Abstract
The ghrelin-producing ε cell represents the fifth endocrine cell type in human pancreatic islets. The abundance of ε cells in adult pancreas is extremely low, which has hampered the investigation on the molecular pathways regulating the development and the function of this cell type. In this study, we explored the molecular features defining the function of pancreatic ε cells isolated from adult nondiabetic donors using single-cell RNA sequencing technology. We focus on transcription factors, cell surface receptors, and genes involved in metabolic pathways that contribute to regulation of cellular function. Furthermore, the genes that separate ε cells from the other islet endocrine cell types are presented. This study expands prior knowledge about the genes important for ε cell functioning during development and provides a resource to interrogate the transcriptome of this rare human islet cell type.
Ghrelin is a hormone secreted by specialized cells in the stomach and intestine as well as from rare ε cells in the islets of Langerhans of the endocrine pancreas (1–5). As a ligand for the GH secretagogue receptor (GHSR), ghrelin is an important stimulator of GH synthesis and secretion (6, 7). Ghrelin also regulates energy homeostasis, body weight and composition, appetite, glucose control, and gastrointestinal motility (8–11). During development, the pancreatic ε cells account for ∼10% of the human islet cells and likely contribute to circulating ghrelin levels in the body (4, 5). In adult humans, the number of ε cells is reduced to <1% (4). Because of their scarcity in the adult state, it is unlikely that pancreatic ε cells contribute to the circulating ghrelin pool; however, it could be speculated that the locally produced ghrelin regulates islet function. In line with this, it has been shown that human δ cells and partly β cells express GHSR (12–14). Furthermore, ghrelin stimulates somatostatin secretion and suppresses glucose-stimulated insulin secretion (15, 16). The study of pancreatic ε cells in animal models is difficult because of extreme scarcity (e.g., in mice 2 weeks of age, few ε cells can be detected) (17, 18).
Ultrastructural studies have shed light on the features of the pancreatic ε cells. They have a round or ovoid shape with occasional cytoplasmic extensions. Their small and spherical granules vary in electron density and show some similarity to α-cell granules (7, 19). Single-cell studies reported a small number of ε cells (13, 20), but detailed information regarding the human pancreatic ε-cell transcriptome is not available. Instead, comparable knowledge has originated from purified gastric ghrelin cells or immortalized cell lines derived from ghrelinomas in the stomach [stomach-derived ghrelinoma (SG-1), mouse ghrelinoma 3 (MGN3-1)] and pancreas [pancreas-derived ghrelinoma (PG-1)] (21–27). In this study, we have obtained the transcriptome of adult human ε cells from 12 nondiabetic donors through a large-scale RNA sequencing effort of single human islet cells. We report on the genes enriched in ε cells when compared with the other endocrine islet cell types as well as the expression of transcription factors, cell surface receptors, and genes pertaining to metabolic pathways.
Materials and Methods
Human islets
Human islets were obtained (Prodo Laboratories) from 12 nondiabetic donors. Donor information and procedure for islet processing have been described previously (28, 29). Briefly, islet samples from each donor were processed independently as they became available. After receipt, islets were cultured overnight followed by enzymatic dissociation and filtering. Afterward, cells were immediately loaded on a Chromium Single Cell Instrument (10X Genomics) and sequenced. Their viability was assessed using trypan blue staining (91.2 ± 3.3% cell viability; n = 12). An online repository contains relevant donor information (30).
RNA fluorescence in situ hybridization of dissociated islet cells
Cytospin was used to place dissociated islet cells into slides. Afterward, cells were fixed in 10% formalin for 35 minutes, followed by permeabilization and hybridization with mRNA probes (GHRL, INS, GCG, PPY, and SST) per the manufacturer’s instructions (Advanced-Cell Diagnostics). Signal was amplified using a fluorescent kit and detected using a microscope slide scanner (Zeiss Axio-Scan.ZI). An RNA fluorescence in situ hybridization analysis module from HALO software (Indica Laboratories) was performed to determine the intensity of the signal followed by quantification analysis.
Single-cell RNA sequencing, read mapping, and ERCC Spike-In
Single cells suspended in PBS with 0.04% BSA were loaded on a Chromium Single Cell Instrument (10X Genomics). RNA sequencing libraries were prepared using Chromium Single Cell 3′ Library, Gel Beads & Multiplex Kit (10X Genomics). Paired-end sequencing was performed on Illumina NextSeq500 [Read 1 for unique molecular identifier (UMI) and cell barcode; Read 2 55-bp transcript read]. Cell Ranger Single-Cell Software Suite (10X Genomics) was used to perform sample demultiplexing, alignment, filtering, and UMI counting. The Human B37.3 Genome assembly and UCSC gene model were used for the alignment.
ERCC Exfold Spike-In Mix 2 from Ambion (ThermoFisher, catalog no. 4456739) was used at 1:10 dilution. Diluted ERCC Mix 2 (2.3 μL) was combined with Chromium Single Cell 3′ Library, Gel Beads & Multiplex Kit (10X Genomics) and loaded to Chromium Single Cell Instrument (10X Genomics). Gel beads in emulsion (GEM) were subsampled (1.3 μL) and then processed with 10X Genomics GEM-RT cleanup, amplification, and library construction protocols (10X Genomics).
Single-cell data analysis
Cell clustering analysis and cell type identification were conducted as described previously (28, 29). Briefly, cells were excluded if they fell in one of the following groups: (i) detected with >1 hormone gene; (ii) high mitochondrial gene ratio (>0.2) (31); (iii) detected <500 genes; or (iv) <3000 total UMI. Seurat package was used to cluster single islet cells and identify enriched genes in each cell type. The 11 ε cells were compared with α, β, δ, and PP cells separately using FindMarkers in Seurat. Enriched genes in ε cells compared with another cell type were identified by Bonferroni-adjusted P < 0.05, log-scale fold change >0.25, and detection rate in 11 ε cells >0.25.
Pathway analysis
Gene pathway annotated datasets were obtained for citrate cycle tricarboxylic acid (KEGG-hsa00020), fatty acid metabolism (KEGG-hsa01212), glycolysis and gluconeogenesis (KEGG-hsa00010), pentose phosphate pathway (KEGG-hsa00030), G protein–coupled receptors (GPCRs) (Hugo Gene Nomenclature Committee), and transcription factors (31).
Accession number
Single-cell sequencing data described in this study have been made available previously (28, 29) and can be found within the Gene Expression Omnibus database (accession no. GSE114297).
