5 | 11,189 | 20.6 | ||
| Arabs | 70 | 3.3 | 3,876 | 7.2 | ||
| Multiplicity of birth | ||||||
| Singleton | 1,936 | 92.3 | 51,442 | 94.9 | ||
| Twins or more | 162 | 7.7 | 2,749 | 5.1 | ||
| Recent immigration to Israel (<5 years) | ||||||
| Neither parent | 1,901 | 90.6 | 50,925 | 94.0 | ||
| 1 parent | 54 | 2.6 | 1,172 | 2.2 | ||
| Both parents | 143 | 6.8 | 2,094 | 3.9 | ||
| Sibling with ASD | 219 | 10.4 | 383 | 0.7 | ||
| Missing SSA geocoding | 44 | 2.1 | 2,494 | 4.6 | ||
Abbreviations: ASD, autism spectrum disorder; IQR, interquartile range; NIS, new Israeli shekel; SSA, small statistical area.
a Higher scores in the census poverty index represent higher socioeconomic status.
Table 2.
Characteristics of the Control Population by Quartile of Nitrogen Dioxide Exposure During Pregnancy, Israel, 2005–2009
| Characteristic | Quartile of Nitrogen Dioxide Exposure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First (7.48–13.6 ppb) (n = 13,548) | Second (13.7–16.8 ppb) (n = 13,548) | Third (16.9–19.5 ppb) (n = 13,547) | Fourth (19.6–31.2 ppb) (n = 13,548) | |||||||||
| Median (IQR) | No. of Persons | % | Median (IQR) | No. of Persons | % | Median (IQR) | No. of Persons | % | Median (IQR) | No. of Persons | % | |
| Birth weight, g | 3,240 (620) | 3,220 (640) | 3,220 (610) | 3,230 (640) | ||||||||
| Gestational age at birth, weeks | 39 (2) | 39 (2) | 39 (2) | 39 (2) | ||||||||
| Maternal age at birth, years | 30.1 (7.5) | 30.5 (7.3) | 30.8 (7.0) | 30.6 (7.5) | ||||||||
| Paternal age at birth, years | 32.9 (7.7) | 33.0 (7.7) | 33.2 (7.3) | 32.8 (7.9) | ||||||||
| Census poverty index (z score)a | 0.06 (1.9) | 0.15 (1.8) | 0.37 (1.5) | 0.22 (1.6) | ||||||||
| Male sex | 6,877 | 50.8 | 6,956 | 51.3 | 6,917 | 51.1 | 6,917 | 51.1 | ||||
| Year of birth | ||||||||||||
| 2005 | 981 | 7.2 | 1,052 | 7.8 | 760 | 5.6 | 474 | 3.5 | ||||
| 2006 | 2,702 | 19.9 | 3,565 | 26.3 | 2,705 | 20.3 | 3,419 | 23.2 | ||||
| 2007 | 3,250 | 24.0 | 3,066 | 22.6 | 3,118 | 23.0 | 2,786 | 20.6 | ||||
| 2008 | 2,698 | 19.9 | 2,905 | 21.4 | 3,525 | 25.4 | 3,950 | 29.2 | ||||
| 2009 | 3,917 | 28.9 | 2,960 | 21.8 | 3,439 | 25.4 | 3,189 | 23.5 | ||||
| Maternal income, NIS/month | ||||||||||||
