Abstract
Overconsumption of biotin (5–100 mg daily) as a supplement by the general population poses a significant problem for clinical immunoassays (IAs) based on biotin–streptavidin (SA) interactions. This affinity pair has been exploited in immunoassays because of its avidity, sensitivity, specificity, and stability. The elevated biotin level in plasma varies from patient to patient, and its severe interference cannot easily be predicted and quantified. Thus, immunoassay manufacturers must investigate the biotin interference in the developed immunoassays to satisfy the threshold of 3510 ng/mL (14 367 nM), as stipulated by the FDA. There is no concrete solution to circumvent the biotin interference without extra costs and technical difficulties, albeit different strategies have been attempted. They include the IA format with biotinylated reagents prebound to streptavidin, the removal of biotin from the specimen, sample treatment, and biotin interference-free assays. The general public has been instructed to stop taking biotin supplements for 48 h or even weeks before the test, depending on the specific test, dose, and frequency of biotin uptake. As lab-based techniques cannot accommodate an enormous number of public samples, a rapid analytical procedure for biotin is urgently needed to quantify for its interference in immunoassays.
1. Introduction
Immunoassays (IAs) play a critical role in hospital settings, which cover both small and large biomolecules of clinical significance in oncology, cardiology, bone metabolism, hepatic fibrosis, anemia, infectious diseases, sepsis/inflammation, toxicology, forensics, drug screening/therapeutic drug monitoring, diabetes (fructosamine and hemoglobin A1c), reproductive endocrinology (hCG), specific proteins, etc. Assays for large molecules include thyroid-stimulating hormone, pituitary glycoprotein hormones, human chorionic gonadotropin, parathyroid hormone, insulin-like growth factor-1, insulin, thyroglobulin, C-peptide, ferritin, N-terminal proB-type natriuretic peptide (BNP), prolactin, prostate-specific antigen, etc. Example assays for small molecules encompass free triiodothyronine (T3), free thyroxine (T4), total T3, total T4, cortisol, 25 hydroxyvitamin D, etc. Beyond the above biomolecules and organic molecules, IAs have been extended for analysis of heavy metals, an emerging area, which is known as heavy metal immunoassays (https://www.ncbi.nlm.nih.gov/pubmed/17168306).
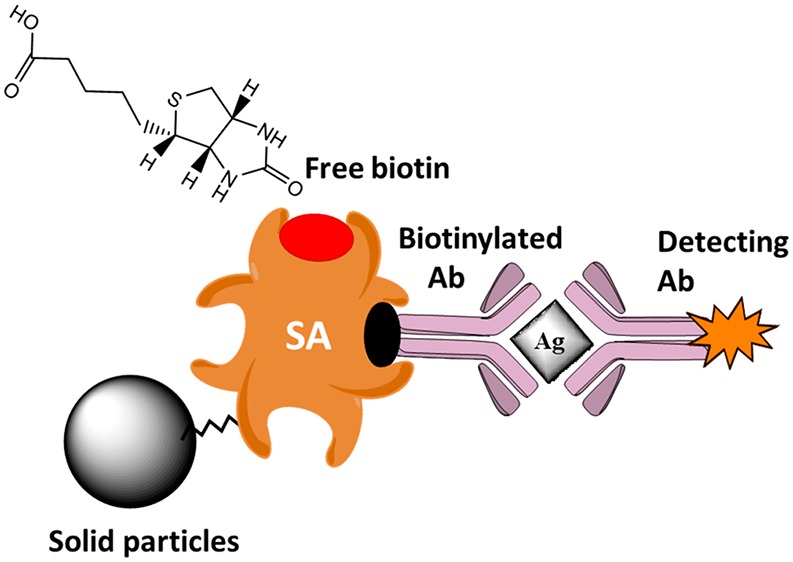
IAs involve the specific binding of an antibody to its antigen, i.e., the target analyte. There are two popular formats, known as “competitive” and “sandwich” IAs for small molecules and large compounds, respectively. In the competitive format, the antibody (Ab), defined as the capture Ab, is anchored on a solid substrate, e.g., magnetic beads. The target analyte if any in the assay sample will compete with the labeled analyte to diminish the signal response. The sandwich format involves a second Ab, i.e., detection Ab, which is labeled with an enzyme or molecule to provoke a signal response. For enhanced detection sensitivity and reproducibility, the capture Ab must be firmly attached to the solid surface with high loading, and biotin–SA chemistry fulfills these requirements.
Elevated biotin in the plasma due to its very high uptake interferes with diagnostic assays that use biotin–streptavidin technology for clinical analytes and biomarkers, e.g., thyroid-stimulating hormone, cardiac troponin, etc. Other clinical analyzers do not involve biotin–SA chemistry; i.e., they are not susceptible to the biotin interference (Table 1).
Table 1. Biotin Effect on the Performance of Some Representative Commercial Immunoassay Analyzersa.
| target analyte | biotin interference (+) | biotin interference (−) |
|---|---|---|
| pituitary ACTH pg/mL | sandwich IA, Roche Cobas e601 | |
| FSH mIU/mL | sandwich IA, OCD Vitros5600 | |
| LH mIU/mL | sandwich IA, OCD Vitros5600 | |
| prolactin ng/mL | sandwich IA, OCD Vitros5600 | |
| TSH mIU/mL | sandwich IA, OCD Vitros5600 | |
| TSH mIU/mL | sandwich IA, Beckman Coulter UniCel DXI 800 | |
| free T3 pg/mL | sandwich IA, OCD Vitros5600 | |
| free T4 ng/dL | sandwich IA, OCD Vitros5600 | |
| adrenal cortisol mg/dL | competitive IA, OCD Vitros5600 | |
| DHEA–sulfate mg/dL | competitive IA, OCD Vitros5600 | |
| total testosterone ng/dL | competitive IA, Immulite 2000 | |
| SHBG nmol/L (nM) | sandwich IA, Immulite 2000 |
Adrenocorticotropic hormone (ACTH; follicle-stimulating hormone (FHS); luteinizing hormone (LH); thyroid-stimulating hormone (TSH); triiodothyronine (T3); thyroxine (T4); dehydroepiandrosterone sulfate (DHEA); sex hormone-binding globulin (SHBG).
