Abstract
The electrochemical interface is an ultrathin interfacial region between the electrode and solution where electrochemical reactions occur. The study of the electrochemical interface continues to be one of the most exciting directions in modern electrochemistry research. Much of our existing knowledge about the electrochemical interface comes from ensemble measurements and ex situ imaging of the electrode surface. Due to its enormous complexity and highly dynamic nature, however, new imaging tools that can probe the interface in situ with ultrahigh spatial and temporal resolution and single-molecule sensitivity are apparently needed. Single-molecule fluorescence microscopy (SMFM) has emerged as a powerful tool that is uniquely suited for studying the electrochemical interface. In this mini-review, we first give a brief overview of various existing SMFM methods for studying electrochemical problems. We then discuss several exciting research topics involving the use of SMFM methods for studying surface-immobilized molecules, single freely diffusing molecules, single molecules as catalytic reaction indicators, and single-molecule labeling and imaging of interfacial nanobubbles. We anticipate that we will continue to see a rapid increase in publications on stochastic electrochemistry of single molecules and nanoparticles. The increased use of SMFM will likely bring new information to our study of the electrochemical interface.
1. Introduction
As a basic science for studying electrical charge and chemical reactions, electrochemistry has numerous applications in diverse research fields, including fuel cells, batteries, (bio)chemical sensing, metal corrosion, organic synthesis, and so on. The electrochemical interface, where redox reactions take place, plays an essential role in nearly all aspects of electrochemistry. Rapid advances in electron microscopy make it possible to image surface features of a solid electrode down to nanometers or even atomic scale under proper conditions, e.g., ultrahigh vacuum, clean electrode, etc. However, how the surface heterogeneity of an electrode may affect its electrochemical property remains largely unclear for numerous Faradaic processes. As such, developing a more complete understanding of the electrochemical interface is pivotal to the advancement of modern electrochemistry. Understanding the structure and function relationship can help design new and more efficient electrochemical systems and materials.1,2
It is challenging to probe the electrochemical interface at high spatial and temporal resolution when the electrode is used in actual reaction conditions, e.g., ambient and fluid environment. Scanning electrochemical microscopy (SECM) is an effective imaging tool to characterize the spatial heterogeneity of the electrode surface.3 Using a nanoelectrode probe, SECM can achieve very high spatial resolution down to ∼10 nm. Despite its high spatial resolution, however, SECM could be limited in its temporal resolution due to the need to scan the probe tip across the surface at a finite speed. For example, it may take several seconds to minutes to acquire an SECM image if nanometer resolution is desired. Other challenges may include the fast and inevitable surface contamination and electrode fouling when a nanoelectrode probe is exposed to an electrolyte solution. Thus, it is demanding to develop new imaging methods, which can allow one to obtain both real-time and high-resolution information at the interface.
Among various imaging methods, single-molecule fluorescence microscopy (SMFM) provides a number of unique features.4 It enables real-time probing of the electrochemical interface with both high temporal resolution and high detection sensitivity (single molecules!).5 Using super-resolution microscopy, one can achieve both millisecond temporal resolution and nanometer spatial resolution in electrochemical imaging. Moreover, optical microscopy allows one to monitor electrochemical processes under ambient conditions.6 Single photons can be detected with EMCCD (electron multiplying charge-coupled device) and sCMOS (scientific complementary metal-oxide-semiconductor) camera devices.7 Considering a single fluorescent molecule can generate 105–107 photons in its lifetime,8 SMFM can have exceedingly high sensitivity and has been increasingly used as a unique analytical technique to probe the electrochemical interface with applications ranging from studying basic electron-transfer kinetics and imaging catalytic heterogeneities to studying interfacial nanobubble generation.
In this mini-review, we will focus on the electrochemical application of SMFM. We will discuss the advantages and challenges of various SMFM methods, including confocal laser scanning microscopy, total internal reflection microscopy, optical confinement, and super-resolution microscopy. We will then summarize research topics in four areas: (1) electrode surface-immobilized single molecules; (2) single freely diffusing molecules; (3) single molecules as catalytic reaction indicators; and (4) single-molecule labeling and imaging of interfacial nanobubbles.
2. Single-Molecule Fluorescence Microscopy
2.1. Basics
Single-molecule fluorescence microscopy is based on conventional fluorescence microscopy with its own unique characteristics. A key factor of SMFM is its ability to distinguish and resolve single fluorescent molecules from the background signal. Traditional epi-fluorescence microscopy illuminates the whole sample volume at the same time without selection, yielding a higher fluorescence background. Therefore, it could be challenging to observe single fluorescent molecules with epi-fluorescence. Some of the most common strategies for detecting single molecules, especially in the electrochemistry systems, involve minimizing the illumination/sampling volume. The emission from the target fluorophores can be detected more easily with reduced background.4,9 Two major approaches can be adapted for achieving higher signal/noise ratio in the optical microscopy: (1) selectively collecting the emission from the molecules on the focal plane, such as confocal microscopy, and (2) selectively exciting molecules on the focal plane, such as total-internal reflection (TIRF) microscopy. Alternatively, one can reduce the background signal by creating a spatially confined structure, allowing molecules of interest to be detected.
2.2. Confocal Microscopy
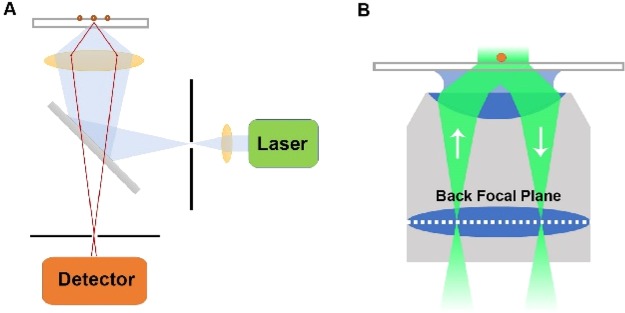
Confocal laser scanning microscopy (or confocal microscopy) is a widely used far-field approach in SMFM and is one of the first methods used for imaging single molecules both immobilized and freely diffusing in solution.9 Confocal, which means “having the same foci”, indicates the use of two conjugated pinholes (light source pinhole and detection pinhole, 50–200 μm (in diameter)) in the same image plane. The light source pinhole is placed at a focused position of the excitation laser as a spatial filter that only permits the main lobe of the light. The detection pinhole on the detection side is used to reject the out-of-focus stray light. The resulting “in-focus” volume is only in femtoliter scale, and the background noise can thus be greatly reduced, enabling detection of single molecules (Figure 1A).
