Abstract
We previously showed that berberine attenuates MexXY efflux-dependent aminoglycoside resistance in Pseudomonas aeruginosa. Here, we aimed to synthesize berberine derivatives with higher MexXY inhibitory activities. We synthesized 11 berberine derivatives, of which 13-(2-methylbenzyl) berberine (13-o-MBB) but not its regiomers showed the most promising MexXY inhibitory activity. 13-o-MBB reduced the minimum inhibitory concentrations (MICs) of various aminoglycosides 4- to 128 fold for a highly multidrug resistant P. aeruginosa strain. Moreover, 13-o-MBB significantly reduced the MICs of gentamicin and amikacin in Achromobacter xylosoxidans and Burkholderia cepacia. The fractional inhibitory concentration indices indicated that 13-o-MBB acted synergistically with aminoglycosides in only MexXY-positive P. aeruginosa strains. Time-kill curves showed that 13-o-MBB or higher concentrations of berberine increased the bactericidal activity of gentamicin by inhibiting MexXY in P. aeruginosa. Our findings indicate that 13-o-MBB inhibits MexXY-dependent aminoglycoside drug resistance more strongly than berberine and that 13-o-MBB is a useful inhibitor of aminoglycoside drug resistance due to MexXY.
Keywords: Pseudomonas aeruginosa, efflux, MexXY, aminoglycoside resistance, berberine
1. Introduction
Pseudomonas aeruginosa is a major cause of nosocomial infections. Treatment of P. aeruginosa infections with antimicrobial concentrations insufficient to inhibit P. aeruginosa growth results in the emergence of new multidrug resistant P. aeruginosa strains [1] that are difficult to eradicate and may increase mortality [2].
Drug efflux is a major mechanism leading to antimicrobial resistance in P. aeruginosa [3]. Four resistance-nodulation-division (RND)-type multidrug efflux pumps (MexAB-OprM [4], MexCD-OprJ [5], MexEF-OprN [6] and MexXY-OprM/OprA [7,8]) have been reported as drug efflux systems involved in the drug resistance of P. aeruginosa. Of these, only MexXY contributes to aminoglycoside drug resistance [8,9]. The MexXY-OprM system comprises a cytoplasmic membrane antibiotic-proton antiporter (MexY), an outer membrane porin (OprM), and a periplasmic membrane fusion protein (MexX) [10]. MexXY has multiple functions, including the expulsion of antibiotics. Wild-type P. aeruginosa expresses low MexXY levels but elevated MexXY has been detected in aminoglycoside-resistant P. aeruginosa strains [11,12]. Therefore, the development of MexXY inhibitors would allow the use of lower concentrations of aminoglycoside drugs that can cause severe side effects such as kidney damage [13].
There have been various reports of inhibitors of RND-type multidrug efflux pumps, but no clinical applications have been published to date [14]. Phenyl-arginine-β-naphthylamide (PAβN, MC-207,110), a well-known efflux pump inhibitor, does not inhibit aminoglycoside resistance due to MexXY [15]. We previously reported that berberine attenuates MexXY-dependent aminoglycoside resistance in P. aeruginosa [15], consistent with a recent report that berberine has high affinity to a MexXY model protein in silico [16].
Berberine is an isoquinoline quaternary alkaloid isolated from many kinds of medicinal plants such as Coptis chinensis, Coptis rhizome, Coptis japonica and Phellondendron amurense [17] and has weak antibacterial activity against Gram-negative bacteria such as P. aeruginosa [18]. Various derivatives of berberine have been developed and studied for their anti-hyperglycemic, anti-cancer, anti-inflammatory, anti-Alzheimer’s disease and anti-microbial activities [19]. Derivatives with multidrug resistance pump inhibitory activity against Staphylococcus aureus [20] and that reduce fluconazole resistance against Candida albicans [21] have been reported. In addition, quaternary ammonium compounds inhibit the biofilm formation in P. aeruginosa and C. albicans have been reported [22].
The optimum concentration of berberine to inhibit MexXY in P. aeruginosa cells is more than 512 µg/mL [15], which is too high for clinical application. In this study, we aimed to synthesize berberine derivatives with higher MexXY inhibitory activities.
2. Results
2.1. Antibacterial Activity of Berberine Derivatives toward P. aeruginosa
We first measured the minimum inhibitory concentrations (MICs) of 11 berberine derivatives (Figure 1) synthesized against P. aeruginosa mutants PAGUg1927, which expresses MexXY, and PAGUg1931, which does not express MexXY. A difference in the activity of a derivative toward the two strains indicates that the MexXY activity is not masked by the other four pumps (MexAB, MexCD, MexEF and MexVW) [15]. The MIC values of the berberine derivatives were lower in both strains compared to berberine (Table 1), suggesting that these berberine derivatives had higher anti-pseudomonas activity compared with berberine. These berberine derivatives showed similar MIC values that differed no more than, 4-fold. Their MIC values against PAGUg1927 were 2-fold greater than against PAGUg1931, indicating that the derivatives are MexXY substrates.
Figure 1.
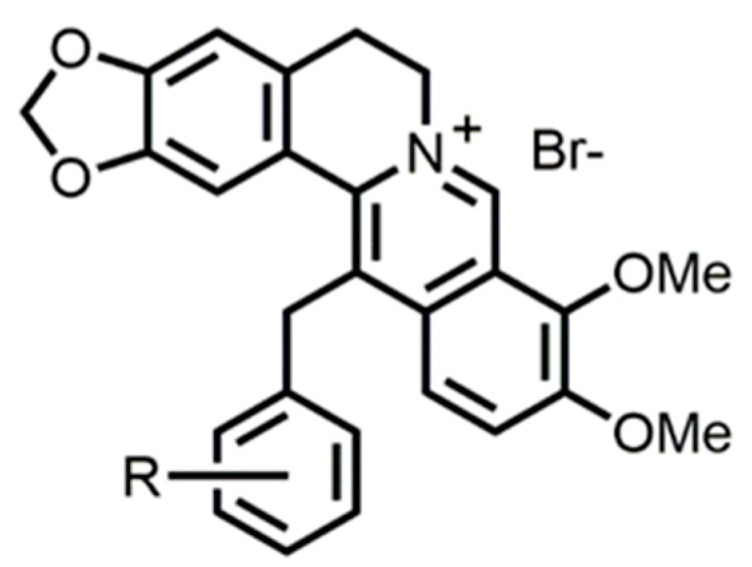
Structure of berberine derivatives.