Biotin supplementation has drastically expanded over the last years as prescribed therapies and vitamin complex preparations for nonmedical purposes. Biotin is an essential vitamin and serves as a cofactor for five carboxylases that catalyze critical steps in the metabolism of fatty acids, glucose, and amino acids. Propionyl-coenzyme A (CoA) carboxylase, pyruvate carboxylase, and methylcrotonoyl-CoA carboxylase are located in mitochondria, whereas acetyl-CoA carboxylase is found in the cytoplasm. Other physiological roles of biotin encompass histone modifications, gene regulation (by modifying the activity of transcription factors), and cell signaling. Out of the eight different stereoisomers, only d-biotin (which is simply referred to as biotin) exhibits biological activity and is abundant in nature. Biotin (vitamin B7, vitamin H, or coenzyme R with MW 244.31 g/mol) binds SA irreversibly with a dissociation constant (Kd) in the order of ≈10–14 M, perhaps one of the strongest noncovalent interactions known in nature.1 The binding is very rapid, and the biotin–SA complex is unaffected by extremes of pH, temperature, organic solvents, and other denaturing agents. In immunoassays, streptavidin (pI = 6.8–7.5, MW 52.8 kDa), a tetramer protein purified from Streptomyces avidinii, is favored over avidin (pI = 10, MW 67 kDa, Kd ≈ 10–15 M), originally isolated from the hen egg white. This protein is glycosylated and positively charged with pseudocatalytic activity and nonspecific binding. NeutrAvidin (pI = 6.3, more neutral than native avidin, MW ∼ 60 Ka),1 deglycosylated avidin, is similar to streptavidin in terms of the size, pI (pI ∼ 5 of streptavidin), and nonspecific binding but still preserves the same biotin-binding properties because the carbohydrate plays no role in biotin binding. SA has four binding sites for biotin, which can be easily tagged to biomolecules without significantly altering the host biological activity. Avidin has a lower binding affinity than streptavidin when biotin is conjugated to another molecule, i.e., biotinylated molecule. Therefore, the interactions of SA and biotinylated molecules have been exploited in various applications including IAs to detect and target biological analytes in a complex sample matrix. Indeed, biotin–SA chemistry is the core of many commercial platforms such as the Beckman Coulter Access/DXI, Siemens Centaur, the Roche Elecsys; Immulite 2000; Ortho Clinical Diagnostics Vitros; and Dimension. In the USA, about 59% of the surveyed IAs are based on biotin–streptavidin chemistry.2 Almost all the commercial IAs developed by Roche and Beijing UniDiag Technology and Diasorin are based on biotin–SA interactions. Similarly, many IAs from Siemens and other IVD companies are also based on biotin–SA interactions. The biotin interference has effectuated in a large number of recalls and clinical reports available online from many healthcare laboratories and authorities during the last two years.
Like any analytical technique, IAs based on biotin–SA chemistry still lack specificity and accuracy stemming from the Ab binding property, the sample matrix, reagent components, and the IA format. In particular, the interference of biotin in the assay sample becomes very pronounced when the sample is introduced together with the capture Ab and the labeled analyte (competitive IA) or the labeled detection Ab (sandwich IA). This format is advocated for “high-throughput” assays, as it reduces the number of steps and costs. The concept of “load and go” has been promoted by several IA providers to process a few hundred samples per hour (40 000–80 000 tests per year) with a minimum of 40–60 samples onboard. The Abbott ARCHITECT i2000 SR can process up to 200 tests/h with a capacity of 135 samples (https://www.corelaboratory.abbott/int/en/offerings/brands/architect/architect-i2000SR). Doubtlessly, the sample capacity will be increased drastically with micro-/nanofabrication technology. In the past, the interference of biotin in IAs based on biotin–SA interactions was very rare considering the normal biotin in blood plasma was significantly below the threshold level that causes interference, and the general population was not consuming the high-dose biotin OTC supplements. This review will highlight the emerging threat of biotin in IAs, the physiological role of biotin, and plausible strategies to circumvent biotin interference.
2. Assay Formats and the Mechanism of Biotin Interference
Over the past 25 years, the SA–biotin interactions have played a significant role in the development of clinical IAs.3,4 Commercial IA systems, i.e., clinical analyzers, are fully automated, which contain slots for the storage of ancillaries and reagents in addition to mechanisms for loading of multiple patient samples. They employ a simple and rapid IA procedure for the high-throughput automated quantitative determination of analytes. In competitive IA, the sample is mixed with the biotinylated Ab, SA-coated magnetic particles (MPs), and the labeled analyte (competitor) in one or two IA process steps. Of interest is the labeling of the analyte with a ruthenium complex for electroluminescence detection (ECL) or with acridinium derivatives for chemiluminescence detection (CL). However, the ECL and CL immunoassays are still being developed based on conventional biotin–SA chemistry by many manufacturers including Beijing Unidiag Technology and Immunodiagnostic Systems. Roche is still using biotin–SA interactions for its commercial IAs. Siemens and Diasorin have already changed or are in the process of changing their IAs to biotin interference-free formats. The development of biotin interference-free formats would require a change in IA chemistry, intensive clinical validation, and regulatory approvals.