Figure 1.
Schematic illustration of confocal microscopy (A) and total-internal reflection fluorescence microscopy (B).
Confocal microscopy has been used in several reports for studying electron-transfer kinetics of single immobilized molecules, which are among the first few examples of probing the electrochemical interface with SMFM. The method has also been used to detect electrochemically generated molecules on the electrode surface. Despite its high sensitivity, however, the scanning mechanism limits its temporal resolution when a large sample is imaged. Moreover, the spatial resolution of the confocal microscopy is still limited by the diffraction limit of conventional light microscopy.
2.3. TIRFM
TIRF microscopy remains as one of the most useful imaging methods for SMFM.9 The key concept of TIRFM is based on illuminating the sample with an ultrathin evanescent field (<200 nm) on the interface generated from the total internal reflection phenomenon. When light is incident on an interface from a medium with a higher refractive index (e.g., glass, 1.5, n1) into another medium with a lower refractive index (e.g., water, 1.33, n2) with an incident angle greater than a certain angle (critical angle, θ = arcsin(n2/n1)), no transmission light can be seen, and the incident light will be totally reflected, i.e., the total internal reflection phenomenon. The electromagnetic field extends across the interface into the medium with a lower refractive index with its intensity exponentially decaying with distance from the interface. The extended electromagnetic field, called the “evanescent field”, can be used to selectively illuminate molecules near the electrode/solution interface, allowing detection of single molecules.
Although TIRFM can be achieved with both prism-based and objective-based setups, objective-based TIRFM (Figure 1B) has been used more frequently for single-molecule studies. Since no scanning is needed, the entire field of view can be imaged simultaneously at a high acquisition speed. Combined with the super-resolution localization techniques, the spatial resolution of TIRFM can be as high as ∼10 nm. All those unique features allow TIRFM to image the electrochemical interface in real time with high spatiotemporal resolution. It is now possible to correlate morphological and structural information obtained from electron microscopy with the electrochemical properties from optical microscopy and reveal the heterogeneity of the electrode surface.10−12
2.4. Nanoconfinement
The confinement method in this context refers to physically limiting the sample volume by creating an extremely small and finite space to exclude irrelevant fluorescent molecules for enhanced signal-to-noise ratio. Thus, compared to confocal microscopy and TIRFM, this method relies more on the unique geometry of the detecting structure than the microscope. Zero-mode waveguides13 and bipolar nanocells14 are two excellent examples of nanoconfinement for probing single molecules generated at the electrochemical interface. At least two dimensions of the confinement device must be within the submicron scale, which could be challenging to fabricate. A simple device that could meet various demands has not been developed.
2.5. Super-Resolution Microscopy
Super-resolution microscopy is a powerful optical approach that circumvents the diffraction limit of conventional optical microscopy to achieve nanometer resolution. Several super-resolution microscopy methods have been developed for imaging ultrafine biological features; these include near-field scanning optical microscopy (NSOM), stimulated emission depletion microscopy (STED), photoactivated localization microscopy (PALM), stochastic optical reconstruction microscopy (STORM), and structured illumination microscopy (SIM).6 In this mini-review, we specifically focus on the use of single-molecule localization microscopy in electrochemistry related research.
A key concept of the approach is that an isolated fluorescent emitter can be precisely located (down to 1 nm precision) by finding the center of the point-spread function, and the precision is mainly dependent on the number of photons collected by the CCD detector. The point-spread function of the fluorescent puncta can be easily obtained through a simple 2D Gaussian fitting of the measured photon signal.15 It could be challenging to use the principle to image complex biological structures, and activation/deactivation (PLAM or STORM) of the fluorophores is required to reduce the number of fluorophores (temperately) to single emitter levels.6 On the other hand, single fluorophore molecules are seen as discrete puncta on an electrochemical interface, which perfectly meet the single emitter requirement. This allows one to locate the molecules with high precision and to image surface heterogeneity at high spatial resolution.5
3. Surface Immobilization
Although SMFM offers some unique opportunities for fluorescent labeling, imaging, and analyzing individual redox molecules, the freely diffusing small molecules can often diffuse too rapidly in solution, making the single-molecule detection very challenging. Therefore, immobilization of single molecules on the electrode surface is a useful method. This section discusses three excellent examples of immobilizing single small molecules onto the electrode surface with polymer embedding, covalent modification, and clay adsorption methods. The use of single redox proteins and polymers is also discussed.
3.1. Single Small Molecules
A redox chromophore 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) has been used to probe the interfacial charge transfer events.18 BODIPY can have temporary fluorescence losses as a result of discrete electron transfer events. Electron injection from BODIPY into to the electrode can lead to a nonfluorescent “off” state, and the fluorescence can be regained by charge recombination. The observation of “on” and “off” events represents single ET events. The average time duration can be analyzed by fitting the distribution with a single exponential decay function, and the rate of electron transfer of single molecules can be determined. In Pan’s work, BODIPY with polystyrene was spin-casted on prepared substrates. The purpose of this inert polystyrene film was to reserve the stability of individual BODIPY molecules. The fluorescence of BODIPY was turned “off” upon electrochemical oxidation, and observations of difference in fluorescence stability and intensity were explained by different charge transfer activities from the chromophore to metal oxide surface. Electron transfer rates were also calculated for further understanding on distinct observations of BODIPY on different substrates.
Zhang et al. studied electrochemical responses of covalently bonded single methylene blue molecules on an electrode.17 Methylene blue (MB), a blue-colored fluorescent dye, can be reduced to a nonfluorescent colorless dye, leuco-methylene blue, by a two-electron process. Unlike BODIPY which is highly fluorescent, MB has a relatively low quantum yield (∼4%). It thus requires an additional fluorescence enhancement for single-molecule detection. They demonstrated that by using gold nanorods (AuNRs) along with longitudinal surface plasmon resonance MB fluorescence could be significantly enhanced (Figure 2A). This 1000-fold enhancement is the result of the overlap between the emission spectrum of MB and the plasmon spectrum. MB concentration was low to allow only one molecule in the electromagnetic near field of a AuNR. Under varied redox potential, fluorescence blinking of MB detections was observed with a confocal microscope, suggesting the dynamic equilibrium of the redox reaction. They also used the Nernst equation as a model to determine the midpoint potential (E0) and found that E0 was higher than that in ensemble-averaged measurement (Figure 2B).