Results
Frequency of ε cells in adult human islets
Single-cell RNA sequencing analysis of 14,779 human islet endocrine cells revealed a population of 11 ε cells (frequency, 0.08%). The cells were identified by high expression of GHRL. Independent detection of GHRL+ cells using RNA fluorescence in situ hybridization revealed a comparable frequency in populations of single human islet cells (average frequency, 0.14%; 160 of 113,109 endocrine islet cells) (30).
We detected a total of 5445 unique genes within the 11 GHRL+ cells at ≥1 UMI, with an average of 1267 genes per cell (30). The total number of genes within the ε-cell population was comparable to the α- and β-cell populations that were also sequenced within this effort (28, 29). Down sampling to 11 cells in each of these populations resulted in detection of 5033 genes in α cells and 4655 genes in β cells. However, when the cell number is increased to 6000 to 7000 α or β cells, we are capable of detecting 17,000 to 18,000 genes, which is comparable to about 18,000 genes detected in whole islets (reads per kilobase per million mapped reads >0.1). The explanation for these differences relies in the low number of cells and low sensitivity of the single-cell RNA sequencing technology. The latter is exemplified in spike-in experiments (see “Materials and Methods”) revealing a gene detection rate of 10% (90% dropout). Consequently, the detection was biased toward higher abundance genes (30). These limitations should be considered when interpreting the dataset. Important implications are that (i) the data may not serve to deduce absolute abundance of a gene and (ii) the absence of expression of a gene should not be considered as a true negative result, especially for low abundant genes. Despite this limitation, the number of detected genes in our ε-cell population was still of considerable measure. This, in addition to our quality control measures, gave us confidence to move forward with the present analysis.
Enriched human ε-cell genes among endocrine islet cell types
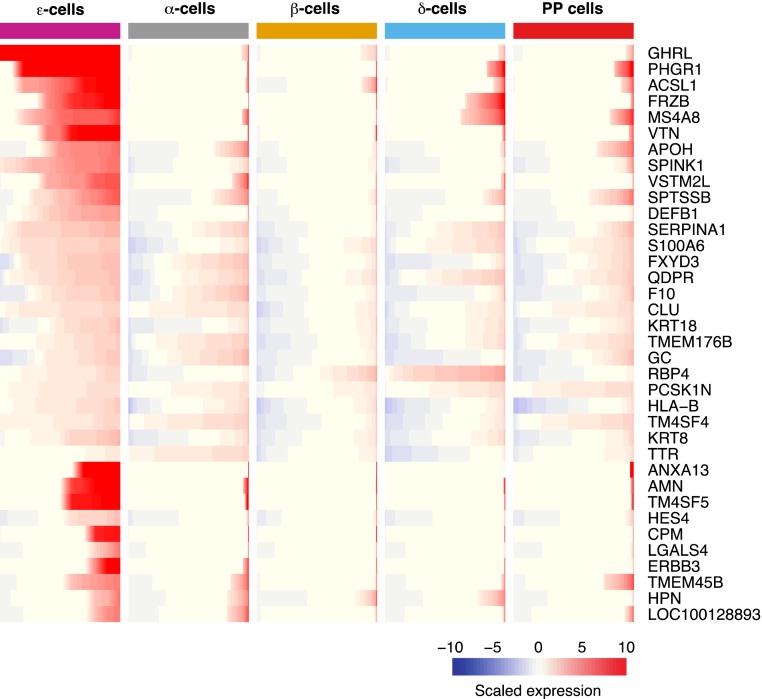
We investigated what sets apart ε cells from the other pancreatic endocrine cell types. In this regard, we found 36 genes to be significantly enriched in ε cells (see “Materials and Methods”) when compared with the other endocrine cell populations (α, β, δ, and PP cells) (Fig. 1; detailed information regarding endocrine cell comparisons can be found in an online repository) (30). On average, each enriched gene showed significantly higher expression in ε cells than one or two other endocrine cell types. Among the genes with a more specific pattern of enrichment in ε cells was acyl-coenzyme A synthetase long chain family member 1 (ACSL1) (ε vs PP cell-adjusted P < 6.12E-04), which has been suggested to have a potential role in the processing of ghrelin. Ghrelin consists of 28 amino acids and can become modified with the addition of an n-octanoyl at its third serine residue. This modification is essential for its binding to the GHSR (6). Acsl1, an isoenzyme that converts long-chain fatty acids (LCFAs) into acyl-coenzyme A esters, was previously found to be expressed at high levels in MGN3-1 (32). Furthermore, results from this study suggested that incorporation of LCFAs, as part of the supply of octanoic acid for ghrelin acylation, was mediated in part by ACSL1 (32).
Figure 1.
Differentially expressed genes in human ε cells vs other endocrine cells (α, β, δ, and PP cells). Enriched genes in ε cells were determined using a Bonferroni-adjusted P value <0.05, log-scale fold change >0.25, and detection rate in 11 ε cells >0.25. Each row represents a gene; each column represents a distinct endocrine cell population. Gene expression is presented in each row as a cumulative percent of the population expressing the gene. The level of expression of each gene is presented as scaled expression.
Surprisingly, we found two ε cell–enriched genes known for their proteinase inhibitory capacity: serine peptidase inhibitor Kazal type 1 (SPINK1) (ε vs α cell-adjusted P < 2.67E-11; ε vs β cell-adjusted P < 3.97E-47; ε vs δ cell-adjusted P < 4.58E-18) and serpin family A member 1 (ε vs β cell-adjusted P < 4E-24). The first is a serine protease inhibitor that binds to erroneously activated trypsin, forming a stable inactive complex. In the islet, SPINK1 is expressed and packaged into acinar cell secretory granules. Its major role in the pancreas relates to the protection from premature trypsinogen activation, which can lead to damage of acinar cells and ultimately development of pancreatitis (33, 34). Serpin family A member 1, a gene encoding the protein α-1 antitrypsin not only inhibits trypsin and elastase proteases, but also has recently been found to inhibit caspase-3 activity (35). It is believed to be involved in tissue repair after injury, with proinflammatory cytokines enhancing its production and release. Administration of α-1 antitrypsin has resulted in islet allograft protection and survival, as well as in prevention of streptozotocin-induced diabetes in mice (36, 37). Similar to our data, immunohistochemistry analysis in human islets has shown this factor to be expressed in α and δ cells, but not in β cells. Unfortunately, its expression in ε cells, where we currently observe its highest enrichment, was, to our knowledge, not assessed (37).