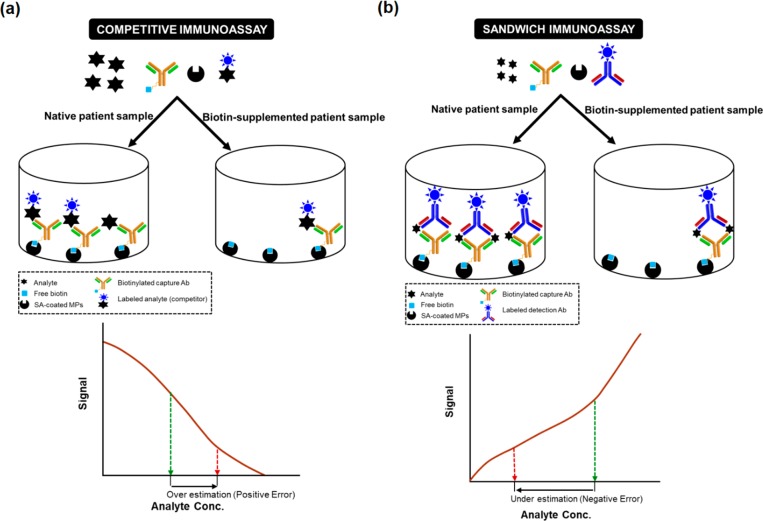
Both competitive and sandwich assays are used in immunoassays. If no analyte is present in the sample, the biotinylated Ab binds the labeled analyte and forms a stable complex with SA–MPs, thereby resulting in a strong signal. However, if the analyte is present, it will compete with the labeled analyte and bind to some of the biotinylated Ab that leads to a decreased signal. Therefore, the analyte concentration is inversely proportional to the signal intensity. The competitive IA format is very susceptible to excess biotin in the sample as the free biotin will compete with the biotinylated Ab for biotin-binding sites of SA. In a “sandwich” IA, excess biotin in the sample binds strongly to the SA–MP that prevents the formation of sandwich immune complexes, which result in falsely low results (Figure 1b).
Figure 1.
Mechanism of the biotin interference in a competitive (a) and sandwich IA (b). The excess biotin in the sample leads to an overestimation of analyte (positive error) in the case of a competitive IA and an underestimation (negative error) in a sandwich IA.
Therefore, as the biotin interference threshold of SA-biotin based IAs is low, excess biotin in the patient sample interferes with the IA when its concentration in the sample is higher than the reported biotin interference threshold of the IA. The erroneous analyte determination due to biotin interference is critical for the emergency department where it can lead to inappropriate medical intervention including surgery due to wrong clinical diagnosis.
In some IAs, where the biotin interference threshold is low, the low levels of biotin in the sample of 40.9 nM5 can interfere with the IAs. In contrast, in IAs with a high biotin interference threshold, the biotin interference is only observed at 818 nM of biotin.6 However, most of the IAs based on SA–biotin interactions have been unable to reach the biotin interference threshold of 1160 ng/mL (4748 nM), which is the maximum level reported so far in multiple sclerosis and inherited metabolic diseases with high biotin uptake, up to 300 mg of biotin/day. The discrepancy between the results obtained by biotin-based assays and nonbiotin-based assays is shown in Table 2. In some cases, the difference is very significant, e.g., a 315-fold difference for progesterone between the two different methods.7 A well-known case is the misdiagnosis of children with metabolic diseases subjected to high-dose biotin treatment. Due to biotin interference in immunoassays, such patients were misdiagnosed with Graves’ disease, as the clinical results indicated elevated levels of free thyroxine (T4) and total triiodothyronine (T3) together with low levels of thyrotropin and high levels of antithyrotropin receptor antibodies.8
Table 2. Some Selected Biotin-Based Assays That Are Highly Susceptible to Elevated Biotin.
| analyte | nonbiotin-based assay (Architect/Abbott) | biotin-based assay (Roche) | bias (+) positive/(− negative) |
|---|---|---|---|
| progesterone | 0.4 nM | 125.4 nM | (+) 315-fold increase |
| FT4 | 11.3 pM | >100 pM | (+) >8.8-fold increase |
| TSH | 1.30 mIU/L | 0.02 mIU/L | (−) 65-fold decrease |
| FSH | 8.5 IU/L | 0.4 IU/L | (−) 21.3-fold decrease |
3. Uptake and Pharmacokinetics of Biotin
Biotin is a water-soluble B-complex vitamin that is essential for the metabolism of proteins, fats, carbohydrates, and glucose as it is an essential coenzyme for several carboxylases. It occurs in every living cell mainly bound to proteins or polypeptides but is abundant in the liver, kidney, and pancreas. The recommended daily requirement for biotin intake is 30 μg, as recommended by the US Department of Agriculture. However, there is no need to take biotin OTC supplements as biotin is abundant in common foods such as egg yolk, whole cereals, soybeans, avocado, cauliflower, leafy greens, pork, liver, etc. The normal dietary intake of biotin in western populations is 35–70 μg/day. A higher dose of biotin is used to treat patients with biotinidase deficiency (5–10 mg/day), holocarboxylase synthetase deficiency (30–40 mg/day), biotin-thiamine-responsive basal ganglia disease (100–300 mg/day), and multiple sclerosis (MS) (300 mg/day).9,10 Biotin is absorbed from the gastrointestinal tract and reaches its peak level in the blood after about 2 h of oral intake. Another study (biotin dose of 75, 150, and 300 μg) confirmed the peak level occurred after 2 h of ingestion, fell between 2 and 4 h, and was only slightly higher than predosing levels after 24 h.11 Only a limited amount of catabolism of biotin occurs, whereas excess biotin is discharged in urine.
Biotin is nonmutagenic, nontoxic, and 100% bioavailable as biotin absorption and excretion are rapid. Therefore, it is one of the topmost selling nutritional supplements worldwide. As the plasma half-life of biotin is 110 min, biotin should be cleared from the circulation within five half-lives, i.e., about 9 h. However, the washout period of biotin is impacted by impaired renal function.
The biotin concentration in serum or plasma of healthy subjects with a normal dietary intake of biotin is less than 2.05 nM (0.5 ng/mL), compared to 4748 nM (1160 ng/mL), the peak level of biotin that has been observed after an intake of a biotin megadose of 300 mg in multiple sclerosis patients. The reported value of plasma biotin in the literature only reflects the concentration of free biotin in plasma. Indeed, biotin binds proteins reversibly; ∼12% of total biotin in plasma is covalently bound; 7% is reversibly bound; and 81% is free biotin (https://www.ncbi.nlm.nih.gov/pubmed/1636621).