Table 1.
Antibacterial activities of berberine derivatives against PAGUg1931 and PAGUg1927.
| Compound | -R | MIC of (µg/mL) | |
|---|---|---|---|
| PAGUg1927 | PAGUg1931 | ||
| GM2 | - | 1024 | 8 |
| Ber3 | - | >512 | >512 |
| 1 | -H | 256 | 128 |
| 2 | o-Br | 256 | 128 |
| 3 | p-Br | 128 | 64 |
| 4 | o-F | 512 | 256 |
| 5 | o-Cl | 256 | 128 |
| 6 | p-Cl | 256 | 128 |
| 7 | o-CH3 | 512 | 256 |
| 8 | m-CH3 | 256 | 128 |
| 9 | p-CH3 | 256 | 128 |
| 10 | o-NO2 | 512 | 256 |
| 11 | 2,6-Cl | 128 | 64 |
Note: R, side chain of the benzyl group of 13-benzylberberine derivatives; GM, gentamicin; Ber, berberine.
2.2. Inhibition of Drug Resistance in P. aeruginosa Using Combined Berberine Derivatives
We investigated the MexXY inhibitory activities of the berberine derivatives by measuring the MICs of gentamicin in the presence of the derivatives against P. aeruginosa mutants PAGUg1927 and PAGUg1931 (Table 2). The concentrations of the berberine derivatives were 1/2, 1/4, or 1/8 that of the MICs for PAGUg1931.
Table 2.
Increase in sensitivity to gentamicin by combination with berberine derivatives.
| Concomitant Compound | -R | GM MIC with Berberine Derivative (µg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAGUg1927 | PAGUg1931 | ||||||||||||
| 256 * | 128 | 64 | 32 | 16 | 8 | 256 | 128 | 64 | 32 | 16 | 8 | ||
| Ber | − | 128 | 256 | 256 | 512 | - | − | 8 | 8 | 8 | 8 | − | − |
| 1 | -H | − | − | 32 | 64 | 128 | − | − | − | 8 | 8 | 8 | − |
| 2 | o−Br | − | − | 32 | 64 | 64 | − | − | − | 4 | 8 | 8 | − |
| 3 | p−Br | − | − | − | 128 | 256 | 512 | − | − | − | 4 | 8 | 8 |
| 4 | o−F | − | 32 | 64 | 128 | − | − | − | 4 | 8 | 8 | − | − |
| 5 | o−Cl | − | − | 32 | 64 | 128 | − | − | − | 4 | 8 | 8 | − |
| 6 | p−Cl | − | − | 256 | 256 | 256 | − | − | − | 4 | 8 | 8 | − |
| 7 | o−CH3 | − | 16 | 32 | 64 | − | − | − | 4 | 8 | 8 | − | − |
| 8 | m−CH3 | − | − | 256 | 256 | 512 | − | − | − | 4 | 8 | 8 | − |
| 9 | p−CH3 | − | − | 256 | 256 | 512 | − | − | − | 4 | 8 | 8 | − |
| 10 | o−NO2 | − | 128 | 128 | 256 | − | − | − | 8 | 8 | 8 | − | − |
| 11 | 2,6−Cl | − | − | − | 128 | 256 | 512 | − | − | − | 4 | 8 | 8 |
Note: R, side chain of the benzyl group of 13−benzyl−berberine derivatives; GM, gentamicin; Ber, berberine, *; combined concentration (µg/mL).
The MIC of gentamicin for PAGUg1927 in the presence of 256 µg/mL berberine was 128 µg/mL (Table 2), which is one-eighth that of gentamicin alone (Table 1). Compounds 1–5 and 7 exhibited apparently increased MexXY inhibitory activity, with compound 7 reducing the MIC of gentamicin 64-fold. Compound 7 was named 13-o-MBB. Compounds 8 and 9, which are regioisomers of 13-o-MBB, increased sensitivity to gentamicin by up to 4-fold and were the weakest MexXY inhibitors.
We also examined changes in sensitivity to drugs other than gentamicin by combination with the berberine derivatives (Table 3). The combined use of 13-o-MBB 128 µg/mL reduced the MIC values of various substrate drugs (amikacin, tobramycin, kanamycin, gentamicin, spectinomycin, norfloxacin, ciprofloxacin, erythromycin, carbenicillin, ethidium bromide, tetracycline, chloramphenicol, azithromycin and cefepime) targeting MexXY by 2-fold to 16-fold (Table 3). The regiosomer 13-(3-methylbenzyl) berberine bromide (13-m-MBB) increased the spectinomycin sensitivity of PAGUg1927 8-fold and that of cefepime 4-fold at 64 µg/mL, whereas the other derivatives did not change the sensitivity to spectinomycin more than 2-fold. In addition, the combined use of 64 µg/mL of 13-(4-methyl-benzyl)-berberine bromide (13-p-MBB) increased sensitivity t cefepime 4-fold for PAGUg1927. Moreover, the sensitizing action of 13-(3-methyl-benzyl)-berberine bromide and 13-(4-methyl-benzyl)-berberine bromide did not exceed that of 13-o-MBB for PAGUg1927. Taken together, these results suggest that the o-methyl group of 13-o-MBB increases antimicrobial sensitivity in a MexXY-dependent manner.
Table 3.