| Not working | 4,829 | 35.6 | 4,697 | 34.7 | 3,844 | 28.4 | 3,726 | 27.5 | ||||
| <4,000 | 3,122 | 23.0 | 2,552 | 18.8 | 2,498 | 18.4 | 2,997 | 22.1 | ||||
| 4,000–5,999 | 2,209 | 16.3 | 2,173 | 16.0 | 2,310 | 17.1 | 2,332 | 17.2 | ||||
| 6,000–9,999 | 2,053 | 15.2 | 2,415 | 17.8 | 2,647 | 19.5 | 2,480 | 18.3 | ||||
| ≥10,000 | 1,335 | 9.9 | 1,711 | 12.7 | 2,248 | 16.6 | 2,013 | 14.8 | ||||
| Paternal income, NIS/month | ||||||||||||
| Not working | 4,124 | 30.4 | 3,361 | 24.8 | 3,125 | 23.1 | 4,465 | 33.0 | ||||
| <4,000 | 1,229 | 9.1 | 1,339 | 9.9 | 1,181 | 8.7 | 1,145 | 8.5 | ||||
| 4,000–5,999 | 1,447 | 10.7 | 1,520 | 11.2 | 1,365 | 10.1 | 1,227 | 9.1 | ||||
| 6,000–7,999 | 1,441 | 10.6 | 1,493 | 11.0 | 1,458 | 10.8 | 1,227 | 9.1 | ||||
| 8,000–9,999 | 1,243 | 9.2 | 1,222 | 9.0 | 1,279 | 9.4 | 1,076 | 7.9 | ||||
| 10,000–13,999 | 1,613 | 11.9 | 1,668 | 12.3 | 1,695 | 12.5 | 1,478 | 10.9 | ||||
| 14,000–19,999 | 1,228 | 9.1 | 1,323 | 9.8 | 1,494 | 11.0 | 1,194 | 8.8 | ||||
| ≥20,000 | 1,223 | 9.0 | 1,622 | 12.0 | 1,950 | 14.4 | 1,736 | 12.8 | ||||
| Population group | ||||||||||||
| General population | 8,712 | 64.3 | 10,158 | 75.0 | 10,789 | 79.6 | 9,467 | 69.9 | ||||
| Ultraorthodox Jews | 3,018 | 22.3 | 2,131 | 15.7 | 2,189 | 16.2 | 3,851 | 28.4 | ||||
| Arabs | 1,818 | 13.4 | 1,259 | 9.3 | 569 | 4.2 | 230 | 1.7 | ||||
| Multiplicity of birth | ||||||||||||
| Singleton | 12,892 | 95.2 | 12,826 | 94.7 | 12,871 | 95.0 | 12,853 | 94.9 | ||||
| Twins or more | 656 | 4.8 | 722 | 5.3 | 676 | 5.0 | 695 | 5.1 | ||||
| Recent immigration to Israel (<5 years) | ||||||||||||
| Neither parent | 12,745 | 94.1 | 12,768 | 94.2 | 12,720 | 93.9 | 12,692 | 93.7 | ||||
| 1 parent | 303 | 2.2 | 273 | 2.0 | 336 | 2.5 | 260 | 1.9 | ||||
| Both parents | 500 | 3.7 | 507 | 3.7 | 491 | 3.6 | 596 | 4.4 | ||||
| Sibling with ASD | 98 | 0.7 | 96 | 0.7 | 104 | 0.8 | 85 | 0.6 | ||||
| Missing SSA geocoding | 1,665 | 12.3 | 657 | 4.8 | 152 | 1.1 | 14 | 0.1 | ||||
Abbreviations: ASD, autism spectrum disorder; IQR, interquartile range; NIS, new Israeli shekel; ppb, parts per billion; SSA, small statistical area.
a Higher scores in the census poverty index represent higher socioeconomic status.