Biotin doses of 5 mg, 10 mg, and 20 mg typically result in biotin peak levels of 168 nM (41 ng/mL), 372 nM (91 ng/mL), and 753 nM (184 ng/mL), respectively. Peak biotin levels vary between patients, depending on renal function, age, sex, and frequency of dosing. Serum biotin concentrations reach a plateau after a few days of constant dose ingestion. Subjects with four uptakes per day of 20 mg of biotin will have their biotin level fall below 40.9 nM after 73 h. As most of the first generation of IAs from IVD giants had a lower biotin threshold of about 30 nM, the biotin levels above this concentration have been shown to provoke noticeable interference in such IAs.12
4. Strategies for the Prevention of Biotin Interference
High-dose biotin OTC supplements have been consumed by the general population for various nonmedical reasons. This is a serious health concern as such overconsumption of biotin could lead to potential errors in analyte determination that could lead to the wrong clinical diagnosis, undesirable medical interventions, and adverse events. Therefore, there is a need to implement effective strategies to obviate biotin interference in clinical IAs.
4.1. Biotin Prebound to Streptavidin
At first glance, IAs based on the SA–biotin pair are thought to be susceptible to interference from excess biotin in blood samples. This truism might be valid if the sample is introduced together with the required soluble reagents. The SA-biotinylated Ab interaction enables the separation of the immune complex from the reaction. Therefore, any substance that interferes with this process will affect both IA formats. Excess biotin in the blood sample interferes with this process, thus causing erroneous laboratory results as mentioned earlier. Biotin-based assays can be easily designed with biotinylated antibody prebound to SA before the sample is introduced, so they are not vulnerable to biotin interference.13 This expectation stems from the formation of a highly stable SA-biotinylated Ab complex, which cannot be dissociated by free biotin. Of course, this protocol requires a longer analysis time and increases the costs associated with testing. However, the reagent manufacturers will be obliged to redesign the current format with soluble biotinylated reagents to mitigate the risk of biotin interference. The SA and biotinylated reagents must be combined during the manufacturing process.14 All IA methods based on biotinylated reagents and biotin-binding proteins must be tested and proved to be free from biotin interference. The Siemens ADVIA Centaur TnI-Ultra assays (cardiac troponin) exhibited a greater than 10% change in results in samples with biotin levels up to 10 ng/mL (41 nM) (https://publications.jrc.ec.europa.eu/repository/bitstream/JRC105914/kjna28571enn2.pdf). The company has been improving the assay procedure to circumvent the biotin interference up to 1000 ng/mL or 4093 nM. A typical example is the “Intact PTH Assay on the ADVIA Centaur CP, XP, and XPT Systems” for parathyroid hormone (PTH), the most important endocrine regulator of circulating calcium and phosphorus concentrations. This system is claimed to be free from biotin interference up to 1000 ng/mL (https://static.healthcare.siemens.com/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/groups/public/@global/@clinicalspec/documents/download/mda2/njgz/~edisp/advia_centaur_intact_pth_assay_a91dx-cai-161031-xc1-4a00_final-03665267.pdf). However, a large number of other IAs from Siemens are also affected by biotin interference, such as dehydroepiandrosterone, cyclosporine, HBc IgM, Hepatitis A total Ab, Hepatitis B core IgM, total testosterone, estradiol, hepatitis B surface antigen, Free T3, Free T4, TSH, progesterone, sex hormone-binding globulin, thyroglobulin, troponin I, vitamin B12, NT-proB-type natriuretic peptide, latex allergen, wheat allergen, creatine kinase MB, etc. However, it is a formidable task to overcome the FDA-desired high biotin interference threshold of 3510 ng/mL (14 367 nM) as stated in the CLSI guideline EP37 first edition for interference testing in clinical chemistry. This high biotin threshold deserves a brief discussion here, as it has been implemented for patient safety. This level is indeed 3 times higher than 1170 ng/mL, the highest physiological biotin concentration observed in patients with high dose biotin uptake. The new CLSI guideline is being strictly followed and referred to by FDA and healthcare authorities. Therefore, this requirement is a new challenge for immunoassay platforms based on biotin–SA chemistry. Thus, the manufacturers have devoted serious efforts and put effective strategies in place to fulfill this requirement. Moreover, it is an essential requirement for global clinics and hospitals. The IVD manufacturers have been approached by hospitals to issue customer notifications on the biotin interference. To date, none of the IVD manufacturers with commercial IAs based on biotin–SA interactions have been able to achieve the desired high biotin interference threshold of 3510 ng/mL, as mentioned in the recent CLSI guidelines. Accordingly, several IAs have already been shifted to biotin-interference-free formats in hospital and clinic settings to ensure patient safety.
From a practical and economic viewpoint, it could be unwise or even mistaken to switch platforms every time an issue of interference like biotin arises. This solution is cost-prohibitive, especially for small clinics and hospitals, and the new platform might not solve the issue of mitigating all interferences or provoke a new interference as blood and blood plasma contain various compounds and unknown drug metabolites.
4.2. Removal of Biotin
Another obvious solution is the removal of biotin from the specimen by passing the sample through a small column, prepacked with SA-bound insoluble beads or polymers, e.g., SA–agarose beads. The removal of biotin using SA–MPs is an alternative. The major advantage of magnetic MPs in IAs is ease of separation of free biotin in the sample by adsorption to SA–MPs. MPs can be prepared in any wet chemistry laboratories using well-established procedures, and they are also commercially available. They are made of nanometric-sized iron oxide particles decorated with polymers with diameters ranging from 35 nm up to 4.5 μm with the component MPs from 5 to 50 nm. They exhibit a unique quality referred to as superparamagnetism in the presence of an external magnetic field.