Increase in sensitivity to antibiotic resistance due to 13-o-MBB and its regioisomers.
| Drug | MIC in the Presence of Berberine Derivative (µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PAGUg1927 | PAGUg1931 | |||||||||
| Ber | 13-o-MBB | 13−m−MBB | 13−p−MBB | − | Ber | 13-o-MBB | 13−m−MBB | 13−p−MBB | − | |
| AMK | 4 | 2 | 4 | 4 | 8 | 1 | 0.5 | 0.5 | 0.5 | 1 |
| TOB | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | 0.25 | 0.5 | 0.125 | 0.25 |
| KM | 128 | 64 | 256 | 256 | 512 | 64 | 32 | 32 | 64 | 64 |
| SPCM | 256 | 64 | 128 | 128 | 1024 | 64 | 8 | 8 | 32 | 64 |
| NLFX | 0.25 | 0.0625 | 1 | 1 | 1 | 0.015625 | 0.015625 | 0.015625 | 0.015625 | 0.015625 |
| EM | 128 | 64 | 512 | 256 | 512 | 16 | 16 | 16 | 8 | 16 |
| CBPC | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| EtBr | 512 | 128 | 256 | 256 | 256 | 64 | 8 | 8 | 16 | 64 |
| Tc | 2 | 1 | 8 | 8 | 16 | 0.125 | 0.125 | 0.125 | 0.125 | 0.25 |
| Cp | 4 | 2 | 8 | 8 | 8 | 2 | 2 | 2 | 1 | 2 |
| AZM | 64 | 64 | 256 | 256 | 512 | 8 | 8 | 8 | 4 | 8 |
| CEF | 1 | 0.25 | 0.125 | 2 | 8 | 0.0625 | 0.125 | 0.125 | 0.125 | 0.125 |
Note: Ber, combined berberine 256 µg/mL; 13-o-MBB, combined 13-o-MBB 128 µg/mL; 13−m−MBB, combined 13−m−MBB 64 µg/mL; 13−p−MBB, combined 13−p−MBB 64 µg/mL; AMK, amikacin; TOB, tobramycin; KM, kanamycin; SPCM, spectinomycin; NLFX, norfloxacin; EM, erythromycin; CBPC, carbenicillin; EtBr, ethidium bromide; Tc, tetracycline; Cp, chloramphenicol; AZM, azithromycin; CEF, cefepime.
We investigated whether the inhibitory action of 13-o-MBB against MexXY-dependent drug resistance can be observed in PAGU 1606, a multidrug resistant P. aeruginosa clinical strain, and its MexXY-deficient strain PAGUg1659 (Table 4). The MIC of amikacin alone against PAGU 1606 was 256 µg/mL and 64 µg/mL when combined with berberine. In contrast, the combined use of 13-o-MBB and amikacin decreased the MIC to 16 µg/mL. Thus, 13-o-MBB inhibits amikacin resistance 4-fold more effectively than berberine in a MexXY-dependent drug resistant strain. Another aminoglycoside drug, 13-o-MBB, inhibited drug resistance two to four times stronger than berberine but had no greater effect on the drug resistance of PAGU 1606 than the other aminoglycosides. However, the MICs of norfloxacin, erythromycin and azithromycin were increased towards PAGUg1659, a pump-deficient strain.
Table 4.
Inhibited resistance to aminoglycoside-based drugs by 13-o-MBB in PAGU 1606
| Drug | MIC (µg/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAGU 1606 | PAGUg1659 | |||||||||||||
| - | Ber (256) 1 | Ber (128) | Ber (64) | 13-o-MBB (256) | 13-o-MBB (128) | 13-o-MBB (64) | - | Ber (256) | Ber (128) | Ber (64) | 13-o-MBB (256) | 13-o-MBB (128) | 13-o-MBB (64) | |
| AMK | 256 | 64 | 128 | 128 | 16 | 32 | 32 | 16 | 8 | 8 | 8 | 8 | 8 | 8 |
| TOB | 256 | 64 | 128 | 128 | 16 | 32 | 32 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| KM | >2048 | 1024 | 1024 | 2048 | 256 | 512 | 1024 | 256 | 256 | 256 | 256 | 256 | 256 | 256 |
| GM | 64 | 4 | 8 | 16 | 2 | 4 | 4 | 0.5 | 0.25 | 0.25 | 0.5 | 0.25 | 0.25 | 0.5 |
| SPCM | >2048 | >2048 | >2048 | >2048 | 2048 | >2048 | >2048 | 2048 | 2048 | 2048 | 2048 | 1024 | 2048 | 2048 |
| NLFX | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 64 | 256 | 256 | 256 | 128 | 128 | 128 |
| CPFX | 64 | 64 | 64 | 64 | 32 | 32 | 32 | 64 | 64 | 64 | 64 | 32 | 32 | 32 |
| EM | 256 | 128 | 256 | 256 | 128 | 256 | 256 | 128 | 256 | 256 | 256 | 256 | 256 | 256 |
| CBPC | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| EtBr | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| Tc | 32 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| Cp | 128 | 64 | 128 | 128 | 64 | 64 | 64 | 128 | 128 | 128 | 128 | 128 | 128 | 128 |
| AZM | 256 | 64 | 64 | 128 | 64 | 64 | 128 | 32 | 256 | 256 | 256 | 128 | 128 | 128 |
| CEF | 512 | 512 | 512 | 512 | 512 | 512 | 512 | 512 | 512 | 512 | 512 | 512 | 512 | 512 |
Note: 1, values in parentheses are combined concentrations (µg/mL); Ber, berberine; AMK, amikacin; TOB, tobramycin; KM, kanamycin; GM, gentamicin; SPCM, spectinomycin; NLFX, norfloxacin; CPFX, ciprofloxacin; EM, erythromycin; CBPC, carbenicillin; EtBr, ethidium bromide; Tc, tetracycline; Cp, chloramphenicol; AZM, azithromycin; CEF, cefepime.
The sensitizing action of 13-o-MBB for various aminoglycosides was compared with that of berberine at the same concentrations as tested against P. aeruginosa clinical strains but using Burkholderia cepacia PAGU 0013 and Achromobacter xylosoxidans PAGU 0002 (Table 5). The two non-P. aeruginosa strains are naturally resistant to aminoglycosides due to the presence of MexXY orthologs [7,23]. 13-o-MBB at the same concentration as berberine increased the sensitivity to the aminoglycosides more than 4-fold over that of berberine. In addition, comparison of the MICs of the aminoglycosides in combination with 13-o-MBB towards a clinical strain of P. aeruginosa and its mexXY-deficient strain provided similar MIC values. 13-o-MBB greatly increased the sensitivity to aminoglycoside drugs for P. aeruginosa, B. cepacia, and A. xylosoxidans, increasing the sensitivity to amikacin more than 128-fold and to gentamycin more than 512-fold for A. xylosoxidans.
Table 5.
