Abstract
Bone tissue engineering involves the combined use of materials with functional properties to regenerate bone. Nanohydroxyapatite (nHA) can influence the behavior of cells. The functional and structural properties of nHA can be controlled during nanoparticle synthesis. This review defines the relationship between the attributes of nHA nanoparticles and their biological effects, focusing on biocompatibility, surface-area-to-volume ratio, bonding chemistry, and substrate functionality. The paper explores how these aspects have been applied in the development of scaffolds for the repair of damaged bone or regeneration of missing bone.
1. Introduction
Advances in the field of biomaterials and tissue engineering have led to novel engineering strategies for improved regeneration of bone.1 Bone tissue engineering (BTE) has now taken on an interdisciplinary perspective, in the search for new and better ways to solve the biomedical engineering challenges of regenerating bone.
Hydroxyapatite (HA) is the major mineral in natural bone and constitutes up to 65% of bone by weight. The solubility of these crystals is influenced by their size and by variations in their chemical composition (e.g., the presence of carbonate ions in the crystal lattice). HA found in normal bone differs from synthetic HA. The former has a more variable composition in terms of minor elements such as magnesium and strontium that may be incorporated into it. Synthetic HA has a high purity with a known chemical composition (especially in terms of the Ca/P ratio) and a high crystallinity.2 Synthetic HA used in a block form as a graft can exfoliate after surgical replacement. This limits its use in bone regeneration procedures. Also, the bulk material is brittle and has low tensile strength and fracture toughness.3
The recent development of nanohydroxyapatite (nHA) materials has been driven by the desire to overcome issues with bulk HA or microparticle HA and to improve bone integration and regeneration of bony defects. Indeed, moving to nanotechnology approaches has become a major direction in BTE. The application of nanotechnology concepts to the problems of designing and fabricating bone scaffolds allows more precise control of the regenerative features of scaffolds.4 This paper focuses on the properties of nHA and the applications of this material in generating biocompatible, bone-like matrices with functional properties that are ideal for BTE. The connections between the production methods of nHA, the properties of the resulting material, and the relavant clinical applications will be explored. A recurring theme is that artificial matrices supported by nHA can generate the desired nanointerface and enhance the regenerative function of scaffolds by influencing cell behavior.
Key requirements of bone regenerative substitutes are that bone growth is stimulated (osteoinduction) and that a micronetwork of pores and channels is present to allow cellular migration, transport of nutrients, and gaseous exchange. The composition and geometry of a scaffold should promote the development of new blood vessels and facilitate the passage of the molecular signals that drive growth and repair. From a purely mechanical aspect, the scaffold must support bone deposition and tolerate applied forces during the time periods that repair or regeneration are occurring.5
2. Clinical Demands for BTE
Bone-related diseases and bone-related physiological conditions account for a sizable proportion of medical costs. For most developed countries, conditions such as osteoporosis and its associated sequelae account for up to 15% of the cost of health care services. This level of expenditure is expected to increase in the future as a result of increasing numbers of cases of fractures in weight-bearing bones due to obesity, osteoporosis, falls, and other accidents.6 Globally, hip fractures could reach 6.3 million per annum by 2050, a 4-fold increase from the current rate.7 Research that focuses on cost-effective treatments for these bone conditions is, therefore, of interest not only to clinicians but also to biomedical scientists and engineers.
There are a number of challenges associated with current “gold standard” methods of treatment. For example, bone repair or replacement requires immediate postoperative fixation to promote and guide bone healing. The healing process can readily be compromised by postsurgical infection or by reactions to the metal components used for fixation. If bone autografts are used to treat large bone defects, there is significant donor site morbidity. If allografts or xenografts are used, these may transfer infection from the source or elicit immunological reactions from the host.8
3. Biological Properities of Bone Associated with the Design of Nanocomposite Scaffolds
Several fabrication strategies that are used with bone nanocomposite scaffolds have been inspired by the biological features of natural bone. The resilience of natural bone is due to its unique microstructure. Bone contains both HA for rigidity and collagen fibrils for reinforcing and flexibility. As shown in Figure 1, the biological building blocks within the dense outer parts of weight-bearing bones are arranged for maximum structural support, with juxtaposed assemblies of distinct patterns, from folded structures through to larger superstructural units.9 Similar concepts can be applied in composite materials, including lamination and parallel fibrillary arrays, taking the key biological features of natural bone and transforming them into an engineering strategy for BTE. The optimal physical properties can then be informed by finite element analysis and other computational approaches.
Figure 1.
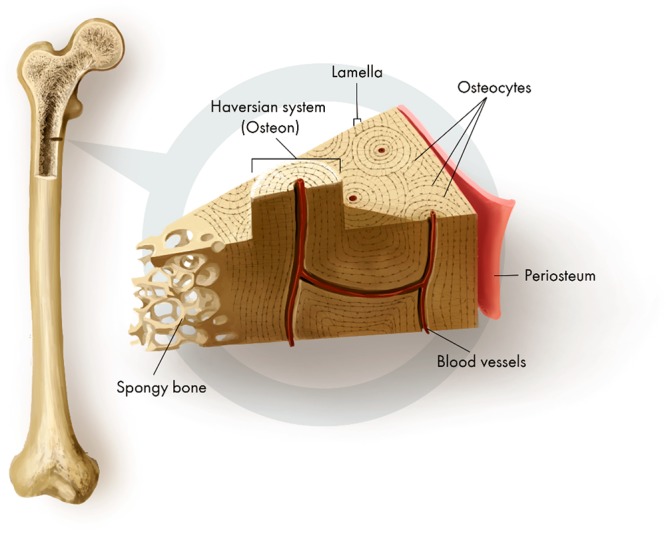
Schematic representation of the internal structure of bone showing its unique and intricate organization.
In bone, the unique combination of organic and inorganic components allows the bone to have stiffness but also to bend, rather then fracture, under normal loading conditions. A parallel approach can be used in BTE, employing materials to give the desired resistance to tensile and compressive forces.