Magnetic beads can be coated with a carboxylated polymer or stearic acid to facilitate bioconjugation. The amino groups of biomolecules are covalently coupled to the COOH group on the surface of magnetic particles by well-known carbodiimide chemistry. Roche’s SA–MPs have a mean diameter of 1 μm with a density of 1.1–1.4 g/cm3. According to the supplier, SA is coupled to carboxyl groups on the surface of superparamagnetic polystyrene particles via a linker molecule. The binding capacity of these particles for free biotin is 1.8 nmol/mg but considerably lower for biotin-labeled oligonucleotide (0.150 nmol/mg) and only 10 pmol/mg for biotin-labeled dsDNA fragments. The adsorption kinetics depends upon the initial biotin in blood/blood plasma, which varies from specimen to specimen. The kinetics of biotin removal also depends on the quantity of streptavidin-coated beads, the average diameter of beads, the density of SA on beads, and the available binding sites for biotin. To date, there is no sufficient kinetic data on this subject, and the quoted incubation time is a conservative number. This pretreatment might be up to a 1 h incubation of the sample with the SA–MPs.15 Streptavidin–biotin affinity interaction is instantaneous as indicated from the work of Schrapp et al.16 in the assay of high-sensitivity cardiac troponin T. A complete suppression of biotin interference is achieved following mixing the sample and streptavidin-coated microparticles without incubation, ranging from 0 to 60 min. It is technically feasible to remove biotin within a very short time, but the cost is also a deciding factor because SA is very expensive. Furthermore, the centrifugation step of 10 min is still required to provide the biotin-free supernatant for subsequent analysis. This requirement might be problematic as the IA systems are designed to provide the first result within 30 min and cannot accommodate the centrifugation step. Carboxyl magnetic beads with free carboxyl groups can also be prepared and even commercially available (https://www.raybiotech.com/carboxyl-magnetic-beads/). These beads are ready to couple with proteins/Ab and other ligands using various simple procedures reported in the literature. Such beads, a ferric oxide core functionalized with various silane groups, are nonporous with an average diameter of ∼0.5 μm.
A novel approach being tried by Roche is the use of a proprietary monoclonal Ab against free biotin for the scavenging of free biotin in the patient sample.17 The developed Ab is specific only to the free biotin and does not bind to the biotin–Ab conjugate. The effort to remove biotin from the specimen by Roche is not surprising because Roche has utilized biotin in almost all its assays. However, this novel approach is expected to incur high costs and could not obviate completely the biotin interference from the sample. In this context, synthetic biotin-binding polymers were prepared by molecular imprinting based on the polymerization of methacrylic acid and ethylene glycol dimethacrylate in the presence of biotin methyl ester.17 The binding of biotin methyl ester by the imprinted was demonstrated; however, the assay was performed in acetonitrile or chloroform.
As mentioned earlier, the typical biotin concentration in serum or plasma is less than 21 nM, but the patients with a mega-dose of 300 mg might have the peak biotin concentration above 4834 nM in their blood. Thus, this upper limit must be used to tailor the quantity of SA–MPs and the adsorption kinetics of biotin. Plausible nonspecific adsorption of the target analyte on SA–MPs must be tested and established accordingly. The extra steps in IAs will increase the costs associated with testing and the potential financial burden of high-dose biotin on the health care system. The Clinical Laboratory Standards Institute (CLSI) has recommended that testing for biotin interference is now performed by manufacturers at biotin concentrations up to 3510 ng/mL (14 367 nM). However, this step is difficult to implement in an automated clinical analyzer and must be performed externally. Most IA methods in clinical settings use specimens without any pretreatment and are run on fully automated, continuous, high-throughput, and random-access systems. The IA manufacturers might need to redesign their assay procedures and hardware to accommodate the addition of biotin to the assay sample.
4.3. Platform without the Biotin–Streptavidin Pair
It is quite possible to eliminate the biotin–SA pair and chemistry in IAs by employing functionalized MPs. The capture Ab can be directly bound covalently to the surface of MPs via different chemistries. It is very unlikely that biotin will bind to such MPs. Such functionalized MPs are commercially available. An alternative is commercial polystyrene-coated paramagnetic particles that are simply activated by 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide and N-hydroxy-2,5-pyrrolidinedione. Functionalized MPs then serve as a versatile platform for bioconjugation with a pertinent Ab. An example is Abbott ARCHITECT assays that are based on direct Ab-coated MPs without employing any biotin–SA interactions. Thus, the assay is free from biotin interference (https://www.corelaboratory.abbott/us/en/offerings/assays/biotin-laboratorians.html). Currently, all assays marketed by Abbott Laboratories do not use biotin in the assay design. Of course, a new technology requires intensive development efforts, extensive validation, a change in manufacturing procedures, and new regulatory approvals due to the involved design changes.
4.4. Sample Treatment
Nonlinearity during dilution is an indication of biotin interference. However, serial dilution may not eliminate biotin interference. This common practice is also limited by the concentrations of the target analyte and biotin in the assay sample, i.e., the detection limit of the assay platform. As an example, the concentration of cardiac troponin I (cTnI) in blood plasma ranges from 11 to 79 ng/L, whereas biotin can be up to 1160 ng/mL (1.16 mg/L).19 Some biotin metabolites do not bind to biotin-binding sites of SA. At least two of them are identified as tetranorbiotin and tetranorbiotin d,l-sulfoxide.20 They are originated from oxidation, sulfur oxidation, or a combination. The treatment is a subject of future endeavors as it requires intensive efforts and resources. The treatment must be performed under mild conditions, and enzymatic oxidation is a distinct possibility. There will be high extra costs associated with the pertinent enzymes and technical difficulties to implement this step into automated IA systems. It is unknown whether the procedure will change the analyte in the sample or impact the analytical performance of IAs.
4.5. Biotin Washout
Biotin has a circulating half-life of 1.5–2 h, and it should be cleared from the circulation within 5 half-lives. However, the clearance period is also dependent upon doses and the frequency of uptake.21 Based on the half-life of biotin in human beings, a washout of 8–10 h is considered sufficient to mitigate the risk of biotin interference in subjects taking up to 5 mg of biotin twice a day or 10 mg of biotin once a day. The washout period ensures the biotin level falls back to within the physiological levels (<21 nM), which causes no interference with the IAs. Biotin exhibits linear pharmacokinetics over the range of doses studied (5–20 mg). It is rapidly absorbed with a serum half-life of 15 h and only reaches a steady state after 3 days.21 Several other studies unravel a washout period up to 73 h for multiple sclerosis patients on 300 mg/day biotin doses. Therefore, there is insufficient evidence to support recommendations for safe testing in patients, taking high levels of biotin on a daily basis.