Figure 2.
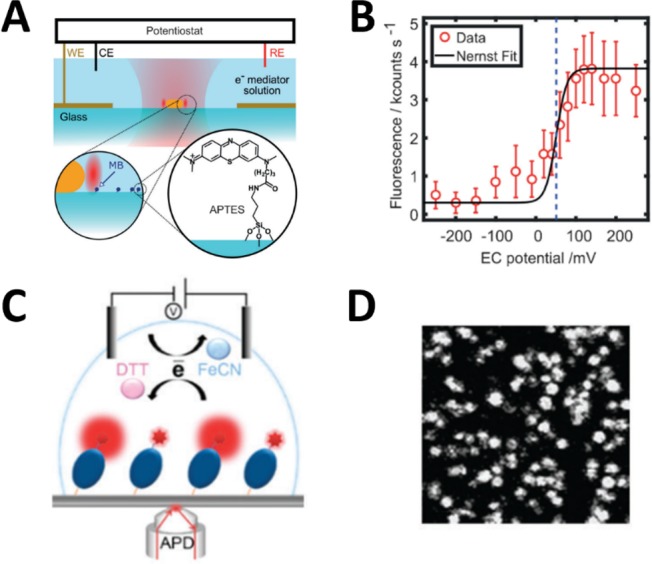
Detection of surface-immobilized single molecules on an electrode. (A) Schematic illustration of the combined electrochemical–confocal setup with immobilized AuNRs (not to scale) and MB molecules on the modified glass surface. (B) Ensemble fluorescence response of around 260 unenhanced MB molecules versus electrochemical potential. The black curve is Nernst fit, and the dashed line indicates the obtained midpoint potential. (A), (B) Reproduced from Zhang, W.; Caldarola, M.; Pradhan, B.; Orrit, M. Angew. Chem. Int. Ed. 2017, 56, 3566–3569 (ref (17)). Copyright 2017 Wiley-VCH. (C) Schematic illustration of the detection of single azurin-Cy5 molecules immobilized on a modified glass surface using confocal microscopy. (D) A confocal fluorescence image of immobilized azurin-Cy5 molecules on glass. (C), (D) Reproduced from Akkilic, N.; Van Der Grient, F.; Kamran, M.; Sanghamitra, N. J. M. Chem. Commun. 2014, 50, 14523–14526 (ref (16)). Copyright 2014 Royal Society of Chemistry.
Sodium montmorillonite (SM), a member in the smectite group of clays, is a good binding material for single molecules because of its high chemical stability and high surface area.19 SM colloid, the dispersions of nanoscale SM particles in water, was deposited on an ITO coverslip, followed by the formation of a thin transparent film. This transparent clay-modified surface not only allowed single-molecule immobilization but also enabled electrochemical measurements coupled with optical detection. The Ackerman group used this clay-modified ITO electrode to study single redox events of cresyl violet: a nonfluorescent cresyl violet can be oxidized to a fluorescent cresyl violet+ by a one-electron oxidation process. The fluorescent and nonfluorescent responses were observed and correlated to cyclic voltammetry scans, corresponding to the oxidized and reduced states of cresyl violet. Intensity fluctuations and blinking represented for the intermittency of the interfacial electron-transfer processes modulated by a potential scan. Meanwhile, the duration time of “on” and “off” states could be used to determine electron-transfer rates of reduction and oxidation, which was comparable to their ensemble-averaged measurements.
3.2. Single Redox Proteins
SMFM can also be applied to larger molecules, such as proteins. A notable mechanism called Förster (fluorescence) resonance energy transfer (FRET) is widely used in studying single proteins. FRET, developed by Theodor Förster, is a process in which an excited donor transfers energy to an acceptor via a nonradiative pathway.20 The Aartsma group developed a FRET-based method to probe the immobilized redox proteins on a gold surface. Probing a fluorophore near the metal surface can be challenging because both the enhancement and quenching effect may be present from the fluorophore–metal interaction. This interaction is highly dependent on distance, and its true effect can be quite complicated to interpret. The Aartsma group focused on the short distance regime (<2 nm), where fluorescence enhancement and quenching coexisted and used azurin, a 14K Da redox protein, in their study.21 Originated from Pseudomonas aeruginosa, azurin is a blue copper protein with a single copper atom (nonfluorescent site) in the center. The oxidized state of azurin shows an intense absorption at ∼600 nm, while it is absent in the reduced state. Voltage can be applied to manipulate the redox state of individual azurin molecules (Figure 2C,D).16 Their FRET-based method was used in an electrochemical cell: after immobilization on a glass substrate, the redox state of azurin was regulated by the reducing and oxidizing reagents, with varying concentrations of the reagents through altering electrode potential. Fluorescence–time traces were obtained with confocal microscopy at different redox potentials, which show discrete “on” and “off” behavior. The authors studied reaction kinetics and thermodynamics by examining fluorescence traces of individual azurin molecules.
Akkilic et al. further developed their method into a direct voltage control experiment such that the redox state of azurin was regulated by varying the chemical potential of the surrounding buffer solution (100 mM K3(PO4) at pH 7.0). Azurin, tagged with an organic fluorophore Cy5, was immobilized on a transparent gold electrode.22 By utilizing the FRET mechanism, fluorescence response was “off” in the oxidized states (Cu2+) because the absorption band of azurin (excited donor) overlaps with the emission band of Cy5 (fluorophore acceptor), leading to fluorescence quenching, whereas fluorescence response was recovered in the reduced state (Cu+) caused by the absence of the absorption band. After surface immobilization on a gold-coated slide, the authors performed optical recordings on single Cy5-labeled azurins while scanning the electrode potential. This allowed them to observe “on” and “off” fluorescence behaviors in real time at varying potentials. This study demonstrated thermodynamic dispersion in midpoint potentials of single azurin molecules.