In addition to physical properties, biological attributes also need to be considered, such as how the material used will promote the adhesion and differentiation of nonforming cells, and thus achieve bone regeneration. Such aspects will be affected by features such as surface area, surface energy, hydrophilicity, functional groups, and conjugants. The nature of the surface and its interaction with cells can be explored using gradient energy technologies assisted by genome sequencing to optimize the materials used in BTE.10
4. Forms of Nanocomposite Materials Used in BTE
Compared to microparticles, nanoparticles have a greater surface area to volume ratio and a stronger energy gradient profile at the surface. The shape of nanoparticles and the behavior of the scaffold can be optimized using three-dimensional modeling and simulation, to align with the size and shape of the bone defect that is being treated. Computational modeling can simulate the response to normal loads and other stresses.
Structural engineering requirements may dictate the need for particular nanoscale design features such as nanorods, nanospheres, nanowires, nanoclusters, nanofibers, and nanofilms as shown in Figure 2. To fabricate such nanostructures, a range of methods can be used, including electrospinning, phase separation, self-assembly, thin-film deposition, nanoimprinting, sol–gel, and photolithography.11
Figure 2.
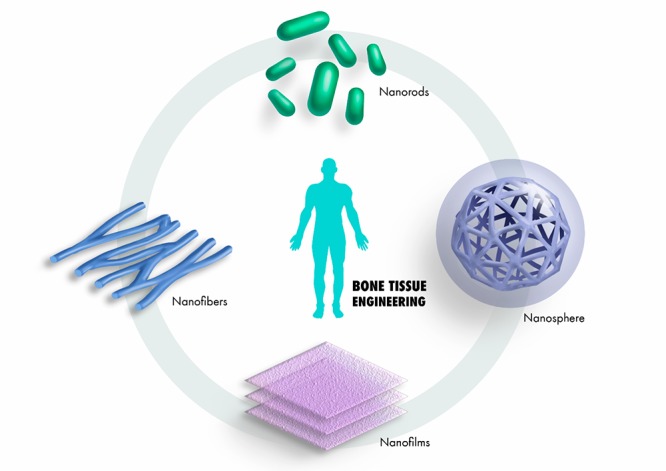
Different structural combinations of nanomaterials integrated to construct a bone-regenerative nanocomposite material.
The large surface area of a nanoparticle, coupled with optimized wettability, can promote nutrient sequestration and protein adsorption. When assembling a composite material, nHA has been incorporated as a reinforcing filler, to enhance the mechanical stability of the composite material, facilitate interactions with cells, and promote the proliferation, adhesion, and differentiation of cells, including stem cells. It can also facilitate cell–material surface interactions via selective protein adsorption.12
5. Processing of Nanohydroxyapatite
HA can be isolated from a range of natural sources, and nHA can be synthesized using a range of chemical methods. Depending on the method used, the final product will differ in terms of particle size, chemical composition (such as the presence or absence of carbonated groups), and crystallinity. This in turn will influence the behavior of the nanoparticles when placed into a bony defect, where aspects such as the rate of resorption become very important. Physical properties of nHA can be controlled by influencing the particle size during synthesis13
6. nHA in Bone-Regenerative Scaffolds
For effective BTE, it is imperative the scaffold matrix supports the growth of cells, from division through to maturation, without causing deleterious changes to cell morphology or behavior. nHA may be used alone or combined with other materials, to achieve the necessary support infrastructure (physical and biological) for the desired bone regenerative responses, as shown in Figure 3.
Figure 3.
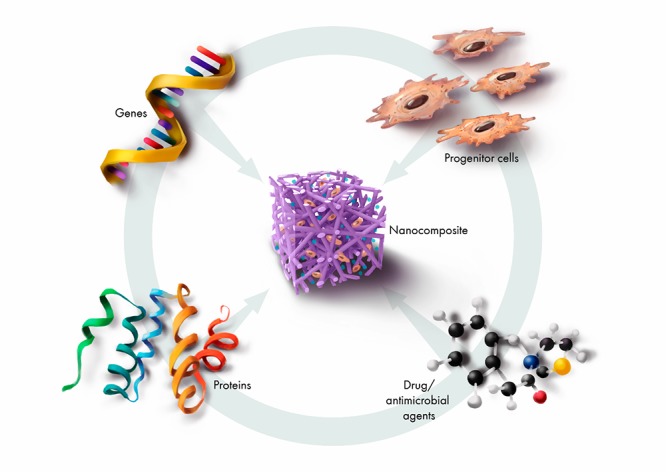
Combinatorial factors applied in bone-regenerative nanocomposites for targeted regenerative responses.
The materials used for BTE need to produce signals that drive the desired cell behavior. At present, there is limited data regarding how the materials used in BTE influence the behavior of cells, for example, how preosteoblasts interact with the nanocomposite interfaces and how the genetic markers associated with their transition toward an osteogenic lineage change over time because of this interaction.
Varying the nHA content and the physical properties of the scaffold (such as its porosity) can alter the regenerative profile of the scaffold. nHA acts at the site of initiation of nucleation. As nHA content rises, more nucleation sites are activated, and the rate of apatite nucleation accelerates. In this way, coating nHA onto scaffold matrices can enhance their biomimetic properties and ultimately drive the outcome of greater bone formation.14 If nHA is used in this way, it is essential to achieve a uniform distribution of nHA particles throughout the entire scaffold. Using an in situ precipitation method can achieve this objective. Nanocomposites comprising nHA can activate the activity of bone biomarkers. The extent of upregulation in the activity of these biomarkers, which is driven by nHA, is summarized in Table 1.