Another highly enriched gene that encodes a defense peptide is defensin beta 1 (ε vs β cell-adjusted P < 2.30E-02; ε vs PP cell-adjusted P < 2.28E-02). This β-defensin protein has the ability to kill a wide variety of microorganisms including bacteria, virus, and yeast. Furthermore, it binds several receptors and enhances the immune activity. Interestingly, β-defensin has been shown to have anti- and proinflammatory actions, depending on the disease and pathogen exposure (38). Interestingly, we also detected enrichment of retinol-binding protein 4 (RBP4) (ε vs αcell-adjusted P < 1.82E-03; ε vs PP cell-adjusted P < 1.17E-03) as well as of transthyretin (TTR) (ε vs δ cell-adjusted P < 6.38E-04). Among the known functions of TTR are the transport of thyroid hormone and formation of a complex with retinol-binding protein that transports retinol in the bloodstream (39). Both RBP4 and TTR have been found to be highly expressed in enriched pools of gastric ghrelin cells, in the gastric cell line (SG-1), but more important in a ghrelinoma cell line of pancreatic origin (PG-1) (24). Last, transmembrane 4 L six family member 4 (TM4SF4), a member of the L6 domain tetraspanin family, was found to be enriched in ε cells (ε vs β cell-adjusted P < 1.87E-05), and the transmembrane 4 L six family member 5 (ε vs β cell-adjusted P < 8.87E-15; ε vs δ cell-adjusted P < 1.53E-02). Although to our knowledge no function is known regarding 4 L six family member 5 in ε cells, the function of TM4SF4 in pancreatic islets has been associated with cell migration and endocrine differentiation. In NK2 homeobox 2 (Nkx2.2)–null mice, in which there is an abundance of ghrelin cells, its expression was found to be significantly elevated. In this same study, knockdown of tm4sf4 in zebrafish resulted in a decrease in the ghrelin cell population, highlighting its potential importance for the ε-cell fate (40).
Thus, we found that genes differentially expressed in ε cells when compared with the other pancreatic endocrine populations span functions related but not limited to hormone processing, proteinase inhibition, cell migration, and antimicrobial activity. Although we have highlighted some of these genes, there are several others whose function in ε cells merit further investigation.
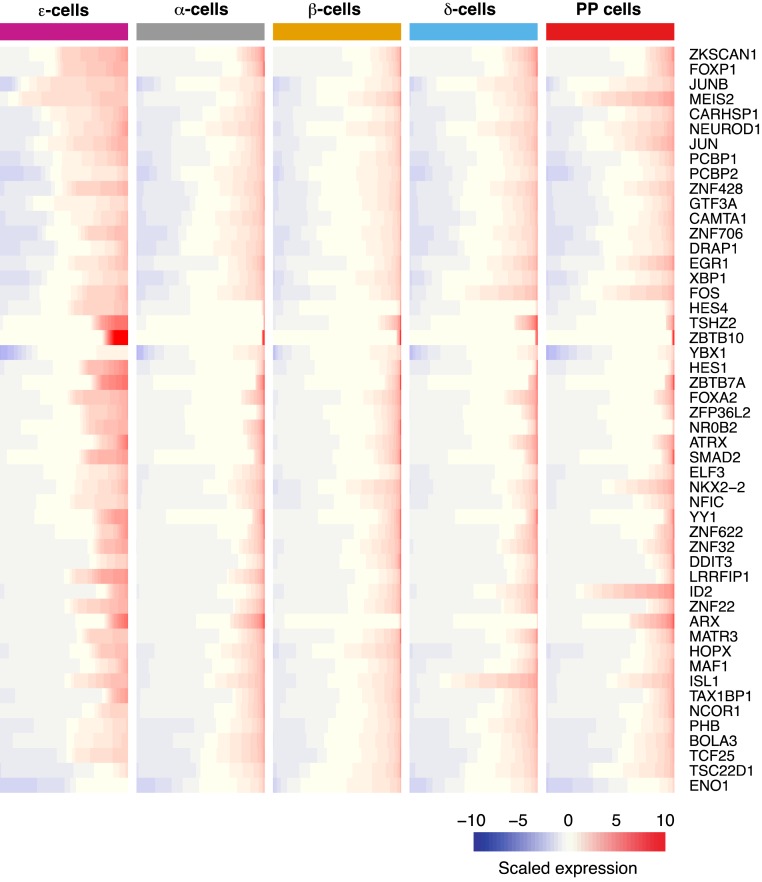
Pancreatic ε cells express relevant islet cell transcription factors
Prior studies in mice have given us insights into the regulatory networks influencing the development of ε cells (17, 18, 41–45). To complement this knowledge, we now report on 366 transcription factors expressed in human adult ε cells (30). Figure 2 shows the top 50 transcription factors ranked based on their average expression. Among them, we observed expression of known genes essential for the differentiation and maintenance of islet endocrine cells including aristaless-related homeobox, ISL LIM homeobox 1, neuronal differentiation 1, and NKX2.2 (46–50). Although not much is known regarding transcriptional regulation of human pancreatic ε cells, immunohistochemistry analysis of fetal samples is largely consistent with our findings because they have shown expression of ISL LIM homeobox 1 and NKX2.2 in this cell population (4). Interestingly, NKX2.2 has been found to activate the mouse and human ghrelin promoter that, together with our findings, points to its potential importance in regulating ghrelin expression in the mature state (51, 52). We also found members of the HES family to be expressed: hes family bHLH transcription factor (HES) 1, HES4, and HES6. To our surprise, HES4 was the only transcription factor found to be significantly enriched in ε cells (ε vs δ cell-adjusted P < 1.71E-02) (Fig. 1). Unfortunately, not much information exists on HES4 because it is expressed in humans only and not mice. However, a member of its family HES1 is a factor known to be involved in the regulation of pancreatic progenitor cell differentiation and maintenance (53). Last, we found aristaless-related homeobox to be detected in ε cells, a finding consistent with developmental mouse studies showing this factor to be expressed within this cell population (30, 54, 55). The function in ε cells of these and other transcription factors found in the current study is an area of biology that merits further investigation.
Figure 2.
Top 50 transcription factors expressed in human ε cells. Average gene expression was used to select the highest expressed transcription factors within the ε-cell population. Each row represents a gene; each column represents a distinct endocrine cell population. Gene expression is presented in each row as a cumulative percent of the population expressing the gene. The level of expression of each gene is presented as scaled expression.