3.3. Single Redox Polymers
Larger molecules and organic polymers could also be examined at a single-molecule level. Barbara, Bard, and co-workers studied the oxidation of polymer poly(9,9-dioctylfluorene-co-benzothiadiazole) (F8BT) on an ITO electrode.23 F8BT, generally used in solar cells, is intrinsically fluorescent. Individual F8BT molecules were dispersed in a polystyrene film deposited onto an ITO surface. Their oxidation was induced by a potential scan on the ITO, resulting in a nonfluorescent state. Aggregation of F8BT was suppressed by the use of the polystyrene layer. Using a wide-field TIRF microscopy, fluorescence traces were recorded in correspondence with the applied potential. Their results statistically revealed the dynamics of oxidation and reduction events of single large polymer molecules.
4. Freely Diffusing Single Molecules
Developing imaging strategies for the study of single freely diffusing molecules could allow us to correlate molecular motion and collision with heterogeneous electron transfer. The ability to directly probe single freely diffusing redox molecules on an electrode may also enable the development of ultrasensitive electroanalytical sensors. This section will discuss several interesting approaches, which are categorized by detection methods.
4.1. Confocal Microscopy
Single molecules diffuse in and out of the laser excitation volume rapidly and randomly.9 A fluorescent burst would be recorded as a fluorophore enters the excitation volume in a confocal recording. The Ackerman group employed a conventional three-electrode cell to monitor the redox behavior of cresyl violet in aqueous solutions using confocal microscopy coupled with potential scan.25 A low cresyl violet concentration (1.2 nM) was used to ensure that less than one molecule on average was in the laser focal volume at a given moment, so single-molecule detection could be achieved. Nonfluorescent cresyl violet could be oxidized to enter in a fluorescence state, and changes of fluorescence intensity were recorded during a potential scan. A strong correlation between fluorescence intensity and potential was illustrated for the reversible redox reaction of single cresyl violet molecules.
Godin et al. reported the usage of confocal microscopy to probe single fluorogenic molecules in organic solvents.26 A redox-active and antioxidant fluorogenic molecule H2B–PMHC was developed by Cosa and co-workers. The fluorescence of this molecule can be activated by direct electrochemical oxidation of H2B–PMHC or electroreduction of O2 to produce superoxide radical anion O2•–, which could initiate chemical oxidation of H2B–PMHC in the presence of O2. Both pathways were confirmed by an observation of the increased fluorescence intensity along with the potential scan.
4.2. TIRFM
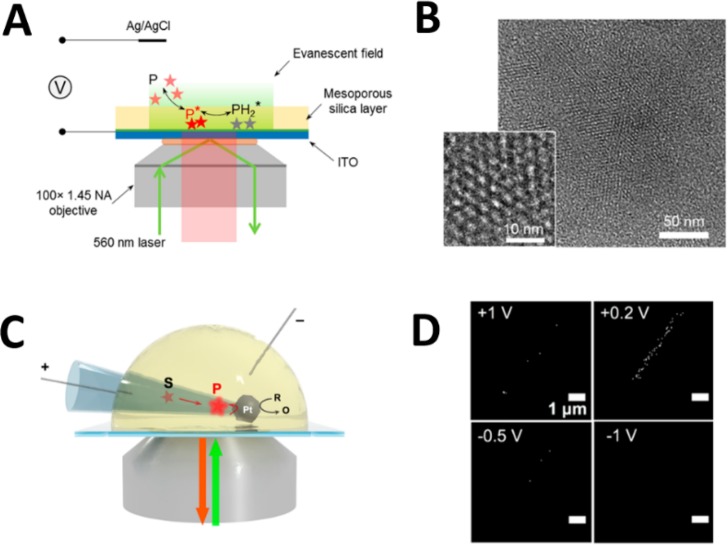
TIRF microscopy is another elegant technique to reduce fluorescence background using the evanescent wave. Only molecules within ∼200 nm above a transparent electrode can be excited. To detect freely diffusing excited molecules, however, their motions must be restricted near the electrode surface. Our group used a modified ITO electrode to confine motions of single molecules in solutions, allowing us to explore single redox events using TIRF microscopy.24 An ultrathin mesoporous silica film (∼70 nm in thickness) containing highly ordered parallel nanochannels (∼3 nm in diameter), was electrochemically deposited onto a transparent ITO electrode (Figure 3A,B). Such narrow channels allowed access of redox molecules while restricting their diffusional motions. Only when single molecules were transiently trapped in a nanochannel could they be fluorescently detected.
Figure 3.
Detections of single freely diffusing molecules at the electrochemical interface. (A) Schematic illustration of SME on a mesoporous silica-modified ITO electrode using TIRF microscopy. Single resorufin molecules are detected when they are temporally trapped in small silica nanochannels. (B) TEM image of mesoporous silica film (top view) deposited on a carbon-coated TEM grid. Inset: high-magnification top view. (A), (B) Reproduced from Lu, J.; Fan, Y.; Howard, M. D.; Vaughan, J. C.; Zhang, B. J. Am. Chem. Soc. 2017, 139, 2964–2971 (ref (24)). Copyright 2017 American Chemical Society. (C) Schematic illustration of the TIRFM setup to image single resorufin molecules in a Pt nanocell. Resazurin reduction at the inner Pt surface is coupled to FcMeOH oxidation at the outer Pt surface. (D) A series of fluorescence images of the nanocell at different voltages. (C), (D) Reproduced from Fan, Y.; Hao, R.; Han, C.; Zhang, B. Anal. Chem. 2018, 90, 13837–13841 (ref (14)). Copyright 2018 American Chemical Society.
Herein, adsorption, desorption, and redox dynamics of transiently immobilized molecules could be studied on one electrode platform. It is known that weakly fluorescent resazurin (S) can be irreversibly reduced to form strongly fluorescent resorufin (P), which can be further reduced reversibly to nonfluorescent dihydroresorufin (PH2). First, to examine the fluorescence response during a potential scan, a high concentration (e.g., μM) of resazurin was used on a carbon film electrode. A nice correlation was observed between the fluorophore’s optical response and its Faradaic current response, confirming that resorufin can be used for single-molecule electrochemistry study using fluorescence microscopy. The reversible reaction of P to PH2 was chosen for subsequent study owing to its distinct on–off responses. On the silica-modified ITO electrode, single resorufin molecules could be easily detected, yielding a burst of fluorescent trajectory. The residence time (τon) was defined as the time of a single fluorescent burst lasted in a fluorescence trace. There were three pathways that contributed to the length of the “on state”: photobleaching, blinking, and desorption. It was demonstrated that only desorption contributed significantly to the transition from the “on state” to the “off state”. In this case, adsorption and desorption kinetics could be extracted from fitting the distribution of τon using a single-exponential decay function.