Table 1. Different Nanocomposites That Upregulate the Activities of Key Bone Biomarkers and Their Dependence on nHAa.
| nanocomposite | method of fabrication | cells | bone-related biomakers | condition | dependence on nHA | average particle size | ref |
|---|---|---|---|---|---|---|---|
| poly(e-caprolactone)-poly(ethylene glycol)/chitosan/nHA | electrospinning | human dental pulp stem cells | Runx2; BMP2; DSPP; BGLAP | in vitro | particle size | 190–260 nm | (40) |
| poly(ε-caprolactone)/poly(lactide-co-glycolide)/nHA | melt-blending method | human mesenhcymal stem cells | Runx2; OPN; OCN; BMP-2; Col I | in vitro and in vivo | particle size | 20–40 nm | (41) |
| Fe/nHA | 3D printing | rabbit bone marrow mesenchymal stem cell | OCN; ALP; BMP-2 | in vitro | combinatorial ratio of coated nHA | agglomerated nanorods | (42) |
Keys: N/A: No data to support theme; Runx 2 (runt-related transcription factor 2); ALP (alkaline phosphatase); BMP-2 (bone morphogenetic protein 2); Col I (collagen I); DSPP (dentin sialophosphoprotein); BGLAP (bone gamma-carboxyglutamate protein); osteonectin (OCN); osteopontin (OPN).
A further aspect of scaffold design is the physical form of the scaffold. It must create a microenvinoment that facilitates the formation and ingress of new blood vessels, to achieve optimal perfusion. To achieve this, the scaffold matrix must promote the adhesion of cells and integrate with the surrounding tissues. How these various aspects work together is discussed further below.
6.1. Mechanically Stable Composites for Repair of Load-Bearing Bone
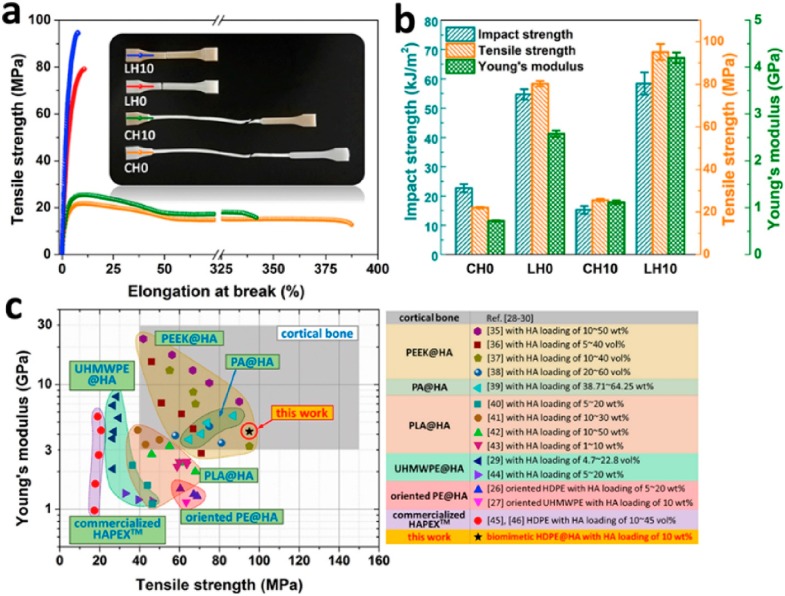
Nanoparticles can readily be added as building blocks to achieve mechanically stable bone scaffold matrices. The influence of HA loading on the mechanical properties of some polymers used in BTE is shown Figure 4.
Figure 4.
Mechanical properties of various samples: (a) Typical stress–strain curves; (b) impact strength, tensile strength and Young’s modulus; (c) comparison of tensile strength and Young’s modulus of the HDPE/HA substitute used in current work, compared to human cortical bone and polymer/HA composites for bone replacement applications reported in the literature. Reprinted with permission from ref (39). Copyright (2019), Elsevier.
A range of synthetic materials have been shown to successfully integrate with nHA, with good mechanical performance for BTE. When nHA is added to polymeric materials, the mechanical properties of the final composite can be compared favorably to those of cortical bone. As shown in Table 2, both the fabrication technique and the molecular weight of the polymer influence the mechanical strength of the resulting nanocomposite material.
Table 2. Comparison of Mechanical Properties of nHA-Integrated Polymers with Cortical Bone and Their Regenerative Propertiesa.
| nanocomposite | method of fabrication | physical nature of composite | regenerative indicators | particle distribution | in vivo or in vitro test | physical dimension of nHA | mechanicalstrength | ref |
|---|---|---|---|---|---|---|---|---|
| nHA/polyethylene (ultrahigh molecular weight) | melt-extrusion method | pellets | apatite nucleation, cell adhesion and proliferation | yes | in vitro | average particle size: 150 nm | ↑ | (43) |
| polylactide/nHA | template-assisted self-assembly | 3D | cell viability, cell adhesion and proliferation | yes | in vitro | N/A | ↓ | (44) |
| l-lactide/nHA | surface modification and ring-opening polymerization | pellets | N/A | yes | N/A | neddle-like 100–200 nm length; 20–30 nm width | ↓ | (45) |
Keys: NA: no data to support theme; ↑ within range of cortical bone mechanical profile; ↓ below range of cortical bone mechanical profile.
Depending on the combination that is used, the mechanical and chemical properties of the synthetic polymers may be improved or degraded. The chemical integration of nHA with polyester compounds can generate porous scaffold networks that support the growth of cells and influence their phenotype. nHA buffers the pH of the local environment as polyester byproducts are formed during degradation. This reduces the extent of associated inflammation during this degradation.15
Rather than using only one polymer, multiple polymers can be employed. One interesting approach is to fabricate a triblock scaffold. Two polymers are initially polymerized and reconstituted with another polymer of known mole fraction. This is followed by the addition of nHA. The nHA layer adds desirable properties, such as improved cell adhesion. It also increases the stiffness and the elastic modulus of the scaffold and slows the rate of degration of the scaffold in alkaline conditions.16
For regeneration of bony defects with complex structures and intricate shapes, successful integration is essential. BTE scaffold materials must maintain the most appropriate structural geometry as the process of regeneration proceeds. While chemical methods can be used to incorporate nHA into scaffolds, these can at times be complex and technique sensitive. An alternative approach is selective laser sintering, which is a rapid prototyping technology. This method can be used to control both the geometry of the final scaffold and its content of nHA or other osteoconductive nanofillers.17
With greater emphasis now being placed on nontoxic, biodegradable byproducts, the use of clay-based hybrid materials, especially those using clays from the smectite group (montmorillonites, saponites, and hectorite), has attracted interest. Smectite mineral structures have two tetrahedral silica sheets, overlaying an octahedral sheet of cationic metals such as aluminum or magnesium. Small particles are charged, and the surfaces of these particles are reactive. The ability of clay minerals to delaminate facilitates a variety of interactions (e.g., cation exchange, hydrophobic interactions, hydrogen bonding, cation bridging, anion exchange, and proton transfers) between the clay particles and organic molecules, on both the surface of the clay particles as well as within their layers. These interactions are influenced by environmental pH as well as by the size and electrostatic properties of the interacting molecules. Interactions between clay minerals and biological molecules can be used to encapsulate and retain the latter, for later slow release, in order to stimulate growth and drive the differentiation of progenitor cells. Using particles of clay minerals also alters the mechanical properties of the scaffold matrix. Montmorillonite has a high surface area and can be blended readily with natural polymers and nHA. The strong interfacial interactions increase the mechanical properties of the resulting nanocomposite material.18
6.2. Recapitulating the Features of the Extracellular Matrix (ECM)
This approach employs, within the nanocomposite, analogues of the molecular signals which regulate protein synthesis and influence cell metabolism and tissue regeneration. The peptide sequences are targeted to specific signaling events.