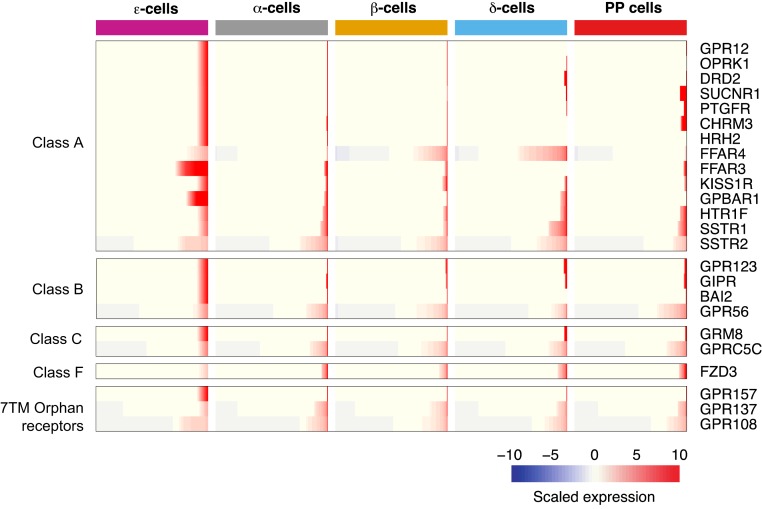
Cell surface receptors present in human pancreatic ε cells
Ghrelin cells respond to nutrients, hormones, and other signaling molecules (21, 56, 57). To gain understanding of how ε cells sense and respond to changes in their environment, we examined the expression of cell surface proteins, including GPCRs (30). We found 24 GPCRs, whose ligands include: neuropeptides, metabolites, hormones, and neurotransmitters, among others (Fig. 3). Of the presently detected GPCRs, we found six reported to be expressed in mouse ghrelin cells of gastric origin: free fatty acid receptor 3 (FFAR3), FFAR4, gastric inhibitory polypeptide receptor (GIPR), KISS1 receptor (KISS1R), somatostatin receptor 1 (SSTR1) and somatostatin receptor 2 (SSTR2). Of these, three of them have been shown to have ghrelin secretion inhibitory functions (SSTR1, SSTR2, and FFAR4) and one with stimulatory effect (GIPR) (21). FFAR4, which recognizes LCFAs, showed higher expression in ε cells as well as in δ and β cells, whereas it was minimally expressed in α and PP cells. Interestingly, whereas in ghrelin cells of gastric origin, FFAR2 was found to be the most enriched short-chain fatty acid receptor (21), our data show instead higher enrichment of FFAR3, pointing to potential tissue differences in fatty acid sensing. Along these lines, G protein–coupled bile acid receptor 1 encoding a bile acid–sensitive receptor, also showed distinctive enrichment in this cell population. In rats, activation of this receptor has been shown to have stimulatory effects on glucagon-like peptide-1, peptide YY, and neurotensin secretion (58). Its significance in human pancreatic ghrelin secretion remains to be evaluated.
Figure 3.
GPCRs expressed in human ε cells. GPCRs are categorized based on their respective classes. Each row represents a gene; each column represents a distinct endocrine cell population. Gene expression is presented in each row as a cumulative percent of the population expressing the gene. The level of expression of each gene is presented as scaled expression.
Apart from GPCRs we also found components of the receptors for both type I and type II interferons: interferon-α and interferon-β receptor subunit 1 and interferon-γ receptor 2 (59) as well as a receptor known for its regulation of immunoglobulin G uptake (Fc fragment of immunoglobulin G receptor and transporter, (30, 60). Two receptors known for their modulation of plasminogen activation were also detected: plasminogen receptor with a C-terminal lysine and S100 calcium-binding protein A10. Interestingly, activation of plasminogen plays an important role in cellular migration by degrading extracellular matrixes (61, 62). Last, CD320 molecule, which encodes a receptor responsible for binding and mediating the uptake of trans-cobalamin saturated with vitamin B12, was also observed (63). Collectively, we found a variety of receptors with distinct cellular functions whose ligands include but were not limited to hormones, nutrients, lipids, and cytokines.
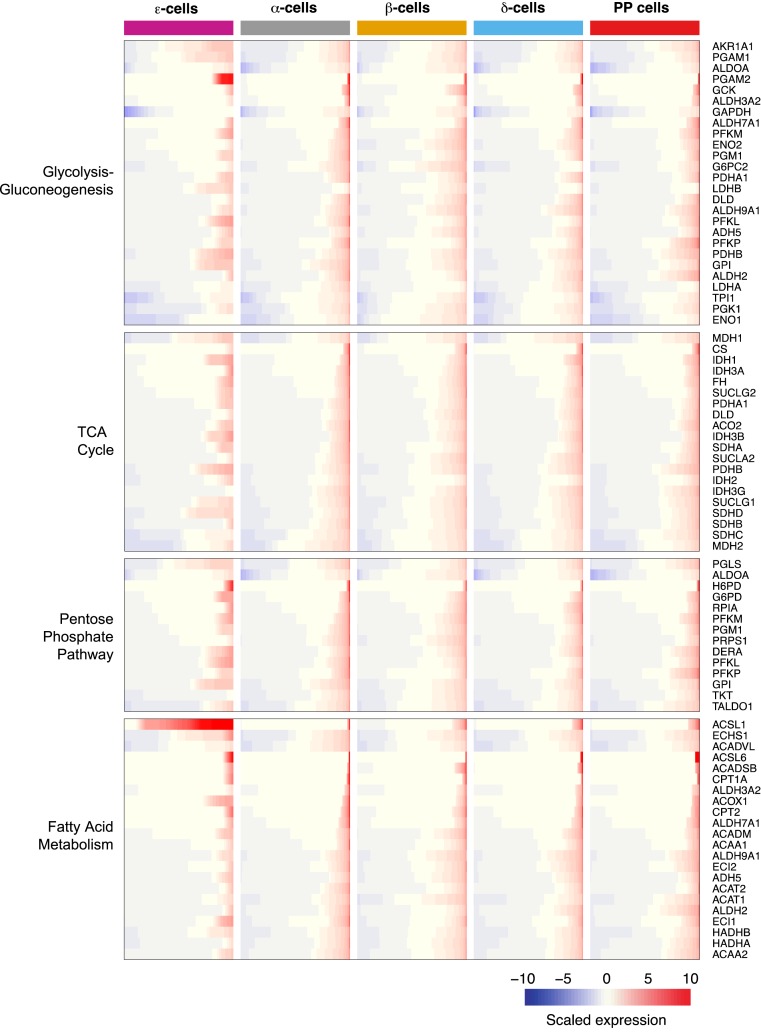
Primary metabolic pathways in human ε cells
Last, we interrogated the expression of factors involved in cellular metabolic pathways. Using annotated gene pathway datasets, we detected expression of genes involved in glycolysis and gluconeogenesis (26/61 annotated genes, 43% detected), tricarboxylic acid cycle (20/30 annotated genes, 67% detected), pentose phosphate pathway (14/27 annotated genes, 52% detected), and fatty acid metabolism (22/42 annotated genes, 52% detected) (Fig. 4). Although not all genes were expressed or detected within the first three pathways, there was a balanced representation of some of the main factors involved in each of them, suggesting their likely participation in regulating ε-cell function. Interestingly, of the members that compose the fatty acid metabolism pathway, ACSL1 was observed to be significantly enriched in ε cells (Fig. 1). Its expression level in this population is in concordance with comparable high expression levels found in MGN3-1 (32). Its function in fatty acid metabolism along with its selective enrichment in this cell population provide support for its further exploration regarding its contribution in ghrelin acylation.