5. Detection of Biotin
Considering the potential interference of biotin in IAs and its sample-dependent concentration, it is desirable to screen biotin together with other target analytes in IAs. As biotin is a small molecule, it is detected by competitive IA. The commercial ELISA kit (mdbioscienes.com) for biotin is based on the competitive binding between biotin and peroxidase-labeled biotin (biotinylated peroxidase) for the biotin-binding sites of avidin, obtained from microorganisms. The literature unravels that dimeric members of the avidin family are found in some bacteria, whereas avidin isolated from the egg whites is a tetramer. The commercial ELISA detects biotin (∼400 ng/L or 1.64 nM) in blood, blood plasma, and urine samples. The biotin level in urine is about 30–40 times higher than the respective serum concentration. Similarly, there are other commercial ELISA test kits employing SA–biotin chemistry.
In addition to manual ELISA, biotin can also be detected via an automated ECL- or CL-based IA via competitive IA, where biotin can be labeled with a ruthenium complex or an acridinium ester. The ECL-based IAs are employed by IVD companies such as Roche and UniDiag, whereas CL-based immunoassays are being used by other IVD players such as Siemens, DiaSorin, Abbott, and Immunodiagnostic Systems.
Miniaturized biosensors with high detection sensitivity, low cost, and multiplexing have the potential to detect biotin in blood. Similarly, lateral flow IA formats and POC tests such as those based on electrochemical sensing would also be highly prospective for the rapid detection of biotin at the point of need. Electroanalysis with low cost, ease of fabrication/miniaturization, and high detection sensitivity might be useful for the detection of biotin in blood. However, the development in this area is very limited with a few literature reports related to electroanalysis/electrocatalysis of biotin. Nevertheless, it is feasible to oxidize biotin by boron-doped diamond as electrode materials22 and the use of captavidin with low affinity to streptavidin toward the development of a reusable sensor for biotin in electrochemistry.23
6. Conclusions and Outlooks
Supraphysiologic biotin intake (10–40 mg daily) in the form of supplements will not be diminished any time soon due to its nontoxicity and availability. In some cases, the patient might consume 300 mg of biotin daily (https://www.endocrine-abstracts.org/ea/0063/ea0063p1173.htm). Biotin sales increased 58% from $219,599,798 in 2014 to $349,101,078 in 2018, and this figure does not include the sales through online retailers. Biotin is formulated in multivitamins, prenatal vitamins, and supplements for hair, skin, and nails. In the USA, about 17% of patients surveyed consume biotin-containing supplements (https://betastatic.fishersci.com/content/dam/fishersci/en_US/documents/programs/healthcare/technical-documents/data-sheets/abbott-biotin-fact-data-sheet.pdf), whereas 7.7% of the 1944 Mayo Clinic surveyed patients took biotin. The market of biotin has been covered rather extensively in the literature (corelaboratory.abbott/us/en/offerings/assays/biotin-laboratorians.html; transparencymarketresearch.com/biotin-market.html; and brandessenceresearch.biz/Chemicals-and-Materials/Biotin-Supplements-Market-Research-on-Global-Analysis-to-2025/Summary).
Thus, the interference of high-dose biotin on IAs in the clinical laboratory is an emerging issue. Biotin interference could lead to serious clinical consequences, such as the misdiagnosis of diseases and surgical interventions. The revised guideline from the Clinical and Laboratory Standards Institute in April 2018, i.e., EP 37 first Edition, mentions the biotin interference threshold testing at 3510 ng/mL (14 367 nM), which is difficult to achieve by any IA developer unless they obviate the biotin–SA interactions.
The simplest and most effective practice must come from the patients and their cooperation. Unless the subjects are prescribed biotin for medical reasons, they should discontinue several days before providing their blood samples for clinical testing. The biotin washout period differs widely among IAs, the dose, and frequency of biotin uptake as well as the patient’s renal function. Patients should stop the intake of biotin supplementation at least 48 h before the test, in agreement with the guidelines issued by many health settings including the Mayo Clinic. However, in patients on high-dose biotin therapy, the clearance period could be up to 73 h or even longer, which corroborates with the findings on multiple sclerosis patients that take a very high biotin dose of 300 mg/day. Clearance also takes longer in patients with poor renal function and high doses of biotin uptake, so the washout period can be several days or weeks for thyroid function tests (TFTs) or months in other cases. For patients with long-term biotin uptake, a washout period of 12 weeks is required for hormone testing except TSH using immunoassays based on biotin–SA chemistry.24
Of course, this scenario is not applicable to emergency department patients who might have taken a high dose of biotin. The ordering physician should consult local laboratory staff to understand that the test menu may be affected by biotin, the magnitude, and the direction of interference (falsely negative or positive). In such cases, the test must be done by an IA that does not have any biotin interference. Lab-based procedures including HPLC-MS can process the samples from MS patients and for patients with other inherited metabolic diseases. Miniaturization of HPLC columns has gained significant progress with the column diameter decreased from 4.6 mm to 7.5 μm. Several clinical and hospital settings have deployed reliable miniaturized LC systems for small-molecule drugs and metabolites, and this technology can be easily adapted for analysis of biotin in emergency situations.
The IA protocols using soluble SA and biotinylated Ab are very susceptible to elevated biotin in the specimen, and such protocols require a major overhaul. The simplest solution for the IAs based on SA–biotin chemistry is to “prebind” reagents to saturate biotin-binding sites of streptavidin before sample addition. This modified protocol should be resistant to biotin interference, but it is still challenging to meet the biotin interference threshold of more than 3510 ng/mL (14 626 nM), as stated in the recent CLSI guidelines for interference testing in clinical IAs. Thorough investigation and validation are still needed to address this issue, which is an essential clinical requirement but a huge burden for IA equipment manufacturers.