Inhibition by 13-o-MBB of aminoglycoside resistance in P. aeruginosa clinical strains.
| Strain | MIC of Aminoglycoside (µg/mL) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMK | GM | TOB | KM | SPEC | |||||||||||
| − | Ber | 13-o-MBB | − | Ber | 13-o-MBB | − | Ber | 13-o-MBB | − | Ber | 13-o-MBB | − | Ber | 13-o-MBB | |
| PAGU 0974 | 4 | 1 | 0.5 | 4 | 0.5 | 0.25 | 0.5 | 0.125 | 0.125 | 128 | 32 | 32 | 512 | 128 | 32 |
| PAGUg 0975 | 1 | 0.5 | 0.5 | 0.25 | 0.125 | 0.25 | 0.25 | 0.25 | 0.125 | 64 | 32 | 32 | 32 | 32 | 32 |
| PAGU 1498 | 32 | 8 | 1 | 1024 | 128 | 8 | 256 | 32 | 8 | >2048 | 512 | 256 | 512 | 128 | 32 |
| PAGUg1565 | 2 | 1 | 1 | 8 | 8 | 8 | 8 | 8 | 8 | 512 | 256 | 256 | 32 | 32 | 32 |
| PAGU 1569 | 256 | 64 | 32 | 256 | 32 | 8 | 16 | 8 | 4 | >2048 | >2048 | 1024 | 512 | 256 | 128 |
| PAGUg1627 | 32 | 32 | 32 | 8 | 8 | 8 | 8 | 8 | 4 | 1024 | 512 | 1024 | 128 | 128 | 128 |
| *PAGU 0013 | 128 | 32 | 4 | 128 | 32 | 4 | 64 | 8 | 1 | 64 | 8 | 2 | 1024 | 128 | 16 |
| PAGU 0002 | >2048 | 256 | 16 | >2048 | 32 | 4 | 512 | 16 | 4 | >2048 | 2048 | 256 | >2048 | 512 | 64 |
2.3. Interaction between 13-o-MBB and Aminoglycoside Drugs
The fractional inhibitory concentration (FIC) values were determined using 13-o-MBB or berberine and gentamicin or amikacin in combination with P. aeruginosa strains PAGU 1606 and PAGUg1927 and their MexXY-defective mutants PAGUg1659 and PAGUg 1931 (Table 6). The combination of 13-o-MBB and amikacin or gentamicin showed a synergistic effect in the MexXY-expressing strain, showing that the MexXY-dependent aminoglycoside resistance inhibitory action of 13-o-MBB is synergistic. In addition, the MICs of 13-o-MBB and berberine were reduced only in combination with amikacin or gentamicin and only in the MexXY-expressing strain, showing that the combination of amikacin or gentamicin in the presence of MexXY increases the accumulation of 13-o-MBB and berberine in the cell.
Table 6.
Antibacterial activities of berberine derivatives against P. aeruginosa.
| Strain | MIC (µg/mL) for AMK in the Presence of: | MIC (µg/mL) for 13-o-MBB in the Presence of: | FIC | Mode of Interaction | ||
| − | 13-o-MBB | − | AMK | |||
| PAGUg1931 | 1 | 1 | 256 | 256 | 2.0 | Indifferent |
| PAGUg1927 | 8 | 2 | 512 | 128 | 0.5 | Synergy |
| PAGUg1659 | 16 | 8 | >512 | >512 | >1.5 | Indifferent |
| PAGU 1606 | 256 | 16 | >512 | 64 | <0.5 | Synergy |
| Strain | MIC (µg/mL) for AMK in the Presence of: | MIC (µg/mL) for Berberne in the Presence of: | FIC | Mode of Interaction | ||
| − | Berberine | − | AMK | |||
| PAGUg1931 | 1 | 1 | >512 | >512 | >2.0 | Indifferent |
| PAGUg1927 | 8 | 4 | >512 | 512 | <1.0 | Synergy or Addition |
| PAGUg1659 | 16 | 8 | >512 | >512 | 1.5 | Indifferent |
| PAGU 1606 | 256 | 64 | >512 | 512 | <0.75 | Synergy or Addition |
| Strain | MIC (µg/mL) for GM in the Presence of: | MIC (µg/mL) for 13-o-MBB in the Presence of: | FIC | Mode of Interaction | ||
| − | 13-o-MBB | − | GM | |||
| PAGUg1931 | 8 | 8 | 256 | 256 | 2.0 | Indifferent |
| PAGUg1927 | 1024 | 32 | 512 | 4 | 0.04 | Synergy |
| PAGUg1659 | 0.5 | 0.5 | >512 | >512 | >1.0 | Indifferent |
| PAGU 1606 | 64 | 2 | >512 | 8 | <0.5 | Synergy |
| Strain | MIC (µg/mL) for GM in the Presence of: | MIC(µg/mL) for Berberine in the Presence of: | FIC | Mode of Interaction | ||
| − | Berberine | − | GM | |||
| PAGUg1931 | 8 | 8 | >512 | >512 | >1.0 | Indifferent |
| PAGUg1927 | 1024 | 128 | >512 | 8 | <0.5 | Synergy |
| PAGUg1659 | 0.5 | 0.5 | >512 | >512 | >1.0 | Indifferent |
| PAGU 1606 | 64 | 8 | >512 | 256 | <0.5 | Synergy |
Note: GM, gentamicin; AMK, amikacin; FIC, fractional inhibitory concentration index.