Integrin-binding peptides, such as the arginylglycylaspartic (RGD) copolymer, can promote osteointegration, as they stimulate the differentiation of mesenchymal stem cells to osteoblasts. Amino acid esters and glucosyl, glyceryl, lactide, and glycolide esters can also be used. These promote biocompatibility and enhance the adhesion of cells and the secretion of bone. The RGD copolymer has been integrated with nHA-grafted poly(l-lactide) and used to reconstruct bone defects, as it promotes bone ingrowth and bone fusion in defects of critical size. The combination of the RGD copolymer with nHA provides a more stable scaffold.19.
6.3. Bioactive Polysaccharides
These polysaccharides have properties that can promote regeneration. They dissolve readily in either water or ethanol, to form a single polymeric phase. They are biocompatible and contain functional groups that are suited for cross-linking.
The first polysaccharide of interest is fucoidan. When used at low concentations, this induces the differentiation of stem cells to osteoblasts.20 Higher concentrations are not suitable as they cause cell death. The regenerative property of fucoidan is associated with its content of l-fucose and with the sulfate ester group in its polysaccharide skeleton. For use in BTE, a single-phase synthesis technique has been developed. This achieves a homogeneous distribution of nHA in a 3D nanocomposite composed of fucoidan and chitosan.21
A second polysaccharide of interest is chondroitin sulfate (CS). This is a glycosaminoglycan which forms part of the extracellular matrix. Its negative charge bind proteins, to form proteoglycans. It can also bind to and modulate the activity of growth factors, cytokines, and chemokines. These features make CS useful for controlling signal transduction pathways and hence desirable for use in BTE scaffold fabrication. CS can be modified chemically and cross-linked with a polymer to form a hydrogel for BTE. Covalent cross-linking gives additional stability to the structure, to support the growth and proliferation of cells.22
Further polysaccharides of note are dextran and pullulan. Both can be incorporated into hydrogels and linked to nHA. These polysaccharides enhance water retention. Dextran and pullulan when cross-linked with nHA have been shown to enhance tissue regeneration and mineralization.23
6.4. Injectable Hydrogels
Hydrogels are an attractive means for enhancing bone regeneration without having to undergo complex surgical procedures. A liquid or semiliquid gel is injected into the defect. The hydrogel hardens slowly, as gelation is triggered by a local increase in temperature.24
Thermosensitive hydrogels are classified in terms of their critical solution temperature, which is the temperature at which the polymer material undergoes separation from one phase to another phase. At the lower critical solution temperature (LCST), the polymer will become insoluble in aqueous solution with any increase in temperature above this threshold.
Polymers that exhibit transitions near body temperatures (35–37 °C) are useful for biomedical applications. Poly(N-isopropylacrylamide) with nHA has been used as a thermosensitive gel in BTE applications. This polymer has a hydrophobic core and a hydrophilic outer shell, and its LCST is 32 °C. Beyond this temperature, the polymer undergoes phase transition into a compact precipitate.25 Other thermosensitive materials that have been combined with nHA to prepare hydrogels for BTE applications include the Pluronics, which are copolymers of poly(ethylene oxide).26
Aside from temperature, local pH can influence the behavior of the hydrogel. Thus, an important aspect of scaffold design is to determine how the local pH will alter the physical properties of the hydrogel. At the same time, the pH must also be optimal for the growth of cells.27
A hydrogel can direct the migration of cells by releasing chemitactic signals. In the same way, it can be designed to release signaling molecules that promote bone growth or drugs for microbial control during bone regeneration The physical properties of the hydrogel also facilitate the proper integration and migration of cells. The addition of nHA to a hydrogel alters its flow properties and its physical stability, as well as stimulating cell proliferation and osteogenesis.28
The regenerative capabilities of injectable hydrogels can be enriched by encapsulating mesenchymal stem cells, which then differentiate into bone forming cells. This increases the bone cell population and is an appealing strategy for regeneration of deep, irregular bony defects.29
6.5. nHA-Based Nanofiber Nanocomposites
Various therapeutic agents can be loaded onto nanofibers for controlled release. By altering the diameter of the fiber, the surface area and porosity can be controlled and thus the rate of drug delivery. Nanofibers loaded with antimicrobial or anti-inflammatory agents could prevent surgical site infection or foreign body reactions, respectively.
Polycaprolactone nanofibers loaded with antibiotic have shown promise for regeneration of bone defects, such as alveolar bone defects caused by periodontitis.30 Likewise, nHA can be coated with a silicate into a core−shell.31 Coaxial electrospinning can integrate a core−shell with a series of nanostructured fiber networks, to create scaffolds for BTE.32
Examples of clinical scenarios where these different kinds of matrices could be used are summarized in Figure 5.
Figure 5.