Figure 4.
Metabolic pathways present in human ε cells. Expression of genes pertaining to distinct metabolic pathways is presented accordingly. Each row represents a gene; each column represents a distinct endocrine cell population. Gene expression is presented in each row as a cumulative percent of the population expressing the gene. The level of expression of each gene is presented as scaled expression. TCA, tricarboxylic acid.
Discussion
Epsilon cells compose a rare population in the adult pancreatic islet. In fact, human is the only species known to still have ghrelin-expressing cells in the adult state (7). This makes the use of animal models impossible, leaving high-throughput RNA sequencing as one of few options to gain unbiased insights into the function and regulation of the islet ε cell. One limitation of the study is the low number of cells. This, coupled with a 90% dropout rate, limits the number of detected transcripts, especially less abundant ones. Within this sequencing effort, we found that analyzing >6000 α or β cells provided us with enough resolution to overcome some of these limitations (28, 29). Unfortunately, having a frequency of 0.08% for ε cells would entail sequencing >10 million islet cells; given the limited access to human tissue, even enriching for this population would be an infeasible approach. Therefore, even with these limitations, the current study provides a resource to study the transcriptome of this rare cell population.
The function and significance of human pancreatic ε cells in the adult stage is still unclear. During gestation, they develop to form an almost-continuous layer around the islet, likely secreting ghrelin into the circulation. Afterward, their numbers decrease, and local actions might be their main function. It could be speculated that ε cells in the adult stage are responsible for regulating intraislet hormone concentration, which would entail other pancreatic endocrine cells expressing Ghsr. In this regard, studies in mouse islets and cell lines have found expression of Ghsr in α, β, and δ cells (14, 64–66). In humans, recent islet single cell RNA sequencing studies have shown an enrichment of GHSR in δ cells (12, 13), a finding we confirm in our dataset. Because islet endocrine cell types contribute to the regulation of whole-body glycemic control, a response to ghrelin translating into such regulation would be expected. However, its direct effect in islet hormone secretion has been difficult to interpret, an issue likely emerging from the difference in study models and hormone treatment concentrations. A considerable number of studies have found ghrelin to inhibit insulin secretion in humans, mice, and rats (67–74); however, these findings have not been unanimously observed, because they have also been shown to have the opposite effect (68, 75, 76). With regard to other islet hormones, studies in humans have shown ghrelin to increase circulating levels of somatostatin and pancreatic polypeptide (72). In vitro studies show that ghrelin potentiates glucose-stimulated somatostatin secretion in mouse and human islets (15); it has the opposite effect in rats (70). Although further studies are needed to refine the effects of this hormone in islet cell types, the present evidence suggests that an effect of ghrelin on hormone secretion is likely. In our study, we found SSTR1 and SSTR2 detected in the ε-cell population and confirmed the enrichment of GHSR in human δ cells, providing supporting evidence for the previously suggested feedback loop between these cell populations in the regulation of insulin secretion.
Ghrelin acyl modification is particularly important for its activation of the GHSR (6). Although the source of medium-chain fatty acids used for this modification has been thought to be primarily derived from the diet, recent findings using PG-1 suggest instead that ε cells are able to obtain them through β-oxidation of LCFAs (77). In the current study, we found ACSL1 to be significantly enriched in ε cells. ACSL1 is an enzyme involved in the first step of fatty acid oxidation. Findings from an in vitro study using MGN3-1 suggested that elevated levels of Acsl1 contributed to the octanoic acid supply derived from LCFAs (32). It is therefore tempting to speculate that β-oxidation of LCFAs is also an important source of the medium-chain fatty acids needed for ghrelin acylation in human pancreatic ε cells.
One of the most relevant questions in the study and understanding of ghrelin cells concerns the signals that regulate the appropriate secretion of this hormone. As an approach to explore this question, we looked into the expressed receptors with an emphasis on GPCRs. Consistently, we detected GPCRs previously shown to be expressed in mouse purified ghrelin cells of gastric origin (FFAR4, FFAR3, KISS1R, SSTR1, SSTR2, and GIPR) (21) as well as in the ghrelinoma cell line MGN3-1 (FFAR4, SSTR2, and BAI2) (22). Intriguingly, we did not detect expression of the adrenoreceptor 1 (ADRB1), which has been found to be highly expressed in purified ghrelin cells as well as in the ghrelinoma cell lines (PG-1, SG-1, and MGN3-1) (21–23). Although differences between mice and humans could explain the absence of expression of this and other potential receptors that were not detected, we believe it is likely that limitations in the sensitivity of the present technology are an important factor to be considered. Interestingly, we also detected expression of orphan receptors whose function in this cell type has not been explored (G protein–coupled receptors 157 GPR157, 137 GPR137, and GPR108). We believe that the variety of receptors identified whose ligands include fatty acids, hormones, neurotransmitters, and neuropeptides, among others, represent a resource to mine in the quest for understanding human islet ghrelin secretion.