2.4. Time-Killing Assay
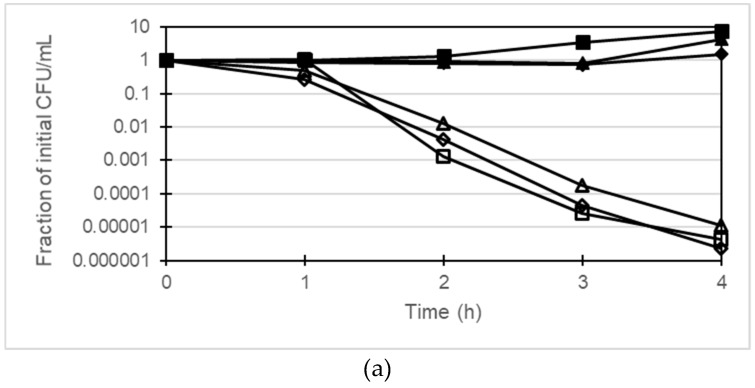
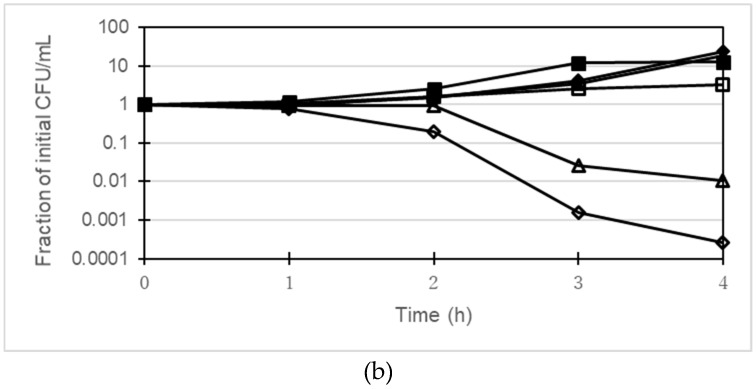
The bactericidal activity of gentamicin together with berberine and 13-o-MBB against P. aeruginosa was investigated using PAGUg1933 and PAGUg1929. PAGUg1933 was killed after 4 h treatment with gentamicin at 2 µg/mL whereas the growth of PAGUg1929 was suppressed but no bactericidal action was observed (Figure 2). Treatment of PAGUg1929 for 4 h with 2 µg/mL gentamicin in combination with 256 µg/mL berberine reduced the number of colonies about 100-fold. In addition, treatment of PAGUg1929 with a combination of 2 µg/mL gentamicin and 64 µg/mL 13-o-MBB enhanced the bactericidal action of gentamicin more than 10-fold over that of 256 µg/mL berberine.
Figure 2.
(a) Time-kill curves by the combination of gentamicin with berberine or 13-o-MBB against PAGUg1933, closed squares, control; closed triangles, berberine 256 µg/mL; closed diamonds, 13-o-MBB 64 µg/mL; open squares, gentamicin 2 µg/mL; open triangles, gentamicin 2 µg/mL with berberine 256 µg/mL; open diamonds, gentamicin 2 µg/mL with 13-o-MBB 64 µg/mL; (b) Time-kill curves by the combination of gentamicin with berberine or 13-o-MBB against PAGUg1929, closed squares, control; closed triangles, berberine 256 µg/mL; closed diamonds, 13-o-MBB 64 µg/mL; open squares, gentamicin 2 µg/mL; open triangles, gentamicin 2 µg/mL with berberine 256 µg/mL; open diamonds, gentamicin 2 µg/mL with 13-o-MBB 64 µg/mL.
3. Discussion
The addition of 128 µg/mL 13-o-MBB increased the sensitivity to aminoglycosides by 2-fold to 8-fold in comparison with 256 µg/mL berberine in the MexXY-positive P. aeruginosa strain PAGUg1927 (Table 2 and Table 3). The antimicrobial activity of 13-o-MBB was not significantly different from that of the 13-o-MBB regioisomers 13-(3-methylbenzyl) berberine bromide and 13-(4-methylbenzyl) berberine bromide, although the drug resistance inhibitory action of 13-o-MBB on the MexXY system is greater than that of these two regioisomers. This indicates that 13-o-MBB has greater inhibitory action against MexXY-dependent drug resistance than berberine and the other berberine derivatives we synthesized.
The deletion of mexXY from PAGU 1606 strain generated the PAGUg1659 strain. The addition of 13-o-MBB increased PAGUg1659 resistance towards norfloxacin, erythromycin and azithromycin 2-fold to 4-fold. Norfloxacin, erythromycin and azithromycin are substrates for MexCD-OprM and increased resistance towards norfloxacin, erythromycin and azithromycin may be due to the induction of MexCD-OprJ [24].
The addition of 13-o-MBB 256 µg/mL increased the efficacies of azithromycin and gentamicin to a Clinical and Laboratory Standards Institute (CLSI) breakpoint (amikacin is 64 µg/mL, gentamycin is 16 µg/mL) in a clinical strain of P. aeruginosa highly resistant to aminoglycosides. Amino acid residue Y613 within the loop of the drug binding pocket of MexY is directly involved in the recognition of aminoglycoside drugs, based on a decrease in sensitivity to aminoglycoside drugs upon mutation of Y613 have been reported [25]. Tobramycin and berberine have been reported to compete for Y613 on the docking simulations of tobramycin or berberine on MexY [16]. Furthermore, they claimed that the results of a combined berberine/tobramycin assay on different clinical isolates of P. aeruginosa were consistent with the in silico findings [16]. The results of our combination assay using berberine and 13-o-MBB with aminoglycosides are consistent with this report [16] and substantiate that the main mechanism of action of berberine and 13-o-MBB is competition for MexY inhibition. Another possible mechanism is suppression of MexY expression. However, Berberine decreased MexY mRNA only 0.8 to 0.9-fold have been reported [26]. Another reported that the MIC of amikacin and gentamicin was increased only up to 4-fold even in a strain P. aeruginosa that expresses 10–21 times more MexY mRNA than the PAO1 strain [12]. Our study of inhibited resistance by berberine showed that the gentamicin MIC for PAGU1606 was reduced 4-fold to 16-fold by berberine (Table 4), suggesting that the inhibition of MexY expression is not the main mechanism of action of berberine and 13-o-MBB.
13-o-MBB showed cytotoxicity against Caco-2 cells, a human epithelial colorectal adenocarcinoma cell line, at 30 µg/mL (data not shown). Thus, a concentration of 256 µg/mL 13-o-MBB could be toxic to human cells. There is thus a need to synthesize a compound that exhibits inhibitory action against MexXY system-dependent drug resistance at a lower concentration than 13-o-MBB and that is non-toxic to human cells.
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
The bacterial strains used in this study are described in Table 7. Bacterial cells were grown in Luria (L) broth and on L agar (1.5%) under aerobic conditions at 37 °C, as previously described [27].
Table 7.