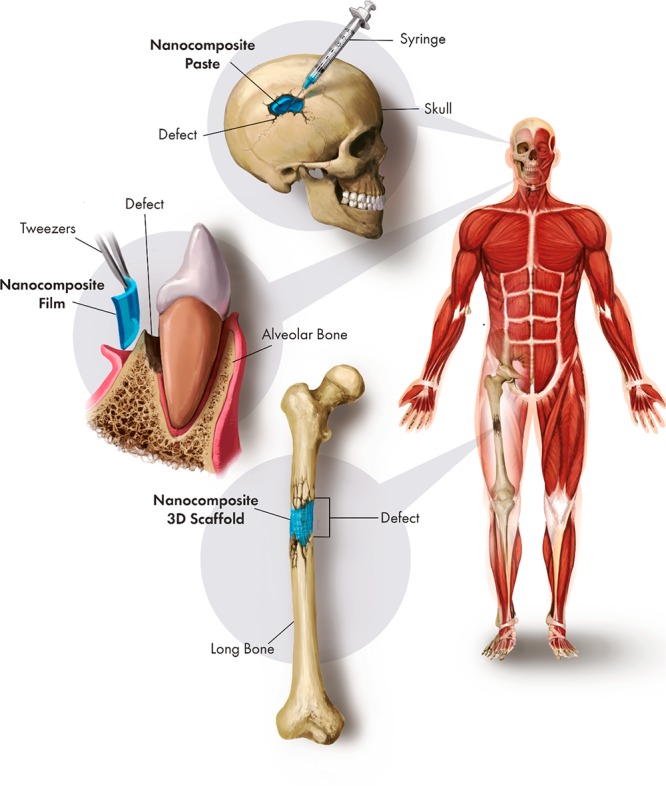
Synergistic application of different forms of nanocomposite for regeneration of clinical bone defects.
7. Computational Modeling of the Regenerative Properties of nHA for BTE
At the macroscopic level, a scaffold must retain the desired shape or geometric configuration. At the microscopic level, the nanoparticles and polymer matrix used in the scaffold must be chosen to trigger the desired patterns of cell growth, fluid flow, and integration with surrounding tissues.
From the known properties of the materials used in scaffolds, finite element analysis (FEA) can be used to assess the physical aspects of the scaffold and its components. The modeling takes into account the boundaries of the scaffold matrix, its porosity, and how particles within the scaffold are dispersed.33
Another useful application of FEA is predicting the effect of changing the properties of the nHA on the physical characteristics of the scaffold. Changes in the apatite composition can be due to altering the variables of the production process. Chemical synthesis, including Mg2+ or other ions in the reaction mixture, will result in their incorporation into the apatite structure. Likewise, altering the temperature and crystal aging time will alter the properties of the nHA as it forms. As the level of crystallinity changes, so will the rate of resportion of the apatite under physiologic conditions. Using such approaches, nonstoichiometric forms of nHA can be created that are more biodegradable and bioactive than stoichiometric HA. Such altered forms may be preferred for certain clinical applications where faster resorption is desired, as part of an overall scaffold design for bone regeneration.34 Key FEA variables that have been used in studies of nHA-based composites are summarized in Table 3.
Table 3. Properties of nHA Composites That Have Been Simulated to Evaluate Their Regenerative Implications in the Repair or Regeneration of Bone.
| scaffold composition | material input parameters | purpose | ref |
|---|---|---|---|
| PLGA/collagen/nHA | nHA content, strand diameter, compressive modulus, strand diameter, spacing, and porosity | topology optimization of three-dimensional scaffold | (46) |
| HA crystals | microporosity, mesoporosity, macroporosity, crack density, granule radius, bone formation rate, and scaffold resorption | estimate load-acting and load-bearing capacity of HA-based biomaterial for mandibular bone replacement | (47) |
| 3D HA scaffold | compressive force | optimizing mechanical performance HA scaffold of different geometric configuration; stress distribution of scaffolds | (48) |
| 3D printed nHA scaffold | uniaxial and biaxial compressions; bending | study stress distribution (compressive loading, biaxial loadings, bending) in different scaffolds; effects of substitution (cationic, anionic) in nHA scaffold | (49) |
A further aspect of computational modeling in BTE is the interactions that occur between the surfaces of materials and proteins. Maximizing this relationship at the submolecular level is important in developing specific nanoscale designs for biomaterials. Molecular dynamics simulation and steered molecular dynamic simulations can jointly be used to study the interactions between material surfaces and bone proteins at the atomic level, such as protein adsorption and desorption on nanotextured surfaces. Several interaction parameters such as binding and desorption energies can be calculated.35
Molecular dynamics simulation can be used to study the interfacial interactions and mechanical behavior of nHA with bone proteins such as osteopontin, where electrostatic interactions occur between the calcium of the nHA and the aspartate, glutamate, and phosphoserine amino acids of osteopontin. During loading, the formation of new bonds between the calcium and the amino acid residues restricts the movement of the osteopontin peptides and generates a stick–slip motion between the two surfaces. This motion explains the high fracture resistance of natural bone.36
Molecular dynamic simulations can explore the orientiation of proteins that have been adsorbed on material surfaces. Changes in this orientiation will alter the adsorption/desorption behavior of the protein. The surface interactions can be influenced by intermolecular hydrogen bonding as well as by the structural characteristics of the protein.37
Another perspective of how nHA interacts with bone proteins is based on changes in the conformation and orientation of the adsorbed proteins, according to which the epitope of the protein has bound onto the nHA surface. This parameter then influences cellular responses. For example, the knuckle epitope of bone morphogenetic protein 2 (BMP-2) absorbs more readily onto nHA surfaces than does the wrist epitope of BMP-2. The exposed wrist epitope interacts with the type I cell membrane receptor on stem cells, and this triggers differentiation of the stem cell to an osteoblast.38 There are numerous other examples of how protein binding sites affect the conformation and activity of proteins bound to surfaces.
Molecular dynamic simulation is a useful tool for studying interfacial interactions between polymers and ceramics. It can facilitate the design of better composite materials by allowing laboratory optimization of the elements of interfacial bonding, protein adsorption, and protein recognition at the atomic level, during the development phase. High-throughput screening methods can then be used to validate the regenerative outcomes at the genomic and proteomic levels in the cell culture setting, to verify that the desired pattern of cellular events is actually being achieved, before then undertaking tests in physiological conditions.