The removal of biotin in the specimen by SA–MPs should be effective, but its implementation will add some extra costs and technical issues. The sample volume in automated clinical immunoassays is about 50 μL, which must accommodate the presence of MPs with very high biotin-binding capacity. The incubation time for the removal of biotin is at least 1 h, whereas high-throughput commercial IA systems are designed to provide the first result within 30 min. Nonspecific adsorption of target analytes on functionalized MPs is hard to predict and must be subject to extensive validation for each target analyte. Nonspecific binding of the target analyte and other endogenous species with SA-coated carriers might diminish both detection reproducibility and sensitivity. This step must be validated using the IAs that do not employ biotin–SA chemistry. The use of functionalized MPs for the direct binding of Ab, as employed in IAs by Abbott, makes the IA completely free from biotin interference.
Interference by biotin metabolites deserves a brief discussion here since some of them also bind biotin-binding sites of SA with high efficiency compared to biotin.18 They are bisnorbiotin methyl ketone (81.4%), bisnorbiotin (61.2%), biotin sulfone (33.2%), and bisnorbiotin d,l-sulfoxide (3.8%). Two other known metabolites do not bind SA:tetranorbiotin d,l-sulfoxide (0.3%) and tetranorbiotin (N/D). In urine, biotin only accounts for 32 ± 12%, compared to 52 ± 15% for bisnorbiotin, 7.9 ± 6 5.8% for bisnorbiotin methyl ketone, 4.0 ± 3.2% for biotin-d,l-sulfoxide, and 3.6 ± 1.9% for biotin sulfone. In biotin interference studies, blood plasma is simply spiked by pure biotin, and the interference of its metabolites has not been investigated. This is not trivial considering biotin accounts for only half of the total avidin-binding substances in human serum.25 Perhaps, this is a subject of future endeavors, which requires extensive investigation and resources. In this context, the manufacturers of IVDs might have shifted the developments of all new assays to biotin-free formats.
A prospective strategy is the development of a fully automated IA for the quantitative determination of free biotin in the patient sample in the range of 5–3510 ng/mL (20.5–14 367 nM). The determination of biotin in the sample would rule out the possibility of wrong clinical diagnosis due to biotin interference, as it will allow the healthcare professionals to choose the most appropriate IAs for the determination of clinical analytes. Albeit biotin concentration in serum can be accurately measured using ultra-HPLC (UHPLC) combined with tandem MS (UHPLC-MS/MS), this lab-based method is not suitable for processing a very large number of public blood samples.
The recent public health safety alerts issued by the FDA and many international authorities have triggered the healthcare authorities and industries to take the necessary measures to tackle this critical global concern. There is no easy solution to overcome biotin interference in IAs to satisfy the new regulatory guidelines. Some key players in the IA business have tried different solutions to mitigate the risk of biotin interference. Further, there is an extensive need for more communication among laboratory personnel, health care providers, and patients. The patients must be advised to stop biotin uptake for several days before blood testing. Laboratory personnel must be trained to process samples with plausible elevated biotin by selecting the right analytical tools. Lastly, health care providers must be aware of the severe biotin interference in several IAs. The FDA is alerting the public, health care providers, lab personnel, and lab test developers that biotin can significantly interfere with certain lab tests and cause incorrect test results which may go undetected. There is also considerable public awareness bulletins and newsletters being issued to the general public and additional stringent norms by the healthcare and hospital authorities in all developed countries. The biotin interference is a big issue for the IA manufacturers and has become an emerging theme of several clinical congresses/events including AACC (American Association of Clinical Chemistry).
IAs for blood are frequently encountered with various interfering species, which cause false-positive and false-negative results from endogenous and exogenous compounds. In general, they can cross-react with IA reagents and skew IA results. Elevated biotin in the blood is an emerging interferent for the IAs based on SA–biotin interaction. Biotin–SA interactions are used by more than 50% of the clinical laboratories worldwide. However, the current regulatory and IVD trend is strongly inclined toward the development of biotin-interference free IAs and obviates the use of biotin–SA interactions. The identification of biomarkers in urine is a subject of future endeavors. Without homeostasis mechanisms, their presence in urine can be used for early diagnosis, prevention, treatment, and prognosis of diseases. The biotin concentration in urine is 30–40-fold higher than the respective plasma concentration; therefore, biotin-based assays are very unlikely to be considered as the prime platform for this special task.
Biographies

John H. T. Luong is associated with University College Cork, School of Chemistry, Cork, Ireland. He has been credited with over 350 published papers, over 20,000 citations, 10 US patents, and 4 books. His current interests include nanotechnology, biosensor technology, immunoassays, POCT, sepatation technology, nanomaterials, and biomaterials.

Sandeep K. Vashist is the Global IVD Director at Pictor Diagnostics and has worked for several large IVD companies in the senior management role. He has developed many CE and FDA approved clinical immunoassays and pioneered several IVD/POCT technologies and devices. He has over 250 manuscripts in journals and conferences, 8 patents, and 3 books. His current interests include IVD, immunoassays, POCT, smart technologies, and multiplex detection.
The authors declare no competing financial interest.
References
- https://www.thermofisher.com/order/catalog/product/31000, accessed December 11, 2019.