Bacterial strains and gene properties.
| Strain Name | Relevant Characteristics | Reference |
|---|---|---|
| Pseudomonas aeruginosa | ||
| PAGU 0974 | PAO1 (K. Poole Lab), wild type | [28] |
| PAGUg0975 | PAGU 0974ΔmexXY | [29] |
| PAGU 1498 | PA7 Non-respiratory clinical isolate | [8] |
| PAGUg1565 | PA7ΔmexXY-oprA | [8] |
| PAGU 1569 | K2162 Pan-aminoglycoside-resistant clinical isolate | [30] |
| PAGU 1606 | NCGM2. S1 Multidrug-resistant clinical isolate | [31] |
| PAGUg1627 | K2162ΔmexXY | [30] |
| PAGUg1659 | PAGU 1606ΔmexXY | [8] |
| PAGUg1927 | YM34 ΔmexZ, mexVW:: gfp-aacC1 | [15] |
| PAGUg1929 | YM34 ΔmexZ, mexVW | [15] |
| PAGUg1931 | PAGUg1927::ΔmexXY | [15] |
| PAGUg1933 | PAGUg1929::ΔmexXY | [15] |
| Others | ||
| PAGU 0002 | ATCC 27061 Achromobacter xylosoxidans subsp. xylosoxidans | [32] |
| PAGU 0013 | ATCC 25416 Burkholderia cepacia | [33] |
4.2. Antibiotic Susceptibility Assay
MICs were assessed in cation-adjusted Mueller–Hinton (MH) broth after about 18–22 h of incubation at 37 °C (for P. aeruginosa) or after about 20–24 h of incubation at 35 °C (for A. xylosoxidans and B. cepacia) using the two-fold serial micro-titer broth dilution method described previously [15]. The categorization as susceptible, intermediate, and resistant was performed according to the interpretive standards of the CLSI.
The FIC index was calculated as described previously [15]. The effects of the drugs were interpreted to be indicative of synergy when the index was ≤0.5.
4.3. Time-Killing Assay
We examined the bactericidal activity of gentamicin monotherapy or combination therapy with berberine or berberine derivatives towards PAGUg1929 and PAGUg1933. Each measurement was started by inoculating between 5 × 106 to 2 × 107 CFU/mL in cation-adjusted MH broth and incubating at 150 rpm at 37 °C on a shaker. Samples were withdrawn to measure the survival counts on MH agar plates at 0, 1, 2, 3 and 4 h. The MH agar plates were incubated at 37 °C for 16–18 h. The concentrations of drugs tested were gentamicin 2 μg/mL, berberine 256 μg/mL, and 13-o-MBB 64 μg/mL. The fraction surviving vs. the control for each sample was determined by taking the average CFU/mL values of the treated samples and dividing by the value for the same sample at 0 h. Each experiment was repeated at least three times, and a representative experiment is shown.
4.4. Synthesis
4.4.1. General Synthesis Information
Melting points were measured on a Yanagimoto micro melting point hot-stage apparatus (MP-S3) and are reported as uncorrected values. 1H-NMR (TMS: δ: 0.00 ppm as an internal standard) and 13C-NMR (CDCl3: δ: 77.00 or DMSO-d6: 39.52 ppm as an internal standard) spectra were recorded on JEOL JNM-AL400 (400 MHz and 100 MHz) spectrometers in CDCl3 or DMSO-d6. Mass spectra were obtained on a JEOL JMP-DX300 instrument (70 eV, 300 mA). Chromatographic separations were accomplished using silica gel 60N (Kanto Chemical Co., Inc., Tokyo, Japan) or aluminum oxide 90 standardized (Merck KGaA., Inc., Darmstadt, Germany). Thin-layer chromatography (TLC) was performed using silica gel 60F254 and aluminum oxide 60F254 neutral (Merck KGaA, Inc., Darmstadt, Germany). All reagents were purchased from Wako Pure Chemical Industry, Osaka, Japan. Kanto Chemical Co., Inc., Tokyo, Japan. Tokyo Chemical Industry Co., Ltd., Tokyo, Japan. Kishida Chemical Co., Ltd., Osaka Japan and Sigma-Aldrich Co., LLC. St. Louis, MO, USA. Dihydroberberine was synthesized by the reduction of berberine according to the reported procedure [21].
4.4.2. 13-Benzylberberine Derivatives; General Procedure
Each benzyl bromide (1.0 mmol) was added in a dropwise manner to a stirred solution of KI (310 mg, 1.86 mmol, 1.86 equiv) and dihydroberberine (337 mg, 1.0 mmol, 1 equiv) in CH3CN (40 mL), and the resulting mixture was held at reflux for 4 h. The reaction mixture was then filtered, and the filtrate was collected and evaporated to dryness in vacuo to give the crude residue. The residue was purified by column chromatography over neutral alumina using CHCl3/CH3OH (50:1 to 20:1) as eluent and recrystallization to give the final compounds 1–11. Compounds 1–10 were known compounds and their characterisation data were identical to those given in the literature. Their melting points (m.p.) were as follows: Compound 1; m.p. 198–200 °C [21], Compound 2; m.p. 179–180 °C [21], Compound 3; m.p. 235–240 °C [21], Compound 4; m.p. 214–216 °C [21], Compound 5; m.p. 210–211 °C [34], Compound 6; m.p. 218–220 °C [35], Compound 7; m.p. 216–220 °C [36], Compound 8; m.p. 222–225 °C [21], Compound 9; m.p. 204–207 °C [36] and Compound 10; m.p. 22–230 °C [21].
4.4.3. Characterisation Data of 13-(2,6-Dichlorobenzyl)berberine Bromide (11)
Compound 11 is a yellow solid. Yield: 41%. 1H-NMR (DMSO-d6) δ: 9.95 (1H, s), 8.11 (1H, d, J = 9.3 Hz), 7.84 (1H, d, J = 9.3 Hz), 7.56 (1H, s), 7.37 (2H, d, J = 7.8 Hz), 7.23 (1H, t, J = 8.3 Hz), 7.15 (1H, s), 6.18 (2H, s), 5.16 (2H, s), 4.84 (2H, br), 4.09 (3H, s), 4.01 (3H, s), 3.08 (2H, br). 13C-NMR (DMSO-d6) δ: 150.0 (s), 149.3 (s), 146.5 (s), 144.2 (d), 144.1 (s), 138.2 (s), 134.9 (s), 134.7 (s), 133.6 (s), 131.9 (s), 131.3 (s), 129.5 (d), 129.2 (d), 125.8 (d), 121.0 (s), 120.5 (d), 120.4 (s), 110.9 (d), 108.1 (d), 102.0 (t), 62.0 (q), 56.9 (q), 56.7 (t), 32.9 (t), 27.4 (t). MS m/z: 494 (M–Br)+, 119, 85. m.p. 228–231 °C.