8. Future Outlook and Conclusions
Bone tissue engineering is a complex task, with numerous complexities from the standpoint of design. nHA holds considerable promise for use in therapeutic BTE interventions. Its properties can be optimized during production to create advanced and cost-effective biomaterials for clinical use. Changes in crystallinity and chemical composition of nHA can be made to alter its rate of resorption. Nanofabrication methods can give the correct shape of the nHA nanoparticles necessary for achieving spatial control over cell behavior, while also imparting the necssary structural properties. Advanced computational modeling can help to optimize the interactions of nHA with bone proteins. Such advanced qualitative and quantitative studies can be a critical element of the design process for new materials and a prelude to cell culture and animal model experiments. It is in the latter phase where the regenerative performance of nHA nancomposite materials can be tested under increasingly stringent conditions to verify that the design intentions in terms of both bone formation and nHA resorption are actually being achieved.
Acknowledgments
This research was supported by an Australian Government Research Training Program Scholarship through The University of Queensland. The authors express thanks to Dr. Craig Neville at the Laboratory for Tissue Engineering and Organ Fabrication, Massachusetts General Hospital, for his valuable comments on the manuscript.
Biographies

Baboucarr Lowe is a final year doctoral student in the University of Queensland under the supervision of Prof. Laurence J. Walsh. He was a postgraduate research fellow in the department of Oral and Maxillofacial Surgery, Massachusetts General Hospital, and Harvard School of Dental Medicine. He completed his master’s and undergraduate degrees from Pukyong National University, South Korea, and University of The Gambia, The Gambia, respectively. His research interests are in the areas of stem cells, biomaterials, biomedical nanotechnology, and tissue engineering. He is a member of the International Association of Dental Research and the American Institute of Chemical Engineers (AIChe).

John G. Hardy received his MSci and PhD in Chemistry from the Universities of Bristol and York, respectively. He subsequently spent 10 years as a postdoctoral researcher working on interdisciplinary projects in France, Germany, Northern Ireland, and the USA and is currently a senior lecturer in the Department of Chemistry at Lancaster University in the UK. His current research spans biochemistry, biomedical engineering, chemistry, materials science, and pharmacy, developing materials for technical and medical applications in collaboration with members of his research group and other researchers in the UK and overseas.

Laurence J. Walsh is a tenured professor in the University of Queensland School of Dentistry, where he is the research group leader for advanced materials and technologies. He received his BDSc, PhD, and DDSc from the same university. Dr Walsh undertook his postdoctoral studies at the University of Pennsylvania School of Medicine. His research interests are in biomaterials for regeneration of dental enamel and bone and for the restoration of teeth. He recently served as the deputy chair of the International Association for Dental Research Asia Pacific Regional meeting in 2019 in Australia. He serves on the editorial board of 5 international journals.
The authors declare no competing financial interest.
References
- Chiara G.; Letizia F.; Lorenzo F.; Edoardo S.; Diego S.; Stefano S.; Eriberto B.; Barbara Z. Nanostructured biomaterials for tissue engineered bone tissue reconstruction. Int. J. Mol. Sci. 2012, 13 (1), 737–757. 10.3390/ijms13010737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossa P. A. F.; Giraldo B. S.; Garcia B. C. G.; Parra E. R.; Arango P. J. A. Comparative study between natural and synthetic Hydroxyapatite: structural, morphological and bioactivity properties. Rev. Mater. 2018, 10.1590/s1517-707620180004.0551. [DOI] [Google Scholar]
- Ryabenkova Y.; Pinnock A.; Quadros P.; Goodchild R.; Möbus G.; Crawford A.; Hatton P.; Miller C. The relationship between particle morphology and rheological properties in injectable nano-hydroxyapatite bone graft substitutes. Mater. Sci. Eng., C 2017, 75, 1083–1090. 10.1016/j.msec.2017.02.170. [DOI] [PubMed] [Google Scholar]
- Nobile S.; Nobile L. Nanotechnology for biomedical applications: Recent advances in neurosciences and bone tissue engineering. Polym. Eng. Sci. 2017, 57, 644–650. 10.1002/pen.24595. [DOI] [Google Scholar]
- Ghassemi T.; Shahroodi A.; Ebrahimzadeh M. H.; Mousavian A.; Movaffagh J.; Moradi A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone. Joint. Surg. 2018, 6 (2), 90. [PMC free article] [PubMed] [Google Scholar]
- Henkel J.; Woodruff M. A.; Epari D. R.; Steck R.; Glatt V.; Dickinson I. C.; Choong P. F.; Schuetz M. A.; Hutmacher D. W. Bone regeneration based on tissue engineering conceptions—a 21st century perspective. Bone Res. 2013, 1 (3), 216. 10.4248/BR201303002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C.; Campion G.; Melton L. J. Hip fractures in the elderly: a world-wide projection. Osteoporosis Int. 1992, 2 (6), 285–289. 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- Salgado A. J.; Coutinho O. P.; Reis R. L. Bone tissue engineering: state of the art and future trends. Macromol. Biosci. 2004, 4 (8), 743–765. 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- Weiner S.; Wagner H. D. The material bone: structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28 (1), 271–298. 10.1146/annurev.matsci.28.1.271. [DOI] [Google Scholar]