Spectroelectrochemical studies of single adsorbed resorufin molecules were also performed. Predicted from the Nernst equation, P would be reduced to the nonfluorescent PH2 with decreased applied potential, and expectedly, it was found that the number of detected fluorescent spots was strongly dependent on potential. A triangular potential waveform, 0 to −1 V, was applied at 50 mV/s, and the results illustrated a reversible fluorescence response with potential scan, as expected from the reversible redox reaction between P and PH2. Furthermore, dN/dt was defined as the time derivative of the detected number of single P molecules to study the Faradaic current response during the redox process, and it was evidenced that dN/dt was linearly correlated to the current signal. The peak values [dN/dt] were found to be proportional to the scan rate, implying surface-controlled electrochemical kinetics.
4.3. Nanoconfinement
Our group has recently developed a diffusion-confined device, a Pt nanocell, to study freely diffusing single redox molecules in solutions.14 To make a nanocell, a Pt nanoparticle was deposited at the orifice of a laser-pulled nanopipette.27 We originally employed this nanocell to study transient collision and oxidation of single silver nanoparticles.28 In the SMFM application, similarly, using an ∼100 nm diameter tip, diffusional motions were constrained, and the probability of transient molecular adsorption on pipet walls was increased (Figure 3C,D). Those adsorbed single molecules, herein, can be more easily detected when they collide and react on the Pt nanoparticle electrode, which functions as a closed bipolar nanoelectrode.
In this study, FcMeOH oxidation and resazurin reduction were coupled on the Pt nanoelectrode. Fluorescence intensity traces were monitored with potential scanning across Pt from +2 V to −2 V and then back to +2 V. By scanning to a negative direction from +2 V, nonfluorescent resazurin started to be reduced to fluorescent resorufin at onset potential of +0.9 V and then reached a fluorescence emission maximum at +0.2 V. Fewer resorufin molecules were detected at more negative potentials due to the occurrence of the second reduction from resorufin to nonfluorescent dihydroresorufin. Also, individual resorufin molecules were observed to be randomly distributed inside the Pt nanocell. Counting individual resorufin molecules at various concentrations and redox potentials offered new insights on detection efficiency. This study could be an excellent demonstration for a future single-molecule, single-site catalytic study in a bipolar electrochemical cell.
Electrochemically active zero-mode waveguides (E-ZMWs) were developed and used by the Bohn group to study single molecules owing to their small volumes (zeptoliters) and excellent optical confinement.13 Their recent work used a fluorogenic redox-active molecule flavin mononucleotide (FMN). FMN exhibits a strong fluorescent emission in the oxidized state, while it is nonfluorescent in the reduced state. E-ZMWs are featured for their recessed dual ring electrode (RDRE). The small volume in each nanowell isolated individual molecules and provided an effective optical confinement, enabling a simultaneous measurement of the optical and electrochemical signal. This confined zeptoliter-volume near the bottom electrode was excited. A freely diffusing FMN molecule entering the nanowell can be repeatedly reduced and oxidized at RDRE, leading to an observation of the molecule’s on–off fluorescence signal, allowing single electron-transfer events to be observed.
5. Single Molecules as Catalytic Reaction Indicators
Nanomaterials are key catalytic materials in electrocatalysis and photoelectrochemistry. To better use such materials, their catalytic property must be thoroughly investigated at both high spatial and high temporal resolution, considering their surface and structural heterogeneity. With SMFM, one can directly image the reactants and/or products of a catalytic reaction one molecule at a time and locate the surface reaction hot spots with nanometer precision. Such information can be used to further understand the structure–property relationship of the catalysts, which could be beneficial for developing more efficient catalysts.
5.1. Electrocatalysis on Individual Single-Walled Carbon Nanotubes
Single-walled carbon nanotubes (SWNTs) attract tremendous attention and are widely used in electrochemistry and electrocatalysis owing to their good conductivity, chemical stability, and catalytic activity.29 Due to their structural heterogeneity, catalytic property on a single nanotube can vary at different locations. The Chen group reported the use of wide-field SMFM to study electrocatalysis on SWNTs with single-reaction resolution. The aforementioned two-step electro-reduction of the nonfluorescent molecule, resazurin, was used in their study. To study electrocatalysis of SWNTs at a single-molecule level, they deposited individual SWNTs onto an ITO working electrode. Under a constant reductive potential, fluorescence bursts were observed due to electrochemical reduction of resazurin to resorufin. The fact that reactions occurred at discrete locations instead of on the entire SWNTs was visually confirmed. They later discussed rate constants for possible corresponding reduction and oxidation processes, and the results suggested that interfacial electron-transfer kinetics between SWNTs and adsorbed molecules are dependent on applied potential.
5.2. Photoelectrocatalysis on Single TiO2 Nanorods
By utilizing wide-field SMFM coupled with the super-resolution technique, the Chen group recently reported an innovative work for optimization of the catalyst-modified photoanode for the water-splitting process.11 Due to the limited efficiency of water splitting of photogenerated holes, oxygen evolution catalysts (OECs) are necessarily modified to achieve higher photocatalytic efficiency. Chen and co-workers mapped both hole- and electron-based active sites, with 30 nm and 15 ms spatial and temporal resolutions, respectively, of water splitting on single TiO2 nanorods with selective laser irradiation.
To achieve their goal, the authors used a microfluidic photoelectrochemical cell, with rutile TiO2 nanorods deposited on an ITO electrode. A solution with two fluorogenic subtractrates, photogenerated hole- and photogenerated electron-induced amplex red (AR) and resazurin, respectively, was loaded and flowed through the cell. When the applied potential was higher than −0.3 V, hole-induced reaction occurred nonuniformly on individual nanorods. Specifically, most reactions appeared at the “hot spots”, sites with relatively high hole activity. A similar phenomenon was observed for applied potential less than −0.4 V, where electron-induced reactions took place nonhomogeneously. Both observations suggested the heterogeneous distribution of the active sites, where surface structural defects were more active than the {100} facet sites. Notably, hole-induced activity was strongly correlated to electron-induced activity spatially.