5. Conclusions
Eleven berberine derivatives were synthesized and tested for MexXY-dependent inhibition of gentamicin resistance using a Pseudomonas aeruginosa positive-MexXY strain and a negative-MexXY strain. 13-o-MBB showed the greatest inhibitory effect on MexXY-dependent gentamicin resistance. Regioisomers of 13-o-MBB exhibited no greater MexXY-dependent inhibition of gentamicin resistance than berberine. 13-o-MBB inhibited resistance to aminoglycosides 4-fold to 16-fold compared with berberine against the four tested P. aeruginosa clinical strains, and Achromobacter xylosoxidans and Burkholderia cepacia. These results indicate that 13-o-MBB inhibits the resistance to aminoglycosides in a MexXY-dependent manner more strongly than berberine. 13-o-MBB is thus a useful inhibitor of aminoglycoside drug resistance due to MexXY.
Acknowledgments
This work was performed under the Research Program of “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” in “Network Joint Research Center for Materials and Devices”.
Author Contributions
K.K. and Y.M. have both significantly contributed to the creation of this manuscript. All authors read and approved the final manuscript. conceptualization, K.K. and Y.M.; methodology, K.K., M.M. and S.Y.; Investigation, K.K. and Y.M.; writing—original draft preparation, J.T. and R.K.; writing—review and editing, K.K.; visualization, Y.K. and K.N.; supervision, Y.K.; project administration, Y.K. and Y.M.; funding acquisition.
Funding
This research was funded by a research grant from Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan (to K.K.) and by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP26460080.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Nair C.G., Chao C., Ryall B., Williams H.D. Sub-lethal concentrations of antibiotics increase mutation frequency in the cystic fibrosis pathogen Pseudomonas aeruginosa. Lett. Appl. Microbiol. 2013;56:149–154. doi: 10.1111/lam.12032. [DOI] [PubMed] [Google Scholar]
- 2.Dantas R.C., Ferreira M.L., Gontijo-Filho P.P., Ribas R.M. Pseudomonas aeruginosa bacteraemia: Independent risk factors for mortality and impact of resistance on outcome. J. Med. Microbiol. 2014;63:1679–1687. doi: 10.1099/jmm.0.073262-0. [DOI] [PubMed] [Google Scholar]
- 3.Poole K. Pseudomonas aeruginosa: Resistance to the max. Front. Microbiol. 2011;5:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsutsumi K., Yonehara R., Ishizaka-Ikeda E., Miyazaki N., Maeda S., Iwasaki K., Nakagawa A., Yamashita E. Structures of the wild-type MexAB-OprM tripartite pump reveal its complex formation and drug efflux mechanism. Nat. Commun. 2019;10:1520. doi: 10.1038/s41467-019-09463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcalde-Rico M., Olivares-Pacheco J., Alvarez-Ortega C., Cámara M., Martínez J.L. Role of the Multidrug Resistance Efflux Pump MexCD-OprJ in the Pseudomonas aeruginosa Quorum Sensing Response. Front. Microbiol. 2018;9:2752. doi: 10.3389/fmicb.2018.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juarez P., Broutin I., Bordi C., Plésiat P., Llanes C. Constitutive Activation of MexT by Amino Acid Substitutions Results in MexEF-OprN Overproduction in Clinical Isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2018;62:e02445-17. doi: 10.1128/AAC.02445-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morita Y., Tomida J., Kawamura Y. MexXY multidrug efflux system of Pseudomonas aeruginosa. Front. Microbiol. 2012;28:408. doi: 10.3389/fmicb.2012.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morita Y., Tomida J., Kawamura Y. Primary mechanisms mediating aminoglycoside resistance in the multidrug-resistant Pseudomonas aeruginosa clinical isolate PA7. Microbiology. 2012;158:1071–1083. doi: 10.1099/mic.0.054320-0. [DOI] [PubMed] [Google Scholar]
- 9.Nikaido H., Takatsuka Y. Mechanisms of RND multidrug efflux pumps. Biochim. Biophys. Acta. 2009;1794:769–781. doi: 10.1016/j.bbapap.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair J.M., Piddock L.J. Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: An update. Curr. Opin. Microbiol. 2009;12:512–519. doi: 10.1016/j.mib.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Vogne C., Aires J.R., Bailly C., Hocquet D., Plésiat P. Role of the multidrug efflux system MexXY in the emergence of moderate resistance to aminoglycosides among Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 2004;48:1676–1680. doi: 10.1128/AAC.48.5.1676-1680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam S., Jalal S., Wretlind B. Expression of the MexXY efflux pump in amikacin-resistant isolates of Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2004;10:877–883. doi: 10.1111/j.1469-0691.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Novoa J.M., Quiros Y., Vicente L., Morales A.I., Lopez-Hernandez F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011;79:33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 14.Jürgen A.B., Winfried V.K. Antimicrobail Drug Efflux Pump Inhibitor. In: Xian-Zhi L., Christpher A.E., Helen I.Z., editors. Efflux-Mediated Antimicrobial Resistance in Bacteria. 2nd ed. Springer International Publishing; Basel, Switzerland: 2016. pp. 755–795. [Google Scholar]
- 15.Morita Y., Nakashima K., Nishino K., Kotani K., Tomida J., Inoue M., Kawamura Y. Berberine Is a Novel Type Efflux Inhibitor Which Attenuates the MexXY-Mediated Aminoglycoside Resistance in Pseudomonas aeruginosa. Front. Microbiol. 2016;7:1223. doi: 10.3389/fmicb.2016.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laudadio E., Cedraro N., Mangiaterra G., Citterio B., Mobbili G., Minnelli C., Bizzaro D., Biavasco F., Galeazzi R. Natural Alkaloid Berberine Activity against Pseudomonas aeruginosa MexXY-Mediated Aminoglycoside Resistance: In Silico and in Vitro Studies. J. Nat. Prod. 2019;82:1935–1944. doi: 10.1021/acs.jnatprod.9b00317. [DOI] [PubMed] [Google Scholar]