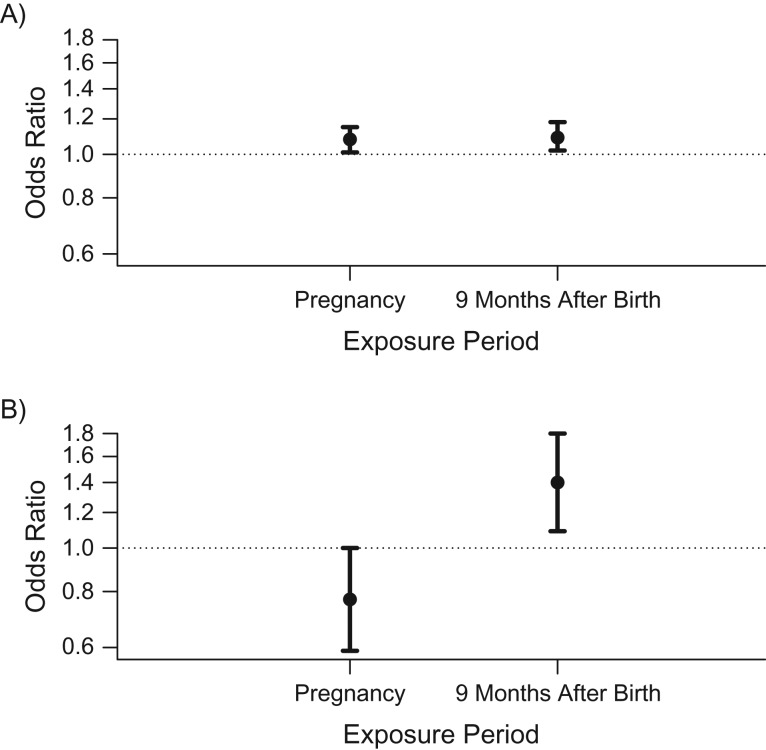
In separate models, average nitrogen dioxide exposures during the entire pregnancy and during the 9-month period after birth were each associated with increased risk of ASD (per 5.85-ppb increment, odds ratio (OR) = 1.08 (95% confidence interval (CI): 1.01, 1.15) and OR = 1.09 (95% CI: 1.02, 1.18), respectively) (Figure 1A). Mutual adjustment for both time periods to account for correlation between periods resulted in higher variance of both estimates, but it revealed a positive association with ASD for the postnatal period (per 5.85 ppb, OR = 1.40, 95% CI: 1.09, 1.80) and a negative association for the pregnancy period (per 5.85 ppb, OR = 0.77, 95% CI: 0.59, 1.00) (Figure 1B).
Figure 1.
Associations between exposure to nitrogen dioxide during pregnancy and during the 9 months after birth and risk of autism spectrum disorder among children born in central coastal Israel during 2005–2009. Odds ratios show the risk of autism spectrum disorder per interquartile-range increment (5.85 ppb) in nitrogen dioxide exposure. A) Results from 2 separate models, each adjusted for year of birth, calendar month of birth, population group, paternal age, and census poverty index. B) Results from 1 model, with mutual adjustment for both exposure periods in addition to all of the covariates listed above. Bars, 95% confidence intervals.
In models restricted to persons with 9 months of exposure data prior to the pregnancy and mutually adjusted for all 3 exposure periods, the associations for the pregnancy and postpregnancy periods were similar and exposure during the prepregnancy period was not associated with ASD (OR = 1.11, 95% CI: 0.83, 1.49; Web Table 2). The findings for the pregnancy and postpregnancy periods appeared to be driven by boys (Web Table 2). There was no suggestion of interaction between exposure during either the pregnancy period (P = 0.6) or the 9 months after birth (P = 0.99) and having a sibling with ASD.
We conducted sensitivity analyses using exposure during one of the 9-month periods (prepregnancy, entire pregnancy, or postpregnancy) mutually adjusted for the other 2 exposure periods, restricted to different subsets of the population (only term infants, excluding subjects missing gestational age data, excluding subjects geocoded at the city level, excluding multiple births, excluding Arabs and ultraorthodox Jews or just Arabs, and excluding subjects who had a sibling with ASD); results were all materially unchanged from those of the primary models (Web Table 3). Further adjustments for either child’s sex, maternal age, paternal income, multiple birth, parents’ immigration status, gestational age, or birth weight also did not have a substantial effect on the main results (Web Table 3).