5.3. Probing Nonfluorescent Reactions via Competition
Chen and co-workers proposed a new method called the competition-enabled imaging technique with super-resolution (COMPEITS) to study nonfluorescent reactions.12 COMPEITS could be described as a competition process of two catalytic reactions at the same particle: one is a fluorescent auxiliary reaction, and the other is a nonfluorescent target reaction. The occurrence of the nonfluorogenic reaction would suppress the fluorogenic reaction, resulting in change of fluorescence intensity. Chen and co-workers used bismuth vanadate (BiVO4) as the photocatalyst for target oxidation of hydroquinone (HQ), generating nonfluorescent quinone. The auxiliary fluorogenic oxidation of AR, on the other hand, would generate fluorescent resorufin molecules. They first demonstrated the competitive inhibition behavior of HQ and AR at the same catalyst surface sites, where at a fixed AR concentration and increased quinone concentration, the rate of quinone formation increased, while the formation rate of resorufin decreased.
To visualize these competitive behaviors, BiVO4 particles were deposited on an ITO electrode in a microfluidic photoelectrochemical cell. BiVO4 particles were tunable with L and a shape parameter ξ (= S/L). AR and HQ were oxidized on the surface of BiVO4 followed by a wide-field laser (405 nm) illumination at BiVO4 to generate charge carriers. Super-resolution fluorescence images of resorufin were taken with an excitation laser (532 nm). Quantitative super-resolution images confirmed that detected resorufin decreased with increasing HQ concentration. Moreover, HQ binding affinity could be determined from detected resorufin, and it was found that HQ had stronger adsorption on the basal {010} facet than the lateral {110} facet of BiVO4 particles. The rates were represented using KHQ{010} and KHQ, with KHQ{010} greater than KHQ. Moreover, it was found that both KHQ{010} and KHQ were negatively proportional to L at fixed ξ. Also, the two adsorption equilibrium constants decreased with increasing ξ. The authors further studied two kinds of edge regions, type I and type II, where type I edges were featured for the surrounding basal and lateral facets while type II edges only focused on lateral effects. The results demonstrated that KHQ was greater for type I edges on average. The particle morphology also affected HQ adsorption equilibrium, and to further examine its ability, ωHQ was used to define the particle’s overall capability of adsorbing HQ on its entire surface at the per-unit-mass level. Overall, ωHQ depended on three types of particle shape. For particles within an intermediate size regime (∼2.3 μm < L < ∼9 μm), ωHQ showed a biphasic behavior, offering insights for designing optimal photocatalysts.
6. Single-Molecule Imaging of Nanobubbles
In water electrolysis, hydrogen and oxygen gases are generated on the cathode and anode, respectively. Insulating gas bubbles generated from these reactions can cover the electrode surface, causing electrode deactivation. Understanding the bubble nucleation and growth therefore may provide valuable information for designing new electrochemical interfaces for improved energy efficiency. At their nucleation stage, interfacial bubbles are small (nanobubble), transparent, and elusive, making it challenging to image.
Ohl and co-workers used a TIRFM-based fluorescence method to study surface bubbles generated by a solvent exchange method. The affinity of the dye molecules to the gas/water interface and the ultrathin evanescent field illumination enabled bubble visualization on the interface.30 Due to the diffraction limit, however, nanobubbles smaller than ∼230 nm in diameter (lateral dimension) could not be resolved. The Wang group used a similar method to monitor photoelectrochemically generated bubbles on single nanocatalysts.31
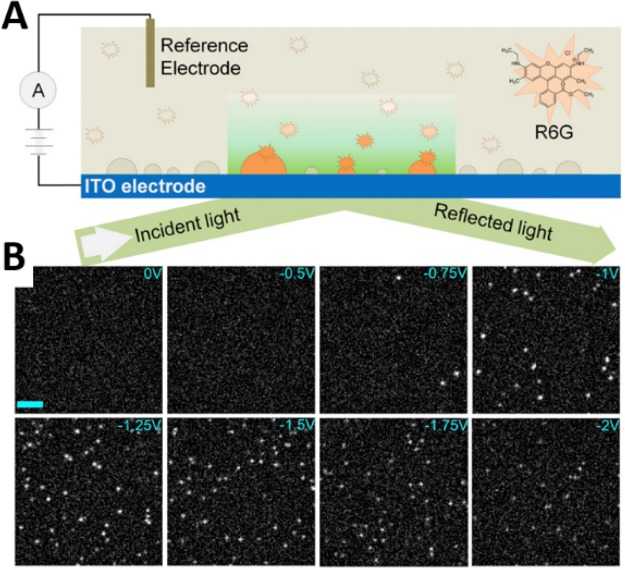
Our group used single fluorophore molecules to label and detect nanobubbles electrochemically generated on an electrode.10 By optimizing the fluorophore concentration, single-molecule occupancy of the nanobubble was achieved. The single-molecule occupancy on the bubble can also be confirmed by the abrupt on/off behavior of the fluorescent puncta. The nanobubble detection was performed with a potential scan from 0 to −2 V vs Pt QRE on an ITO electrode using TIRFM, with rhodamine 6G (R6G) as the labeling molecule (Figure 4A). As seen in Figure 4B, initially, there were no R6G molecules detected due to the absence of nanobubbles. At intermediate potentials, several molecules can be seen, which had the highest fluorescence intensities indicating formation of very small nanobubbles. The number of fluorescence spots increased when the potential was further decreased, indicating more nanobubbles were generated at higher negative potentials. The detection puncta can be located via super-resolution localization. Our results show that nanobubbles could appear 500 mV prior to reaching the thermodynamic potential for water reduction. The intensity of each labeling R6G molecule can also be extracted, from which one can estimate the nanobubble size (assuming a hemispherical shape). The negative correlation between the puncta intensity and the applied potential indicated the growth of the nanobubble at more negative potentials.
Figure 4.
Single-molecule imaging of electrochemically generated nanobubbles. (A) Schematic illustration of rhodamine 6G-labeled H2 nanobubbles at an ITO electrode surface in the TIRFM setup. (B) A series of TIRF images taken from a potential scan from 0 V to −2.0 V at 100 mV/s vs a Pt QRE in water containing 1 M Na2SO4 and 10 nM R6G (scale bar, 5 μm). Reproduced from Hao, R.; Fan, Y.; Howard, M. D.; Vaughan, J. C.; Zhang, B. Proc. Natl. Acad. Sci. 2018, 115, 5878–5883 (ref (10)). Copyright 2018 the US National Academy of Sciences.