- Michelmore A.; Clements L.; Steele D. A.; Voelcker N. H.; Szili E. J. Gradient technology for high-throughput screening of interactions between cells and nanostructured materials. J. Nanomater. 2012, 2012, 1–7. 10.1155/2012/839053. [DOI] [Google Scholar]
- Zhang L.; Webster T. J. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today 2009, 4 (1), 66–80. 10.1016/j.nantod.2008.10.014. [DOI] [Google Scholar]
- Gong T.; Xie J.; Liao J.; Zhang T.; Lin S.; Lin Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. 10.1038/boneres.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan J.; Lowe B.; Manivasagan P.; Kang K.-H.; Chalisserry E. P.; Anil S.; Kim D. G.; Kim S.-K. Isolation and characterization of nano-hydroxyapatite from salmon fish bone. Materials 2015, 8 (8), 5426–5439. 10.3390/ma8085253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowska-Tylman J.; Locs J.; Salma I.; Wozniak B.; Pilmane M.; Zalite V.; Wojnarowicz J.; Kedzierska-Sar A.; Chudoba T.; Szlazak K.; et al. In vivo and in vitro study of a novel nanohydroxyapatite sonocoated scaffolds for enhanced bone regeneration. Mater. Sci. Eng., C 2019, 99, 669–684. 10.1016/j.msec.2019.01.084. [DOI] [PubMed] [Google Scholar]
- Ma P. X.; Zhang R.; Xiao G.; Franceschi R. Engineering new bone tissue in vitro on highly porous poly (α-hydroxyl acids)/hydroxyapatite composite scaffolds. J. Biomed. Mater. Res. 2001, 54 (2), 284–293. . [DOI] [PubMed] [Google Scholar]
- Torabinejad B.; Mohammadi-Rovshandeh J.; Davachi S. M.; Zamanian A. Synthesis and characterization of nanocomposite scaffolds based on triblock copolymer of L-lactide, ε-caprolactone and nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng., C 2014, 42, 199–210. 10.1016/j.msec.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Duan B.; Wang M.; Zhou W. Y.; Cheung W. L.; Li Z. Y.; Lu W. W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6 (12), 4495–4505. 10.1016/j.actbio.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Dawson J. I.; Oreffo R. O. Clay: new opportunities for tissue regeneration and biomaterial design. Adv. Mater. 2013, 25 (30), 4069–4086. 10.1002/adma.201301034. [DOI] [PubMed] [Google Scholar]
- Pountos I.; Panteli M.; Lampropoulos A.; Jones E.; Calori G. M.; Giannoudis P. V. The role of peptides in bone healing and regeneration: a systematic review. BMC Med. 2016, 14 (1), 103. 10.1186/s12916-016-0646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H.-S.; Venkatesan J.; Kim S.-K. Hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2013, 57, 138–141. 10.1016/j.ijbiomac.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Lowe B.; Venkatesan J.; Anil S.; Shim M. S.; Kim S.-K. Preparation and characterization of chitosan-natural nano hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1479–1487. 10.1016/j.ijbiomac.2016.02.054. [DOI] [PubMed] [Google Scholar]
- Kim H. D.; Lee E. A.; An Y.-H.; Kim S. L.; Lee S. S.; Yu S. J.; Jang H. L.; Nam K. T.; Im S. G.; Hwang N. S. Chondroitin sulfate-based biomineralizing surface Hydrogels for bone tissue engineering. ACS Appl. Mater. Interfaces 2017, 9 (26), 21639–21650. 10.1021/acsami.7b04114. [DOI] [PubMed] [Google Scholar]
- Schlaubitz S.; Derkaoui S. M.; Marosa L.; Miraux S.; Renard M.; Catros S.; Le Visage C.; Letourneur D.; Amedee J.; Fricain J.-C. Pullulan/dextran/nHA macroporous composite beads for bone repair in a femoral condyle defect in rats. PLoS One 2014, 9 (10), e110251 10.1371/journal.pone.0110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S.; Hui P. C.-l.; Kan C.-w. Thermoresponsive hydrogels and their biomedical applications: Special insight into their applications in textile based transdermal therapy. Polymers 2018, 10 (5), 480. 10.3390/polym10050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira N.; Ferreira L.; Cardoso V.; Boni F.; Souza A.; Gremião M. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. 10.1016/j.eurpolymj.2017.12.004. [DOI] [Google Scholar]
- Li Z.; Su Y.; Xie B.; Wang H.; Wen T.; He C.; Shen H.; Wu D.; Wang D. A tough hydrogel–hydroxyapatite bone-like composite fabricated in situ by the electrophoresis approach. J. Mater. Chem. B 2013, 1 (12), 1755–1764. 10.1039/c3tb00246b. [DOI] [PubMed] [Google Scholar]
- Rogina A.; Ressler A.; Matić I.; Ferrer G. G.; Marijanović I.; Ivanković M.; Ivanković H. Cellular hydrogels based on pH-responsive chitosan-hydroxyapatite system. Carbohydr. Polym. 2017, 166, 173–182. 10.1016/j.carbpol.2017.02.105. [DOI] [PubMed] [Google Scholar]
- Tan J.; Zhang M.; Hai Z.; Wu C.; Lin J.; Kuang W.; Tang H.; Huang Y.; Chen X.; Liang G. Sustained Release of Two Bioactive Factors from Supramolecular Hydrogel Promotes Periodontal Bone Regeneration. ACS Nano 2019, 13 (5), 5616–5622. 10.1021/acsnano.9b00788. [DOI] [PubMed] [Google Scholar]
- Arun Kumar R.; Sivashanmugam A.; Deepthi S.; Iseki S.; Chennazhi K.; Nair S. V.; Jayakumar R. Injectable chitin-poly (ε-caprolactone)/nanohydroxyapatite composite microgels prepared by simple regeneration technique for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7 (18), 9399–9409. 10.1021/acsami.5b02685. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Jiang Y.; Zhang Y.; Wen S.; Wang Y.; Zhang H. Dual functional electrospun core-shell nanofibers for anti-infective guided bone regeneration membranes. Mater. Sci. Eng., C 2019, 98, 134–139. 10.1016/j.msec.2018.12.115. [DOI] [PubMed] [Google Scholar]