- 17.Imanshahidi M., Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 18.Tegos G., Stermitz F.R., Lomovskaya O., Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002;46:3133–3141. doi: 10.1128/AAC.46.10.3133-3141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jing W., Jie T., Li F.Y., Jia L., Jing Y.L., Fan Y. Berberine Analogues: Progress towards Versatile Applications. Heterocycles. 2015;91:2233–2270. [Google Scholar]
- 20.Dolla N.K., Chen C., Larkins-Ford J., Rajamuthiah R., Jagadeesan S., Conery A.L., Ausubel F.M., Mylonakis E., Bremner J.B., Lewis K., et al. On the Mechanism of Berberine-INF55 (5-Nitro-2-phenylindole) Hybrid Antibacterials. Aust. J. Chem. 2015;67:1471–1480. doi: 10.1071/CH14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H., Wang L., Li Y., Liu J., An M., Zhu S., Cao Y., Jiang Z., Zhao M., Cai Z., et al. Structural optimization of berberine as a synergist to restore antifungal activity of fluconazole against drug-resistant Candida albicans. ChemMedChem. 2014;9:207–216. doi: 10.1002/cmdc.201300332. [DOI] [PubMed] [Google Scholar]
- 22.Makvandi P., Gu J.T., Zare E.N., Ashtari B., Moeini A., Tay F.R., Niu L.N. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2019 doi: 10.1016/j.actbio.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Bador J., Amoureux L., Blanc E., Neuwirth C. Innate aminoglycoside resistance of Achromobacter xylosoxidans is due to AxyXY-OprZ, an RND-type multidrug efflux pump. Antimicrob. Agents Chemother. 2013;57:603–605. doi: 10.1128/AAC.01243-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morita Y., Tomida J., Kawamura Y. Responses of Pseudomonas aeruginosa to antimicrobials. Front. Microbiol. 2014;8:422. doi: 10.3389/fmicb.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau C.H., Hughes D., Poole K. MexY-promoted aminoglycoside resistance in Pseudomonas aeruginosa: Involvement of a putative proximal binding pocket in aminoglycoside recognition. MBio. 2014;5:e01068. doi: 10.1128/mBio.01068-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su F., Wang J. Berberine inhibits the MexXY-OprM efflux pump to reverse imipenem resistance in a clinical carbapenem-resistant Pseudomonas aeruginosa isolate in a planktonic state. Exp. Ther. Med. 2018;15:467–472. doi: 10.3892/etm.2017.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita Y., Tomida J., Kawamura Y. In: Resistance and Response to Anti-Pseudomonas Agents and Biocides, in Pseudomonas: New Aspects of Pseudomonas Biology. 2nd ed. Ramos J., Goldberg J.B., Filloux A., editors. Springer; New York, NY, USA: 2015. pp. 173–187. [Google Scholar]
- 28.Morita Y., Sobel M.L., Poole K. Antibiotic inducibility of the MexXY multidrug efflux system of Pseudomonas aeruginosa: Involvement of the antibiotic-inducible PA5471 gene product. J. Bacteriol. 2006;188:1847–1855. doi: 10.1128/JB.188.5.1847-1855.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy P.H., Tetu S.G., Larouche A., Elbourne L., Tremblay S., Ren Q., Robert D., Derek H., Ryan S., Kisha W., et al. Complete genome sequence of the multiresistant taxonomic outlier Pseudomonas aeruginosa PA7. PLoS ONE. 2010;5:e8842. doi: 10.1371/journal.pone.0008842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobel M.L., Mckay G.A., Poole K. Contribution of the MexXY multidrug transporter to aminoglycoside resistance in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 2003;47:3202–3207. doi: 10.1128/AAC.47.10.3202-3207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi-Akiyama T., Kuwahara T., Tada T., Kitao T., Kirikae T. Complete genome sequence of highly multidrug-resistant Pseudomonas aeruginosa NCGM2.S1, a representative strain of a cluster endemic to Japan. J. Bacteriol. 2011;193:7010. doi: 10.1128/JB.06312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yabuuchi E., Kawamura Y., Kosako Y., Ezaki T. Emendation of genus Achromobacter and Achromobacter xylosoxidans (Yabuuchi and Yano) and proposal of Achromobacter ruhlandii (Packer and Vishniac) comb. nov., Achromobacter piechaudii (Kiredjian et al.) comb. nov., and Achromobacter xylosoxidans subsp. denitrificans (Ruger and Tan) comb. nov. Microbiol. Immunol. 1998;42:429–438. doi: 10.1111/j.1348-0421.1998.tb02306.x. [DOI] [PubMed] [Google Scholar]
- 33.Yabuuchi E., Kosako Y., Oyaizu H., Yano I., Hotta H., Hashimoto Y., Ezaki T., Arakawa M. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol. Immunol. 1992;36:1251–1275. doi: 10.1111/j.1348-0421.1992.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D., Wang L., Liu H., Jiang Z., Li Y., Jiang Y., Zhu S., Li P., Xie W., Cai Z., et al. Preparation of Canadine Derivatives as Synergistic Antifungal Agents. [(accessed on 25 July 2012)];2012 Available online: URL https://worldwide.espacenet.com/publicationDetails/biblio?II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20120725&CC=CN&NR=102603755A&KC=A.
- 35.Mahapatra A., Maheswari V., Kalia N.P., Rajput V.S., Khan I.A. Synthesis and antitubercular activity of berberine derivatives. Chem. Nat. Compd. 2014;50:321–325. doi: 10.1007/s10600-014-0942-8. [DOI] [Google Scholar]
- 36.Wang J., Yang T., Chen H., Xu Y.-N., Yu L.-F., Liu T., Tang J., Yi Z., Yang C.-G., Xue W., et al. The synthesis and antistaphylococcal activity of 9,13-disubstituted berberine derivatives. Eur. J. Med. Chem. 2017;127:424–433. doi: 10.1016/j.ejmech.2017.01.012. [DOI] [PubMed] [Google Scholar]