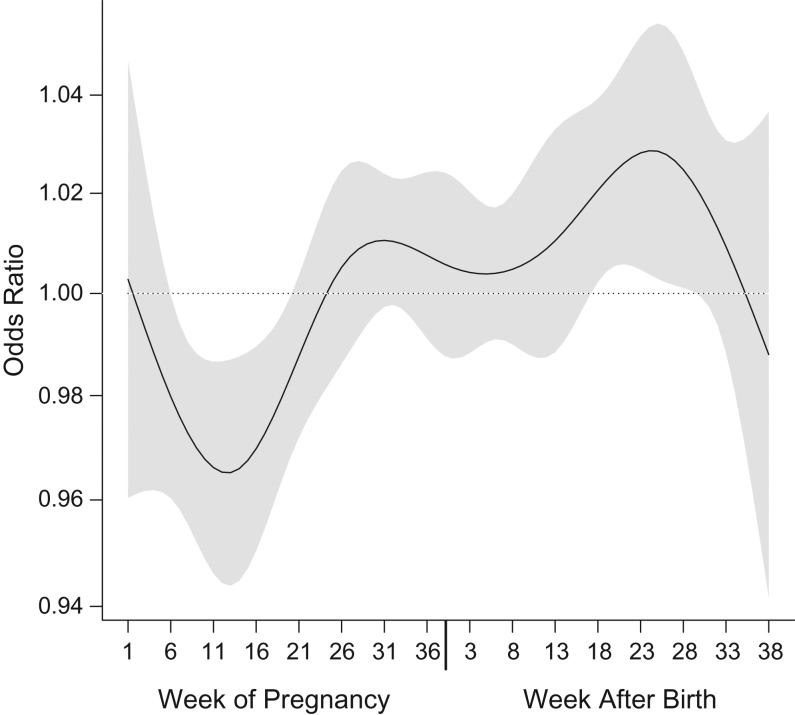
A nonlinear distributed-lag model of weekly exposures revealed significantly reduced odds of ASD with exposures incurred during weeks 5–20 of pregnancy, with a minimum in week 13, and increased odds of ASD with exposures incurred during postnatal weeks 15–30, with a maximum in week 26 (Figure 2).
Figure 2.
Results from a distributed-lag model representing polynomial time-dependent associations between weekly nitrogen dioxide exposure and risk of autism spectrum disorder among children born in central coastal Israel during 2005–2009. The black line represents the time-varying function estimating risk of autism spectrum disorder with weekly exposures during 38 weeks of pregnancy (left) and the first 38 weeks of life (right), and the gray area around it represents its 95% confidence interval. These results are from a nonlinear distributed-lag model with 7 degrees of freedom. A linear association was assumed between the exposure and the outcome at each time point. Results were adjusted for year of birth, calendar month of birth, population group, paternal age, and census poverty index.
DISCUSSION
In this large-scale case-control study nested within the entire population of the central coastal region of Israel, we found that increased residential exposure to nitrogen dioxide during the year after birth was significantly associated with having a child diagnosed with ASD. Our results illustrate the importance of mutual adjustment for different correlated time periods of exposure to identify critical windows of exposure, since in non–mutually adjusted models several time periods appeared to be associated with ASD. Mutual adjustment for different time periods also helps to reduce confounding by factors that are related to several different exposure time periods (19, 20).
Intriguingly, mutually adjusted models revealed a significant association between higher nitrogen dioxide exposure during pregnancy and a reduced chance of having a child with ASD, particularly with exposure in the second trimester of pregnancy and, to a lesser extent, the first trimester of pregnancy. This pattern can be explained if the exposure in question affects the chance of someone entering the cohort. This is related to a form of selection bias that has been described in the occupational health literature as left-truncation bias (21) but has also been described for cohorts in general and birth cohorts specifically (22, 23), as well as one that underlies the birth weight paradox (24). That is, typically birth cohorts condition on having a live birth and do not include pregnancies that were lost. Thus, if the exposure of interest is related to fetal loss (e.g., spontaneous abortion), the exposure could look “protective” for the main outcome. This can arise either because the exposure-related fetal loss could preferentially occur among children who would have had the outcome had they been liveborn or because those who are born despite high levels of the exposure are less likely to have other exposures that are related to both fetal loss and the outcome.