- Holmes E. W.; Samarasinghe S.; Emanuele A.; Meah F. Biotin interference in clinical immunoassay: a cause for concern. Arch. Pathol. Lab. Med. 2017, 141, 1459–1460. 10.5858/arpa.2017-0107-LE. [DOI] [PubMed] [Google Scholar]
- Wilchek M.; Bayer E. A.. Avidin-biotin mediated immunoassays: overview. In Avidin-Biotin Technology: Methods Enzymology; Wilchek M., Bayer E. A., Eds.; Academic Press: New York, 1990; Vol. 184, pp 467–469. [PubMed] [Google Scholar]
- Avery G. Biotin Interference in Immunoassay: A review for the laboratory scientist. Ann. Clin. Biochem. 2019, 56, 424–430. 10.1177/0004563219842231. [DOI] [PubMed] [Google Scholar]
- NACB Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin b12, Pantothenic Acid, Biotin, and Choline. www.nap.edu/catalog/6015.html (accessed 17 June 2018). [PubMed]
- Samarasinghe S.; Meah F.; Singh V.; Basit A.; Emanuele N.; Emanuele M. A.; Mazhari A.; Holmes E. W. Biotin interference with routine clinical immunoassays: understand the causes and mitigate the risks. Endocr. Pract. 2017, 23, 989–998. 10.4158/EP171761.RA. [DOI] [PubMed] [Google Scholar]
- Dasgupta A.Effect of biotin on clinical laboratory test results: how to avoid such interferences. In Biotin and Other Interferences in Immunoassays: A Concise Guide, 1st ed.; Dasgupta A., Ed.; Elsevier BV: Amsterdam, Netherlands, 2019; pp 51–73. [Google Scholar]
- Kummer S.; Hermsen D.; Distelmaier F. Biotin treatment mimicking Graves’ disease. N. Engl. J. Med. 2016, 375, 704–706. 10.1056/NEJMc1602096. [DOI] [PubMed] [Google Scholar]
- Trambas C.; Lu Z.; Yen T.; Sikaris K. Characterization of the scope and magnitude of biotin interference in susceptible Roche Elecsys competitive and sandwich immunoassays. Ann. Clin. Biochem. 2018, 55, 205–215. 10.1177/0004563217701777. [DOI] [PubMed] [Google Scholar]
- Piketty M.; Polak M.; Flechtner I.; Gonzales-Briceno L.; Souberbielle J. C. False biochemical diagnosis of hyperthyroidism in streptavidin-biotin based immunoassays: the problem of biotin intake and related interferences. Clin. Chem. Lab. Med. 2017, 55, 780–788. 10.1515/cclm-2016-0606. [DOI] [PubMed] [Google Scholar]
- Clevidence B. A.; Marshall M. W.; Canary J. J. Biotin levels in plasma and urine of healthy adults consuming physiological doses of biotin. Nutr. Res. (N. Y., NY, U. S.) 1988, 8, 1109–1118. 10.1016/S0271-5317(88)80112-X. [DOI] [Google Scholar]
- Katzman B. M.; Lueke A. J.; Donato L. J.; Jaffe A. S.; Baumann N. A. Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin. Biochem. 2018, 60, 11–16. 10.1016/j.clinbiochem.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Kirkwood J.Meeting the biotin challenge. Am. Ass. Clin. Chem. 2018. https://www.aacc.org/publications/cln/articles/2018/janfeb/meeting-the-biotin-challenge. Published January 1, 2018 (Accessed September 09 and Nov. 27, 2019). [Google Scholar]
- Siemens Healthcare Diagnostics I. Customer Bulletin 078D1052–01 Rev. A. Siemens Advia Centaur XP: Availability of an Enhanced Intact PTH (iPTH) Assay (US), 2011.
- McEnroe R. J.; Dimeski G.; Miller W. G.; Sonntag O.. Supplementary tables for interference testing in clinical chemistry, 1st ed. CLSI EP37; Clinical Laboratory Standards Institute: Wayne, PA, 2017. [Google Scholar]
- Schrapp A.; Fraissinet F.; Hervouet C.; Girot H.; Brunel V. Biotin and high-sensitivity cardiac troponin T assay. Biochem. Med. 2018, 28, 030901. 10.11613/BM.2018.030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerg M.; Heindl D.; Hillringhaus L.; Hirzel K.; Hojer C. D.; Huber F.; Josel H.-P.; Meier T.; Schraeml M.; Voss E.. Novel biotin-specific monoclonal antibody and use thereof. WO2018122205A2, 2018.
- Leary T. P.; Gutierrez R. A.; Muerhoff A. S.; Birkenmeyer L. G.; Desai S. M.; Dawson G. J. A chemiluminescent, magnetic particle-based immunoassay for the detection of hepatitis C virus core antigen in human serum or plasma. J. Med. Virol. 2006, 78, 1436–1440. 10.1002/jmv.20716. [DOI] [PubMed] [Google Scholar]
- Venge P.; Johnston N.; Lindahl B.; James S. Normal plasma levels of cardiac troponin I measured by the high-sensitivity cardiac troponin I access prototype assay and the impact on the diagnosis of myocardial ischemia. J. Am. Coll. Cardiol. 2009, 54, 1165–1172. 10.1016/j.jacc.2009.05.051. [DOI] [PubMed] [Google Scholar]
- Zempleni J.; Mock D. M. Advanced analysis of biotin metabolites in body fluids allows a more accurate measurement of biotin bioavailability and metabolism in humans. J. Nutr. 1999, 129, 494S–497S. 10.1093/jn/129.2.494S. [DOI] [PubMed] [Google Scholar]
- Grimsey P.; Frey N.; Bendig G.; Zitzler J.; Lorenz O.; Kasapic D.; Zaugg C. E Population pharmacokinetics of exogenous biotin and the relationship between biotin serum levels and in vitro immunoassay interference. Int. J. Pharmacokinet. 2017, 2, 247–256. 10.4155/ipk-2017-0013. [DOI] [Google Scholar]
- Buzid A.; McGlacken G. P.; Glennon J. D.; Luong J. H. T. Electrochemical sensing of biotin using nafion modified boron-doped diamond electrode. ACS Omega 2018, 3, 7776–7782. 10.1021/acsomega.8b01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzid A.; Hayes P. E.; Glennon J. D.; Luong J. H. T. Captavidin as a regenerable biorecognition element on boron-doped diamond for biotin sensing. Anal. Chim. Acta 2019, 1059, 42–48. 10.1016/j.aca.2019.01.058. [DOI] [PubMed] [Google Scholar]
- Stieglitz H. M.; Korpi-Steiner N.; Katzman B.; Mersereau J. E.; Styner M. Suspected testosterone-producing tumor in a patient taking biotin supplements. J. Endo. Soc. 2018, 2, 563–569. 10.1210/js.2018-00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock D. M.; Lankford G. L.; Mock N. I. Biotin accounts for only half of the total avidin-binding substances in human serum. J. Nutr. 1995, 125, 941–946. [DOI] [PubMed] [Google Scholar]