Limited information exists with regard to the transcription factors necessary for ε-cell maturation and maintenance of function. In the current study, we inquired into the transcription factors expressed in adult ε cells and found 366 transcriptions factors to be detected, encompassing 22% of all annotated factors. Although the majority of these factors and their function in ε cells has not been studied, a few have been shown to influence the differentiation of this endocrine cell lineage. Developmental mouse studies have found that loss of Nkx2.2 and paired box 6 results in an overabundance of ghrelin-expressing cells (17, 18). Interestingly, we found mature ε cells to express both NKX2.2 and paired box 6. Although counterintuitive, these findings hint at the complexity of their function within ε cells, where, during development, they seem to antagonize the ε-cell lineage, whereas in the mature stage they may be important for the function of this cell type. Evidence supporting this concept has emerged for NKX2.2; it has been shown to be able to activate the mouse and human ghrelin promoter (51, 52). Consistent with mouse studies in which loss of Nkx6.1 did not have an effect on the ε-cell population (78) and human development immunohistochemistry studies that have shown ghrelin-expressing cells to be devoid of NKX6.1,4 we also did not detect expression of this factor within this cell type. Interestingly, we found HES4 to be uniquely enriched in ε cells. To our knowledge, the function of this factor in ε cells has not been described and, along with the other transcription factors, merits further investigation.
Although other studies have inquired into the gene expression signature of ghrelin-expressing cells, they have been possible only in tissues with higher enrichment of this cell type, such as that of gastric origin or through using previously described ghrelinoma cell lines (21–27). Of these, the most relevant for our study is PG-1. Using quantitative RT-PCR analysis, several studies have inquired into its transcriptome, providing useful information regarding genes with potential involvement in ghrelin hormone secretion (23–25). We confirmed the expression of genes composing membrane-trafficking proteins [synaptotagmin (SYT) 4, SYT5, SYT11, SYT14, and SYT17] (25), neuroendocrine markers [CHGA; secretogranin II (SCG2), SCG3, and SCG5; and proprotein convertase subtilisin/kexin type 1 and proprotein convertase subtilisin/kexin type 2] (23), and genes associated with retinol metabolism (RBP4 and TTR) (24).
In addition to complementing previous knowledge acquired in mice, the current study provides a repository containing the genes found in adult human ε cells. We review the potential function and implication of several of these factors in the ε-cell population and provide opportunities for future avenues of investigation.
Acknowledgments
The authors thank Samantha Intriligator for her help with preparing the manuscript and Dr. Lori Sussel for critical reading of the manuscript.
Financial Support: This work was funded by Regeneron Pharmaceuticals, Inc.
Author Contributions: Y.X., G.D.G., and J.G. designed the studies; G.D.G., J.K., Y.W., Y.D., M.N., and C.A. conducted the studies; Y.X., G.D.G., A.-H.L., J.T., and J.G. analyzed the data; Y.X., G.D.G., A.-H.L., A.J.M., J.T., J.N., S.M.G., and J.G. wrote the manuscript.
Disclosure Summary: G.D.G., J.K., A.-H.L., Y.W., Y.D., M.N., C.A., A.J.M., J.G., and Y.X. are employees and shareholders of Regeneron Pharmaceuticals, Inc. The remaining authors have nothing to disclose.
Glossary
- Abbreviations
ACSL1, acyl-coenzyme A synthetase long chain family member 1
- FFAR
free fatty acid receptor
- GEM
gel beads in emulsion
- GHSR
GH secretagogue receptor
- GIPR
gastric inhibitory polypeptide receptor
- GPCR
G protein–coupled receptor
- HES
hes family bHLH transcription factor
- LCFA
long-chain fatty acid
- MGN3-1
mouse ghrelinoma 3
- NKX2.2
NK2 homeobox 2
- PG-1
pancreas-derived ghrelinoma
- RBP4
retinol-binding protein 4
- SG-1
stomach-derived ghrelinoma
- SPINK1
serine peptidase inhibitor Kazal type 1
- SYT
synaptotagmin
- TM4SF4
transmembrane 4 L six family member 4
- TTR
transthyretin
- UMI
unique molecular identifier
References
- 1. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–4261. [DOI] [PubMed] [Google Scholar]
- 2. Dornonville de la Cour C, Björkqvist M, Sandvik AK, Bakke I, Zhao CM, Chen D, Håkanson R. A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept. 2001;99(2-3):141–150. [DOI] [PubMed] [Google Scholar]
- 3. Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T. Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides. 2002;23(3):531–536. [DOI] [PubMed] [Google Scholar]
- 4. Andralojc KM, Mercalli A, Nowak KW, Albarello L, Calcagno R, Luzi L, Bonifacio E, Doglioni C, Piemonti L. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia. 2009;52(3):486–493. [DOI] [PubMed] [Google Scholar]
- 5. Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept. 2002;107(1-3):63–69. [DOI] [PubMed] [Google Scholar]
- 6. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. [DOI] [PubMed] [Google Scholar]
- 7. Wierup N, Sundler F, Heller RS. The islet ghrelin cell. J Mol Endocrinol. 2013;52(1):R35–R49. [DOI] [PubMed] [Google Scholar]
- 8. Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. [DOI] [PubMed] [Google Scholar]
- 9. Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. [DOI] [PubMed] [Google Scholar]
- 10. Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276(3):905–908. [DOI] [PubMed] [Google Scholar]
- 11. Delhanty PJ, van der Lely AJ. Ghrelin and glucose homeostasis. Peptides. 2011;32(11):2309–2318. [DOI] [PubMed] [Google Scholar]
- 12.Muraro MJ, Dharmadhikari G, Grun D, Grün D, Groen N, Dielen T, Jansen E, van Gurp L, Engelse MA, Carlotti F, de Koning EJ, van Oudenaarden A. A single-cell transcriptome atlas of the human pancreas. Cell Syst 2016;3(4):385-394. [DOI] [PMC free article] [PubMed]
- 13. Segerstolpe Å, Palasantza A, Eliasson P, Andersson EM, Andréasson AC, Sun X, Picelli S, Sabirsh A, Clausen M, Bjursell MK, Smith DM, Kasper M, Ämmälä C, Sandberg R. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 2016;24(4):593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, Destefanis S, Annunziata M, Martinetti M, Catapano F, Ghè C, Isgaard J, Papotti M, Ghigo E, Muccioli G. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3′,5′-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-Kinase/Akt signaling. Endocrinology. 2007;148(2):512–529. [DOI] [PubMed] [Google Scholar]