Including highly correlated variables in a model together can lead to unstable effect estimates. However, as Schisterman et al. (25) recently described, bias of effect estimates would only come at extremely high correlations (>0.99), and if the structure of the data requires inclusion of highly correlated variables, they need to be included together. The confidence intervals of effect estimates, in this case, will reflect the instability of the point estimates. In our study, the confidence intervals did get wider when the exposure periods were included together, but they still did not include the null. Thus, we do not believe that model instability accounts for our findings.
Some studies do support the idea that air pollution is a risk factor for stillbirth (26), various pregnancy complications (27), and spontaneous abortion (28). If a prenatal reduced risk and postnatal increased risk exist, this makes it difficult to determine whether the lack of association later in pregnancy is truly due to no biological effect of traffic-related pollution on ASD or due to the “protective” association from selection bias balancing out a harmful association with ASD. Importantly, if these 2 phenomena are occurring, their relative balance could create different overall findings in different study settings, particularly when there is not mutual adjustment for different exposure time periods.
Two prior European studies found no association between nitrogen dioxide and autistic traits, but they either considered only the pregnancy period (13) or evaluated postbirth periods without mutually adjusting for the different exposure time windows (11). In a more recent study from Sweden, Gong et al. (12) used a large-scale nested case-control design similar to that of our study, with clinical diagnoses of ASD, but did not find an association between pregnancy or postpregnancy traffic-related pollution and ASD. However, they did not mutually adjust for the exposure time periods (12). Furthermore, exposure to nitrogen dioxide in Sweden has smaller variability and is generally lower than that in Israel. Namely, levels of nitrogen oxides (i.e., nitrogen monoxide + nitrogen dioxide) in the Swedish study were much lower than nitrogen dioxide levels in the current study (with nitrogen dioxide representing approximately 60% of ambient nitrogen oxides in central Israel). It is also possible that the null findings in the European studies resulted from a canceling out of “protective” and risk associations in different time periods. Mutual adjustment for the different exposure time windows would address this (25).
A harmful association between nitrogen dioxide and ASD was seen in the US studies—all from California, where exposure levels were much higher (range, 17–30 ppb) than those in the Swedish studies—although the models examined only the pregnancy period or did not mutually adjust for different exposure time windows (7, 9, 29). Thus, this result is consistent with the generally elevated association for ASD in our study when mutual adjustment for different exposure periods was not done. It is noteworthy that the only US study that evaluated both pregnancy and postpregnancy exposure to nitrogen dioxide also found stronger results for exposure in the first year of life than during pregnancy (7). The study in Taiwan only evaluated exposure incurred during the 4 years before ASD diagnosis (10). It is also notable that several other US studies also found positive associations between traffic pollution and ASD using other markers, such as distance to the nearest road (8) and other traffic-related air pollutants (30–32).
Mechanisms that could mediate the increased risk of ASD with higher exposure to traffic-related pollution include oxidative stress, microglial activation, and neuroinflammation, as these processes are both induced experimentally by traffic-related pollutants in rodents (33–35), with evidence of male-specific effects (36), and have been found to be associated with ASD in children (37, 38). Specifically, microglia play a role in synaptic pruning, which occurs from the last trimester of pregnancy through the early years of life. Increased microglial activation can be induced by traffic-related pollution (35, 36) and may alter normal pruning (39, 40), which can lead to the altered brain connectivity patterns found in children with ASD (41). Additional support for these mechanisms comes from a recent epigenome-wide meta-analysis of methylation in children related to prenatal nitrogen dioxide exposure, which found differential methylation and expression of genes related to antioxidant defense pathways (42).