- Anitha A.; Menon D.; Sivanarayanan T.; Koyakutty M.; Mohan C. C.; Nair S. V.; Nair M. B. Bioinspired composite matrix containing hydroxyapatite-silica core-shell nanorods for bone tissue engineering. ACS Appl. Mater. Interfaces 2017, 9 (32), 26707–26718. 10.1021/acsami.7b07131. [DOI] [PubMed] [Google Scholar]
- Shao W.; He J.; Sang F.; Ding B.; Chen L.; Cui S.; Li K.; Han Q.; Tan W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite–tussah silk fibroin nanoparticles for bone tissue engineering. Mater. Sci. Eng., C 2016, 58, 342–351. 10.1016/j.msec.2015.08.046. [DOI] [PubMed] [Google Scholar]
- Naderi S.; Dabbagh A.; Hassan M. A.; Razak B. A.; Abdullah H.; Kasim N. H. A. Modeling of porosity in hydroxyapatite for finite element simulation of nanoindentation test. Ceram. Int. 2016, 42 (6), 7543–7550. 10.1016/j.ceramint.2016.01.161. [DOI] [Google Scholar]
- Sarkar K.; Kumar V.; Devi K. B.; Ghosh D.; Nandi S. K.; Roy M. Effects of Sr doping on biodegradation and bone regeneration of magnesium phosphate bioceramics. Materialia. 2019, 5, 100211. 10.1016/j.mtla.2019.100211. [DOI] [Google Scholar]
- Huang B.; Lou Y.; Li T.; Lin Z.; Sun S.; Yuan Y.; Liu C.; Gu Y. Molecular dynamics simulations of adsorption and desorption of bone morphogenetic protein-2 on textured hydroxyapatite surfaces. Acta Biomater. 2018, 80, 121–130. 10.1016/j.actbio.2018.09.019. [DOI] [PubMed] [Google Scholar]
- Lai Z. B.; Wang M.; Yan C.; Oloyede A. Molecular dynamics simulation of mechanical behavior of osteopontin-hydroxyapatite interfaces. J. Mech. Behav. Biomed. Mater. 2014, 36, 12–20. 10.1016/j.jmbbm.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Wu T.; Dong X.; Wang Q.; Shen J. Adsorption mechanism of BMP-7 on hydroxyapatite (001) surfaces. Biochem. Biophys. Res. Commun. 2007, 361 (1), 91–96. 10.1016/j.bbrc.2007.06.169. [DOI] [PubMed] [Google Scholar]
- Gu H.; Xue Z.; Wang M.; Yang M.; Wang K.; Xu D. Effect of Hydroxyapatite Surface on BMP-2 Biological Properties by Docking and Molecular Simulation Approaches. J. Phys. Chem. B 2019, 123 (15), 3372–3382. 10.1021/acs.jpcb.9b01982. [DOI] [PubMed] [Google Scholar]
- Liu T.; Huang K.; Li L.; Gu Z.; Liu X.; Peng X.; Kuang T. High performance high-density polyethylene/hydroxyapatite nanocomposites for load-bearing bone substitute: fabrication, in vitro and in vivo biocompatibility evaluation. Compos. Sci. Technol. 2019, 175, 100–110. 10.1016/j.compscitech.2019.03.012. [DOI] [Google Scholar]
- Hokmabad V. R.; Davaran S.; Aghazadeh M.; Alizadeh E.; Salehi R.; Ramazani A. A Comparison of the Effects of Silica and Hydroxyapatite Nanoparticles on Poly (ε-caprolactone)-Poly (ethylene glycol)-Poly (ε-caprolactone)/Chitosan Nanofibrous Scaffolds for Bone Tissue Engineering. Tissue Eng. Regener. Med. 2018, 15 (6), 735–750. 10.1007/s13770-018-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Zhang S.; Zhang X.; Xie S.; Zhao G.; Zhang L. Biocompatibility and physicochemical characteristics of poly (ε-caprolactone)/poly (lactide-co-glycolide)/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Des. 2017, 114, 149–160. 10.1016/j.matdes.2016.10.054. [DOI] [Google Scholar]
- Yang C.; Huan Z.; Wang X.; Wu C.; Chang J. 3D printed Fe scaffolds with HA nanocoating for bone regeneration. ACS Biomater. Sci. Eng. 2018, 4 (2), 608–616. 10.1021/acsbiomaterials.7b00885. [DOI] [PubMed] [Google Scholar]
- Huang Y.-F.; Xu J.-Z.; Zhou D.; Xu L.; Zhao B.; Li Z.-M. Simultaneous reinforcement and toughening of polymer/hydroxyapatite composites by constructing bone-like structure. Compos. Sci. Technol. 2017, 151, 234–242. 10.1016/j.compscitech.2017.08.026. [DOI] [Google Scholar]
- Wan Y.; Wu C.; Xiong G.; Zuo G.; Jin J.; Ren K.; Zhu Y.; Wang Z.; Luo H. Mechanical properties and cytotoxicity of nanoplate-like hydroxyapatite/polylactide nanocomposites prepared by intercalation technique. J. Mech. Behav.Biomed. Mater. 2015, 47, 29–37. 10.1016/j.jmbbm.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Qiu X.; Hong Z.; Hu J.; Chen L.; Chen X.; Jing X. Hydroxyapatite surface modified by L-lactic acid and its subsequent grafting polymerization of L-lactide. Biomacromolecules 2005, 6 (3), 1193–1199. 10.1021/bm049502l. [DOI] [PubMed] [Google Scholar]
- Uth N.; Mueller J.; Smucker B.; Yousefi A.-M. Validation of scaffold design optimization in bone tissue engineering: finite element modeling versus designed experiments. Biofabrication 2017, 9 (1), 015023 10.1088/1758-5090/9/1/015023. [DOI] [PubMed] [Google Scholar]
- Scheiner S.; Komlev V. S.; Hellmich C. Strength increase during ceramic biomaterial-induced bone regeneration: a micromechanical study. Int. J. Fract. 2016, 202 (2), 217–235. 10.1007/s10704-016-0157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.; Li J.; Shao Z.; Ma K.; Zhang Z.; Wang B.; Zhang Y. Encapsulation of mesenchymal stem cells in chitosan/β-glycerophosphate hydrogel for seeding on a novel calcium phosphate cement scaffold. Med. Eng. Phys. 2018, 56, 9–15. 10.1016/j.medengphy.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Gu Y.; Yasodharababu M.; Nair A. K. A multiscale investigation of mechanical properties of bio-inspired scaffolds. Comput. Methods. Biomech. Biomed. Engin. 2018, 21 (13), 703–711. 10.1080/10255842.2018.1512593. [DOI] [PubMed] [Google Scholar]