- 15. DiGruccio MR, Mawla AM, Donaldson CJ, Noguchi GM, Vaughan J, Cowing-Zitron C, van der Meulen T, Huising MO. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol Metab. 2016;5(7):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschöp MH, D’Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59(9):2145–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heller RS, Jenny M, Collombat P, Mansouri A, Tomasetto C, Madsen OD, Mellitzer G, Gradwohl G, Serup P. Genetic determinants of pancreatic epsilon-cell development. Dev Biol. 2005;286(1):217–224. [DOI] [PubMed] [Google Scholar]
- 18. Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci USA. 2004;101(9):2924–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wierup N, Sundler F. Ultrastructure of islet ghrelin cells in the human fetus. Cell Tissue Res. 2005;319(3):423–428. [DOI] [PubMed] [Google Scholar]
- 20.Baron M, Veres A, Wolock SL, Faust AL, Gaujoux R, Vetere A, Ryu JH, Wagner BK, Shen-Orr SS, Klein AM, Melton DA, Yanai I. A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure. Cell Syst 2016;3(4):346-360. [DOI] [PMC free article] [PubMed]
- 21. Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nøhr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab. 2013;2(4):376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koyama H, Iwakura H, Dote K, Bando M, Hosoda H, Ariyasu H, Kusakabe T, Son C, Hosoda K, Akamizu T, Kangawa K, Nakao K. Comprehensive profiling of GPCR expression in ghrelin-producing cells. Endocrinology. 2016;157(2):692–704. [DOI] [PubMed] [Google Scholar]
- 23. Zhao TJ, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, Goldstein JL, Zigman JM. Ghrelin secretion stimulated by beta1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci USA. 2010;107(36):15868–15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walker AK, Gong Z, Park WM, Zigman JM, Sakata I. Expression of serum retinol binding protein and transthyretin within mouse gastric ghrelin cells. PLoS One. 2013;8(6):e64882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mani BK, Chuang JC, Kjalarsdottir L, Sakata I, Walker AK, Kuperman A, Osborne-Lawrence S, Repa JJ, Zigman JM. Role of calcium and EPAC in norepinephrine-induced ghrelin secretion. Endocrinology. 2014;155(1):98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iwakura H, Li Y, Ariyasu H, Hosoda H, Kanamoto N, Bando M, Yamada G, Hosoda K, Nakao K, Kangawa K, Akamizu T. Establishment of a novel ghrelin-producing cell line. Endocrinology. 2010;151(6):2940–2945. [DOI] [PubMed] [Google Scholar]
- 27. Sakata I, Nakano Y, Osborne-Lawrence S, Rovinsky SA, Lee CE, Perello M, Anderson JG, Coppari R, Xiao G, Lowell BB, Elmquist JK, Zigman JM. Characterization of a novel ghrelin cell reporter mouse. Regul Pept. 2009;155(1-3):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xin Y, Dominguez Gutierrez G, Okamoto H, Kim J, Lee AH, Adler C, Ni M, Yancopoulos GD, Murphy AJ, Gromada J. Pseudotime ordering of single human β-cells reveals states of insulin production and unfolded protein response. Diabetes. 2018;67(9):1783–1794. [DOI] [PubMed] [Google Scholar]
- 29. Dominguez Gutierrez G, Xin Y, Okamoto H, Kim J, Lee AH, Ni M, Adler C, Yancopoulos GD, Murphy AJ, Gromada J. Gene signature of proliferating human pancreatic α cells. Endocrinology. 2018;159(9):3177–3186. [DOI] [PubMed] [Google Scholar]
- 30.Dominguez Gutierrez G, Kim J, Lee AH, Tong J, Niu J, Gray S, Wei Y, Ding Y, Ni M, Adler C, Murphy AJ, Gromada J, Xin Y. Data from: Gene signature of the human pancreatic ε cell. Dryad 2018. Deposited 29 October 2018. 10.5061/dryad.3g2n7dc. [DOI] [PMC free article] [PubMed]
- 31. Xin Y, Kim J, Okamoto H, Ni M, Wei Y, Adler C, Murphy AJ, Yancopoulos GD, Lin C, Gromada J. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab. 2016;24(4):608–615. [DOI] [PubMed] [Google Scholar]
- 32. Bando M, Iwakura H, Koyama H, Hosoda H, Shigematsu Y, Ariyasu H, Akamizu T, Kangawa K, Nakao K. High incorporation of long-chain fatty acids contributes to the efficient production of acylated ghrelin in ghrelin-producing cells. FEBS Lett. 2016;590(7):992–1001. [DOI] [PubMed] [Google Scholar]
- 33. Wang GP, Xu CS. Pancreatic secretory trypsin inhibitor: more than a trypsin inhibitor. World J Gastrointest Pathophysiol. 2010;1(2):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Derikx MH, Geisz A, Kereszturi É, Sahin-Tóth M. Functional significance of SPINK1 promoter variants in chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2015;308(9):G779–G784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang B, Lu Y, Campbell-Thompson M, Spencer T, Wasserfall C, Atkinson M, Song S. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes. 2007;56(5):1316–1323. [DOI] [PubMed] [Google Scholar]
- 36. Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA. 2005;102(34):12153–12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bosco D, Meda P, Morel P, Matthey-Doret D, Caille D, Toso C, Bühler LH, Berney T. Expression and secretion of alpha1-proteinase inhibitor are regulated by proinflammatory cytokines in human pancreatic islet cells. Diabetologia. 2005;48(8):1523–1533. [DOI] [PubMed] [Google Scholar]
- 38. Semple F, Dorin JR. β-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4(4):337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Richardson SJ. Evolutionary changes to transthyretin: evolution of transthyretin biosynthesis. FEBS J. 2009;276(19):5342–5356. [DOI] [PubMed] [Google Scholar]
- 40. Anderson KR, Singer RA, Balderes DA, Hernandez-Lagunas L, Johnson CW, Artinger KB, Sussel L. The L6 domain tetraspanin Tm4sf4 regulates endocrine pancreas differentiation and directed cell migration. Development. 2011;138(15):3213–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arnes L, Hill JT, Gross S, Magnuson MA, Sussel L. Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population. PLoS One. 2012;7(12):e52026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97(4):1607–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heller RS, Stoffers DA, Liu A, Schedl A, Crenshaw EB III, Madsen OD, Serup P. The role of Brn4/Pou3f4 and Pax6 in forming the pancreatic glucagon cell identity. Dev Biol. 2004;268(1):123–134. [DOI] [PubMed] [Google Scholar]
- 44. Sussel L, Kalamaras J, Hartigan-O’Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125(12):2213–2221. [DOI] [PubMed] [Google Scholar]