Furthermore, we used the single-molecule labeling method to probe the catalytic activity of single gold nanoplates supported on an ITO electrode. The electrode potential was scanned from +0.5 to −1.8 V vs Pt QRE to reduce protons to molecular hydrogen. Nanobubbles were observed on both ITO and gold at more positive potentials. When the potential reached ∼−1.6 V, however, significantly more nanobubbles were generated on the gold and its surrounding area. Interestingly, the areas around the gold also had more nanobubble detections, which was likely due to the “hydrogen spillover” effect: the electrochemically generated hydrogen atoms can rapidly diffuse from the gold catalyst to the surrounding area where they form H2 molecules.
7. Perspective
Single-molecule fluorescence microscopy has ultrahigh spatial and temporal resolution and single-molecule sensitivity, making it uniquely suitable for probing the electrochemical interface under in situ ambient conditions. Recent years have seen numerous exciting research applications of SMFM including fundamental single-molecule electrochemistry, photoelectrocatalysis, and imaging of single nanobubbles. Despite these exciting progresses, there are several challenges to be addressed. For example, the fast motion of small fluorophore molecules and the strong quenching effect of the electrode surface make it difficult to observe single freely diffusing redox molecules near the electrode. A second challenge is the somewhat limited number of suitable redox active fluorophores, whose fluorescence property can undergo a drastic change upon oxidation or reduction on the electrode. A few excellent attempts have been made toward probing nonfluorescent reactions, including Chen’s COMPEITS method and single-molecule labeling for nanobubble detection. However, it is still quite challenging to find a more general method to probe many other redox processes. Fluorescence-enabled electrochemical microscopy (FEEM),27,32,33 a method proposed by our group, can be used to couple a conventional redox reaction, e.g., oxidation of ferrocene or dopamine, with a fluorogenic reaction on a bipolar electrode, enabling one to use fluorescence to study any redox reactions. The combination of FEEM and SMFM may thus be a more general approach for studying the electrochemical interface with single-molecule sensitivity and nanometer spatial resolution.
Acknowledgments
This work was supported by the National Science Foundation (CHE-1904426).
Biographies

Rui Hao is an Assistant Professor in the Chemistry Department at Southern University of Science and Technology (SUSTech) of China. He received his B.S. in Chemistry from Tsinghua University and Ph.D. in Materials Science from Peking University. He worked as a postdoctoral associate in Bo Zhang’s lab prior to joining SUSTech in September, 2019. His research interests include developing new electrochemical and microscopic techniques for studying single molecules and nanobubbles.

Zhuoyu Peng received her B.S. in chemistry from University of Michigan in 2015. She is currently a Ph.D. student in Professor Bo Zhang’s lab at the University of Washington and is investigating the formation of single nanobubbles at the electrochemical interface using single-molecule, super-resolution fluorescence microscopy.

Bo Zhang is a Professor of Chemistry at the University of Washington. He finished his Ph.D. training with Henry White at the University of Utah in 2006 followed by a postdoctoral work with Andrew Ewing at Penn State before joining the UW in 2008. His research interest includes the development and use of new electrochemical and microscopy tools to better understand the electrode/solution interface.
The authors declare no competing financial interest.
References
- Oja S. M.; Wood M.; Zhang B. Nanoscale Electrochemistry. Anal. Chem. 2013, 85, 473–486. 10.1021/ac3031702. [DOI] [PubMed] [Google Scholar]
- Oja S. M.; Fan Y.; Armstrong C. M.; Defnet P.; Zhang B. Nanoscale Electrochemistry Revisited. Anal. Chem. 2016, 88, 414–430. 10.1021/acs.analchem.5b04542. [DOI] [PubMed] [Google Scholar]
- Liang Y.; Pfisterer J. H. K.; McLaughlin D.; Csoklich C.; Seidl L.; Bandarenka A. S.; Schneider O. Electrochemical Scanning Probe Microscopies in Electrocatalysis. Small Methods 2019, 3, 1800387. 10.1002/smtd.201800387. [DOI] [Google Scholar]
- Shashkova S.; Leake M. C. Single-Molecule Fluorescence Microscopy Review: Shedding New Light on Old Problems. Biosci. Rep. 2017, 37, BSR20170031. 10.1042/BSR20170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.; Zhou X.; Shen H.; Andoy N. M.; Choudhary E.; Han K.-S.; Liu G.; Meng W. Single-Molecule Fluorescence Imaging of Nanocatalytic Processes. Chem. Soc. Rev. 2010, 39, 4560–4570. 10.1039/b909052p. [DOI] [PubMed] [Google Scholar]
- Sydor A. M.; Czymmek K. J.; Puchner E. M.; Mennella V. Super-Resolution Microscopy: From Single Molecules to Supramolecular Assemblies. Trends Cell Biol. 2015, 25, 730–748. 10.1016/j.tcb.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Von Diezmann A.; Shechtman Y.; Moerner W. E. Three-Dimensional Localization of Single Molecules for Super-Resolution Imaging and Single-Particle Tracking. Chem. Rev. 2017, 117, 7244–7275. 10.1021/acs.chemrev.6b00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathwig K.; Aartsma T. J.; Canters G. W.; Lemay S. G. Nanoscale Methods for Single-Molecule Electrochemistry. Annu. Rev. Anal. Chem. 2014, 7, 383–404. 10.1146/annurev-anchem-062012-092557. [DOI] [PubMed] [Google Scholar]