Our results suggest that the association between nitrogen dioxide and ASD is relevant mostly in boys, although the smaller number of girls led to less stable estimates among them. Some of the prior epidemiologic studies also suggested a similar sex difference (32, 43), and work on the association between prenatal particulate matter exposure and neurodevelopment has suggested that third-trimester exposure is associated with lower intelligence quotient scores in boys but not in girls (44). This is in line with various animal studies of controlled exposure to air pollutants, which demonstrated a differential response by sex, usually attributed to higher expression of the antioxidant/antiinflammatory enzyme paraoxonase 2 (33, 35). This enzyme inversely correlates with susceptibility to toxicants, causing oxidative stress in vitro, and its levels are also higher in human females relative to males (45).
Our study had several limitations. As in most large-scale air pollution studies, we based our exposure assessment on the residential address. The subjects—mothers and children—did not spend all their time at their home address. This may have resulted in exposure measurement error that could have biased our results towards the null, as found in a previous study (43). However, the use of modeled exposure estimates protects from confounding by individual behavioral factors, since they do not influence our exposure model (6, 46). Our results could still reflect residual confounding by factors that affect ambient air pollution levels. However, we found no association with the prepregnancy period in mutually adjusted models. This suggests that time-invariant factors, such as socioeconomic status, do not confound the associations we see, because otherwise associations with other exposure windows that are affected by these factors should also be present (i.e., the negative control exposures principle) (6, 47–49).
Genetics plays an important role in the etiology of ASD, but unfortunately we did not have genetic data for our analyses. Thus, we were not able to explore interesting questions of gene × environment interactions or whether associations differ by genetic predisposition. However, assuming that having a sibling with ASD is an indicator of increased genetic risk for ASD, the lack of interaction by this factor in our findings suggests that the associations with nitrogen dioxide are not modified by genetic predisposition to ASD. Finally, nitrogen dioxide should be considered a marker for a mix of traffic-related air pollutants, including many gases and particles for which detailed exposure data are not available for epidemiologic studies.
The strengths of our study include 1) a very large study population that has been sampled systematically from the total population of the study area and includes all of the relevant cases and 2) case identification based on national health coverage that is independent of service needs. These factors minimize the chances of selection bias that can affect nonnested case-control studies. In addition, we used a validated and detailed exposure model that incorporated nitrogen dioxide variability in both time and space in the study area, where possible associations between air pollution and ASD have not been studied before. Finally, we used objective individual-level demographic and socioeconomic covariates, independent of subject reporting, to adjust for potential confounders.
In summary, we bring new evidence for an association between prenatal and/or perinatal exposure to traffic pollution and ASD from a region of the world not yet analyzed, with a different climate and health-care system and different pollutant sources and confounding structures. The findings suggest that exposure to traffic-related pollution during the postnatal period may be more relevant to ASD than prenatal exposure, as well as the intriguing possibility that prenatal exposure to traffic pollution may be related to fetal loss.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Braun School of Public Health and Community Medicine, The Hebrew University of Jerusalem and Hadassah Ein Kerem, Jerusalem, Israel (Raanan Raz, Hagai Levine); Research and Planning Administration, National Insurance Institute of Israel, Jerusalem, Israel (Ofir Pinto); Faculty of Civil and Environmental Engineering, Technion–Israel Institute of Technology, Haifa, Israel (David M. Broday, Yuval); and Departments of Environmental Health and Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Marc G. Weisskopf).
This study was funded by James Crystal, in honor of Lilian Yaros; by Marilyn B. Hoffman, to support projects that address her vision; and by the US National Institutes of Health (grants R21 ES026900 and P30 ES000002). R.R. was partially supported by the Environment and Health Fund (Jerusalem, Israel) during part of the study.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of any of the authors’ affiliated institutions. The funding sources played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, and approval of the manuscript.