- 45. Wang Q, Elghazi L, Martin S, Martins I, Srinivasan RS, Geng X, Sleeman M, Collombat P, Houghton J, Sosa-Pineda B. Ghrelin is a novel target of Pax4 in endocrine progenitors of the pancreas and duodenum. Dev Dyn. 2008;237(1):51–61. [DOI] [PubMed] [Google Scholar]
- 46. Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385(6613):257–260. [DOI] [PubMed] [Google Scholar]
- 47. Ediger BN, Du A, Liu J, Hunter CS, Walp ER, Schug J, Kaestner KH, Stein R, Stoffers DA, May CL. Islet-1 Is essential for pancreatic β-cell function. Diabetes. 2014;63(12):4206–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gu C, Stein GH, Pan N, Goebbels S, Hörnberg H, Nave KA, Herrera P, White P, Kaestner KH, Sussel L, Lee JE. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab. 2010;11(4):298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mastracci TL, Anderson KR, Papizan JB, Sussel L. Regulation of Neurod1 contributes to the lineage potential of Neurogenin3+ endocrine precursor cells in the pancreas. PLoS Genet. 2013;9(2):e1003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gutiérrez GD, Bender AS, Cirulli V, Mastracci TL, Kelly SM, Tsirigos A, Kaestner KH, Sussel L. Pancreatic β cell identity requires continual repression of non-β cell programs. J Clin Invest. 2017;127(1):244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hill JT, Chao CS, Anderson KR, Kaufman F, Johnson CW, Sussel L. Nkx2.2 activates the ghrelin promoter in pancreatic islet cells. Mol Endocrinol. 2010;24(2):381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shiimura Y, Ohgusu H, Sato T, Kojima M. Regulation of the human ghrelin promoter activity by transcription factors, NF-κB and Nkx2.2. Int J Endocrinol. 2015;2015:580908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li XY, Zhai WJ, Teng CB. Notch signaling in pancreatic development. Int J Mol Sci. 2015;17(1):E48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kordowich S, Collombat P, Mansouri A, Serup P. Arx and Nkx2.2 compound deficiency redirects pancreatic alpha- and beta-cell differentiation to a somatostatin/ghrelin co-expressing cell lineage. BMC Dev Biol. 2011;11(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mastracci TL, Wilcox CL, Arnes L, Panea C, Golden JA, May CL, Sussel L. Nkx2.2 and Arx genetically interact to regulate pancreatic endocrine cell development and endocrine hormone expression. Dev Biol. 2011;359(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87(1):240–244. [DOI] [PubMed] [Google Scholar]
- 57. Al Massadi O, Pardo M, Roca-Rivada A, Castelao C, Casanueva FF, Seoane LM. Macronutrients act directly on the stomach to regulate gastric ghrelin release. J Endocrinol Invest. 2010;33(9):599–602. [DOI] [PubMed] [Google Scholar]
- 58. Kuhre RE, Wewer Albrechtsen NJ, Larsen O, Jepsen SL, Balk-Møller E, Andersen DB, Deacon CF, Schoonjans K, Reimann F, Gribble FM, Albrechtsen R, Hartmann B, Rosenkilde MM, Holst JJ. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol Metab. 2018;11:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–386. [DOI] [PubMed] [Google Scholar]
- 60. Rath T, Baker K, Pyzik M, Blumberg RS. Regulation of immune responses by the neonatal fc receptor and its therapeutic implications. Front Immunol. 2015;5:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lighvani S, Baik N, Diggs JE, Khaldoyanidi S, Parmer RJ, Miles LA. Regulation of macrophage migration by a novel plasminogen receptor Plg-R KT. Blood. 2011;118(20):5622–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Madureira PA, O’Connell PA, Surette AP, Miller VA, Waisman DM. The biochemistry and regulation of S100A10: a multifunctional plasminogen receptor involved in oncogenesis. J Biomed Biotechnol. 2012;2012:353687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jiang W, Nakayama Y, Sequeira JM, Quadros EV. Mapping the functional domains of TCblR/CD320, the receptor for cellular uptake of transcobalamin-bound cobalamin. FASEB J. 2013;27(8):2988–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chuang JC, Sakata I, Kohno D, Perello M, Osborne-Lawrence S, Repa JJ, Zigman JM. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol Endocrinol. 2011;25(9):1600–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem. 2004;52(3):301–310. [DOI] [PubMed] [Google Scholar]
- 66. Adriaenssens AE, Svendsen B, Lam BY, Yeo GS, Holst JJ, Reimann F, Gribble FM. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 2016;59(10):2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reimer MK, Pacini G, Ahrén B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144(3):916–921. [DOI] [PubMed] [Google Scholar]
- 68. Salehi A, Dornonville de la Cour C, Håkanson R, Lundquist I. Effects of ghrelin on insulin and glucagon secretion: a study of isolated pancreatic islets and intact mice. Regul Pept. 2004;118(3):143–150. [DOI] [PubMed] [Google Scholar]
- 69. Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86(10):5083–5086. [DOI] [PubMed] [Google Scholar]
- 70. Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol. 2002;146(2):241–244. [DOI] [PubMed] [Google Scholar]
- 71. Colombo M, Gregersen S, Xiao J, Hermansen K. Effects of ghrelin and other neuropeptides (CART, MCH, orexin A and B, and GLP-1) on the release of insulin from isolated rat islets. Pancreas. 2003;27(2):161–166. [DOI] [PubMed] [Google Scholar]
- 72. Arosio M, Ronchi CL, Gebbia C, Cappiello V, Beck-Peccoz P, Peracchi M. Stimulatory effects of ghrelin on circulating somatostatin and pancreatic polypeptide levels. J Clin Endocrinol Metab. 2003;88(2):701–704. [DOI] [PubMed] [Google Scholar]
- 73. Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes. 2004;53(12):3142–3151. [DOI] [PubMed] [Google Scholar]
- 74. Dezaki K, Kakei M, Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes. 2007;56(9):2319–2327. [DOI] [PubMed] [Google Scholar]
- 75. Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, Kojima M, Kangawa K, Arima T, Matsuo H, Yada T, Matsukura S. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51(1):124–129. [DOI] [PubMed] [Google Scholar]
- 76. Lee HM, Wang G, Englander EW, Kojima M, Greeley GH Jr. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143(1):185–190. [DOI] [PubMed] [Google Scholar]
- 77. Ikenoya C, Takemi S, Kaminoda A, Aizawa S, Ojima S, Gong Z, Chacrabati R, Kondo D, Wada R, Tanaka T, Tsuda S, Sakai T, Sakata I. β-Oxidation in ghrelin-producing cells is important for ghrelin acyl-modification. Sci Rep. 2018;8(1):9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127(24):5533–5540. [DOI] [PubMed] [Google Scholar]