- Moerner W. E.; Fromm D. P. Methods of Single-Molecule Fluorescence Spectroscopy and Microscopy. Rev. Sci. Instrum. 2003, 74, 3597–3619. 10.1063/1.1589587. [DOI] [Google Scholar]
- Hao R.; Fan Y.; Howard M. D.; Vaughan J. C.; Zhang B. Imaging Nanobubble Nucleation and Hydrogen Spillover during Electrocatalytic Water Splitting. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 5878–5883. 10.1073/pnas.1800945115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambur J. B.; Chen T. Y.; Choudhary E.; Chen G.; Nissen E. J.; Thomas E. M.; Zou N.; Chen P. Sub-Particle Reaction and Photocurrent Mapping to Optimize Catalyst-Modified Photoanodes. Nature 2016, 530, 77–80. 10.1038/nature16534. [DOI] [PubMed] [Google Scholar]
- Mao X.; Liu C.; Hesari M.; Zou N.; Chen P. Super-Resolution Imaging of Non-Fluorescent Reactions via Competition. Nat. Chem. 2019, 11, 687. 10.1038/s41557-019-0288-8. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Zaino L. P. III; Bohn P. W. Potential-Dependent Single Molecule Blinking Dynamics for Flavin Adenine Dinucleotide Covalently Immobilized in Zero-Mode Waveguide Array of Working Electrodes. Faraday Discuss. 2013, 164, 57–69. 10.1039/c3fd00013c. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Hao R.; Han C.; Zhang B. Counting Single Redox Molecules in a Nanoscale Electrochemical Cell. Anal. Chem. 2018, 90, 13837–13841. 10.1021/acs.analchem.8b04659. [DOI] [PubMed] [Google Scholar]
- Huang B.; Wang W.; Bates M.; Zhuang X. Three-Dimensional Super-Resolution Reconstruction Microscopy. Science 2008, 319, 810–813. 10.1126/science.1153529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkilic N.; Van Der Grient F.; Kamran M.; Sanghamitra N. J. M. Chemically-Induced Redox Switching of a Metalloprotein Reveals Thermodynamic and Kinetic Heterogeneity, One Molecule at a Time. Chem. Commun. 2014, 50, 14523–14526. 10.1039/C4CC06334A. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Caldarola M.; Pradhan B.; Orrit M. Gold Nanorod Enhanced Fluorescence Enables Single-Molecule Electrochemistry of Methylene Blue. Angew. Chem., Int. Ed. 2017, 56, 3566–3569. 10.1002/anie.201612389. [DOI] [PubMed] [Google Scholar]
- Liu J.; Hill C. M.; Pan S.; Liu H. Interfacial Charge Transfer Events of BODIPY Molecules: Single Molecule Spectroelectrochemistry and Substrate Effects. Phys. Chem. Chem. Phys. 2014, 16, 23150–23156. 10.1039/C4CP02950J. [DOI] [PubMed] [Google Scholar]
- Lei C.; Hu D.; Ackerman E. Clay Nanoparticle-Supported Single-Molecule Fluorescence Spectroelectrochemistry. Nano Lett. 2009, 9, 655–658. 10.1021/nl802998e. [DOI] [PubMed] [Google Scholar]
- Jares-Erijman E. A.; Jovin T. M. FRET Imaging. Nat. Biotechnol. 2003, 21, 1387–1395. 10.1038/nbt896. [DOI] [PubMed] [Google Scholar]
- Elmalk A. T.; Salverda J. M.; Tabares L. C.; Canters G. W.; Aartsma T. J. Probing Redox Proteins on a Gold Surface by Single Molecule Fluorescence Spectroscopy. J. Chem. Phys. 2012, 136, 235101. 10.1063/1.4728107. [DOI] [PubMed] [Google Scholar]
- Akkilic N.; Kamran M.; Stan R.; Sanghamitra N. J. M. M. Voltage-Controlled Fluorescence Switching of a Single Redox Protein. Biosens. Bioelectron. 2015, 67, 747–751. 10.1016/j.bios.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Palacios R. E.; Fan F.-R. F.; Bard A. J.; Barbara P. F. Single-Molecule Spectroelectrochemistry (SMS-EC). J. Am. Chem. Soc. 2006, 128, 9028–9029. 10.1021/ja062848e. [DOI] [PubMed] [Google Scholar]
- Lu J.; Fan Y.; Howard M. D.; Vaughan J. C.; Zhang B. Single-Molecule Electrochemistry on a Porous Silica-Coated Electrode. J. Am. Chem. Soc. 2017, 139, 2964–2971. 10.1021/jacs.6b10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C.; Hu D.; Ackerman E. J. Single-Molecule Fluorescence Spectroelectrochemistry of Cresyl Violet. Chem. Commun. 2008, (43), 5490–5492. 10.1039/b812161c. [DOI] [PubMed] [Google Scholar]
- Godin R.; Cosa G. Counting Single Redox Turnovers: Fluorogenic Antioxidant Conversion and Mass Transport Visualization via Single Molecule Spectroelectrochemistry. J. Phys. Chem. C 2016, 120, 15349–15353. 10.1021/acs.jpcc.6b06183. [DOI] [Google Scholar]
- Hao R.; Zhang B. Nanopipette-Based Electroplated Nanoelectrodes. Anal. Chem. 2016, 88, 614–620. 10.1021/acs.analchem.5b03548. [DOI] [PubMed] [Google Scholar]
- Hao R.; Fan Y.; Zhang B. Imaging Dynamic Collision and Oxidation of Single Silver Nanoparticles at the Electrode/Solution Interface. J. Am. Chem. Soc. 2017, 139, 12274–12282. 10.1021/jacs.7b06431. [DOI] [PubMed] [Google Scholar]
- Xu W.; Shen H.; Kim Y. J.; Zhou X.; Liu G.; Park J.; Chen P. Single-Molecule Electrocatalysis by Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 3968–3973. 10.1021/nl900988f. [DOI] [PubMed] [Google Scholar]
- Chan C. U.; Ohl C.-D. D. Total-Internal-Reflection-Fluorescence Microscopy for the Study of Nanobubble Dynamics. Phys. Rev. Lett. 2012, 109, 174501. 10.1103/PhysRevLett.109.174501. [DOI] [PubMed] [Google Scholar]
- Su H.; Fang Y.; Chen F.; Wang W. Monitoring the dynamic photocatalytic activity of single CdS nanoparticles by lighting up H2 nanobubbles with fluorescent dyes. Chem. Sci. 2018, 9, 1448–1453. 10.1039/C7SC04684G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja S. M.; Guerrette J. P.; David M. R.; Zhang B. Fluorescence-Enabled Electrochemical Microscopy with Dihydroresorufin as a Fluorogenic Indicator. Anal. Chem. 2014, 86, 6040–6048. 10.1021/ac501194j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja S. M.; Zhang B. Imaging Transient Formation of Diffusion Layers with Fluorescence-Enabled Electrochemical Microscopy. Anal. Chem. 2014, 86, 12299–12307. 10.1021/ac5035715. [DOI] [PubMed] [Google Scholar]