Conflict of interest: none declared.
Abbreviations
- ASD
autism spectrum disorder
- CI
confidence interval
- NII
National Insurance Institute of Israel
- OR
odds ratio
REFERENCES
- 1. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2. Robinson EB, St Pourcain B, Anttila V, et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 2015;48(5):552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lichtenstein P, Carlström E, Råstam M, et al. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167(11):1357–1363. [DOI] [PubMed] [Google Scholar]
- 4. Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. 2014;43(2):443–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lam J, Sutton P, Kalkbrenner A, et al. A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PLoS One. 2016;11(9):e0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weisskopf MG, Kioumourtzoglou MA, Roberts AL. Air pollution and autism spectrum disorders: causal or confounded? Curr Environ Health Rep. 2015;2(4):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volk HE, Lurmann F, Penfold B, et al. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 2013;70(1):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Volk HE, Hertz-Picciotto I, Delwiche L, et al. Residential proximity to freeways and autism in the CHARGE Study. Environ Health Perspect. 2011;119(6):873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Becerra TA, Wilhelm M, Olsen J, et al. Ambient air pollution and autism in Los Angeles County, California. Environ Health Perspect. 2013;121(3):380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jung CR, Lin YT, Hwang BF. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. PLoS One. 2013;8(9):e75510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gong T, Almqvist C, Bölte S, et al. Exposure to air pollution from traffic and neurodevelopmental disorders in Swedish twins. Twin Res Hum Genet. 2014;17(6):553–562. [DOI] [PubMed] [Google Scholar]
- 12. Gong T, Dalman C, Wicks S, et al. Perinatal exposure to traffic-related air pollution and autism spectrum disorders. Environ Health Perspect. 2017;125(1):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guxens M, Ghassabian A, Gong T, et al. Air pollution exposure during pregnancy and childhood autistic traits in four European population-based cohort studies: the ESCAPE Project. Environ Health Perspect 2016;124(1):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raz R, Weisskopf MG, Davidovitch M, et al. Differences in autism spectrum disorders incidence by sub-populations in Israel 1992–2009: a total population study. J Autism Dev Disord. 2015;45(4):1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Israel Ministry of Health Director General Guidelines. Diagnosis of Children Suffering from Autism PDD (the Autism Spectrum) [in Hebrew]. Jerusalem, Israel: Ministry of Health; 2007. http://www.health.gov.il/hozer/mk13_2007.pdf. Accessed January 1, 2018. [Google Scholar]
- 16. Yuval, Bekhor S, Broday DM. Data-driven nonlinear optimisation of a simple air pollution dispersion model generating high resolution spatiotemporal exposure. Atmos Environ. 2013;79:261–270. [Google Scholar]
- 17. Yuval, Levy I, Broday DM. Improving modeled air pollution concentration maps by residual interpolation. Sci Total Environ. 2017;598:780–788. [DOI] [PubMed] [Google Scholar]
- 18. Gasparrini A. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw. 2011;43(8):1–20. [PMC free article] [PubMed] [Google Scholar]
- 19. Greenland S. The effect of misclassification in the presence of covariates. Am J Epidemiol. 1980;112(4):564–569. [DOI] [PubMed] [Google Scholar]
- 20. Ogburn EL, Vanderweele TJ. Bias attenuation results for nondifferentially mismeasured ordinal and coarsened confounders. Biometrika. 2013;100(1):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Applebaum KM, Malloy EJ, Eisen EA. Left truncation, susceptibility, and bias in occupational cohort studies. Epidemiology. 2011;22(4):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weisskopf MG, Sparrow D, Hu H, et al. Biased exposure-health effect estimates from selection in cohort studies: are environmental studies at particular risk? Environ Health Perspect. 2015;123(11):1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liew Z, Olsen J, Cui X, et al. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol. 2015;44(1):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hernández-Díaz S, Schisterman EF, Hernán MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006;164(11):1115–1120. [DOI] [PubMed] [Google Scholar]
- 25. Schisterman EF, Perkins NJ, Mumford SL, et al. Collinearity and causal diagrams: a lesson on the importance of model specification. Epidemiology. 2017;28(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siddika N, Balogun HA, Amegah AK, et al. Prenatal ambient air pollution exposure and the risk of stillbirth: systematic review and meta-analysis of the empirical evidence. Occup Environ Med. 2016;73(9):573–581. [DOI] [PubMed] [Google Scholar]
- 27. Yorifuji T, Naruse H, Kashima S, et al. Residential proximity to major roads and obstetrical complications. Sci Total Environ. 2015;508:188–192. [DOI] [PubMed] [Google Scholar]
- 28. Enkhmaa D, Warburton N, Javzandulam B, et al. Seasonal ambient air pollution correlates strongly with spontaneous abortion in Mongolia. BMC Pregnancy Childbirth. 2014;14:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. von Ehrenstein OS, Aralis H, Cockburn M, et al. In utero exposure to toxic air pollutants and risk of childhood autism. Epidemiology. 2014;25(6):851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Windham GC, Zhang L, Gunier R, et al. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco Bay Area. Environ Health Perspect. 2006;114(9):1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalkbrenner AE, Daniels JL, Chen JC, et al. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21(5):631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roberts AL, Lyall K, Hart JE, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect. 2013;121(8):978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Costa LG, Cole TB, Coburn J, et al. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2017;59:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng H, Saffari A, Sioutas C, et al. Nanoscale particulate matter from urban traffic rapidly induces oxidative stress and inflammation in olfactory epithelium with concomitant effects on brain. Environ Health Perspect. 2016;124(10):1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roqué PJ, Dao K, Costa LG. Microglia mediate diesel exhaust particle-induced cerebellar neuronal toxicity through neuroinflammatory mechanisms. Neurotoxicology. 2016;56:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bolton JL, Marinero S, Hassanzadeh T, et al. Gestational exposure to air pollution alters cortical volume, microglial morphology, and microglia-neuron interactions in a sex-specific manner. Front Synaptic Neurosci. 2017;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rose S, Melnyk S, Pavliv O, et al. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry. 2012;2:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frustaci A, Neri M, Cesario A, et al. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med. 2012;52(10):2128–2141. [DOI] [PubMed] [Google Scholar]
- 39. Schafer DP, Stevens B. Brains, blood, and guts: MeCP2 regulates microglia, monocytes, and peripheral macrophages. Immunity. 2015;42(4):600–602. [DOI] [PubMed] [Google Scholar]
- 40. Hong S, Dissing-Olesen L, Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol. 2016;36:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mohammad-Rezazadeh I, Frohlich J, Loo SK, et al. Brain connectivity in autism spectrum disorder. Curr Opin Neurobiol. 2016;29(2):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gruzieva O, Xu CJ, Breton CV, et al. Epigenome-wide meta-analysis of methylation in children related to prenatal NO2 air pollution exposure. Environ Health Perspect. 2017;125(1):104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raz R, Roberts AL, Lyall K, et al. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II cohort. Environ Health Perspect. 2015;123(3):264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chiu YH, Hsu HH, Coull BA, et al. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ Int. 2016;87:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Giordano G, Tait L, Furlong CE, et al. Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 expression. Free Radic Biol Med. 2013;58:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weisskopf MG, Webster TF. Trade-offs of personal versus more proxy exposure measures in environmental epidemiology. Epidemiology. 2017;28(5):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weisskopf MG, Tchetgen Tchetgen EJ, Raz R. Commentary: on the use of imperfect negative control exposures in epidemiologic studies. Epidemiology. 2016;27(3):365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Flanders WD, Klein M, Darrow LA, et al. A method for detection of residual confounding in time-series and other observational studies. Epidemiology. 2011;22(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.