Abstract
Background and purpose
Previous studies have found ischemic stroke is associated with atrial fibrillation. However, the causal association between ischemic stroke and atrial fibrillation is not clear. Furthermore, the network relationship among ischemic stroke, atrial fibrillation and its risk factors need further attention. This study aims to examine the potential causal association between ischemic stroke and atrial fibrillation and further to explore potential mediators in the causal pathway from ischemic stroke to atrial fibrillation.
Methods
Summary statistics from the ISGC (case = 10,307 and control = 19,326) were used as ischemic stroke genetic instruments, AFGen Consortium data (case = 65,446 and control = 522,744) were used for atrial fibrillation, and other consortia data were used for potential mediators (fasting insulin, white blood cell count, procalcitonin, systolic and diastolic blood pressure, body mass index, waist circumference, and height). Under the framework of network Mendelian randomization, two-sample Mendelian randomization study was performed using summary statistics from several genome-wide association studies. Inverse-variance weighted method was performed to estimate causal effect.
Results
Blood pressure mediates the causal pathways from ischemic stroke to atrial fibrillation. The total odds ratio of ischemic stroke on atrial fibrillation was 1.05 (95% confidence interval [CI], 1.02 to 1.07; P = 1.3 × 10−5). One-unit increase of genetically determined ischemic stroke was associated with 0.02 (DBP: 95% CI, 0.001 to 0.034, P = 0.029; SBP: 95% CI, 0.006 to 0.034, P = 0.003) upper systolic and diastolic blood pressure levels. Higher genetically determined systolic and diastolic blood pressure levels were associated with higher atrial fibrillation risk (DBP: RR, 1.18; 95% CI, 1.03 to 1.35; P = 0.012. SBP: RR, 1.18; 95% CI, 1.01 to 1.38; P = 0.04). Specially, we also found the bidirectional causality between blood pressure and ischemic stroke.
Conclusions
Our study provided a strong evidence that raised blood pressure in stroke patients increases the risk of atrial fibrillation and active acute blood pressure lowering can improve the outcome in ischemic stroke patients.
Keywords: Ischemic stroke, Atrial fibrillation, Network Mendelian randomization, Bidirectional causality
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in clinical practice and is associated with increased risk of stroke, dementia, falls, and death, among other outcomes (Chugh et al. 2014; Cerasuolo et al. 2017a). AF is a common cause of stroke, which is biologically plausible. However, AF is sometimes detected after ischemic stroke (IS), and one in every four cases of AF begins with continuous ECG monitoring shortly after the onset of symptoms (Cerasuolo et al, 2017b). The current guidelines recommend anticoagulant therapy for every patient with recurrent AF after stroke. However, in some cases, AF detected after acute IS may be shortlasting and perhaps a nonrecurrent autonomic and inflammatory epiphenomena of stroke, so some patients may face an unnecessary risk of bleeding (Haeusler et al, 2018). In addition, more knowledge is needed to determine the mediating mechanism of AF detected after IS, and to effectively prevent the occurrence of new-onset AF after IS (Sposato et al. 2014; Scheitz et al. 2015; Fauchier et al. 2017). Previous population-based observational studies found that IS has been established as a risk factor of AF (Rizos et al. 2015; Luo et al, 2018). However, the causal association between IS and AF has not been studied yet. Furthermore, the potential pathways involved in the association from IS to AF remain unclear. In the past few years, several traditional and newly emerging risk factors for AF, including thyroid function (Roberts 2019), glycemic traits (Kokubo et al. 2015; Chen et al. 2019), inflammation (Paquet et al. 2018; Karam et al. 2017) and obesity (Tikkanen et al, 2019; Aune et al. 2017; Frost et al. 2014; Karas et al. 2016; Nattel 2017) are also closely related to IS. Thus, these may act as potential mediators that lie in the pathway from IS to increased risk of AF.
In this study, we evaluated the potential causal roles of IS in AF and explored the potential mediators involved in the causal association between IS and AF in a network Mendelian randomization analysis framework based on the summarized genome-wide association study data. The potential mediators include fasting insulin, white blood cell count, procalcitonin, systolic and diastolic blood pressure, body mass index, waist circumference, and height.
Materials and methods
Summary of GWAS data
The GWAS summary statistics datasets we used in this study were from the ISGC Consortium (https://strokegenetics.org/) for ischemic stroke (Malik et al, 2016); MAGIC Consortium (http://www.magicinvestigators.org/) for FastingInsulin; GIANT Consortium (https://www.broadinstitute.org/collaboration/giant/index) for body mass index, waist circumference and height (Locke et al, 2015; Shungin et al, 2015; Wood et al, 2014); Neale Lab (http://www.nealelab.is/) for systolic and diastolic blood pressure (Churchhouse & Neale, 2017); Astle W for white blood cell count (Astle et al, 2016); MRC-IEU Consortium (http://www.bristol.ac.uk/integrative-epidemiology/) for procalcitonin (Mitchell et al, 2017); AFGen Consortium (https://www.afgen.org/) for atrial fibrillation (Roselli et al, 2018). They were commonly used in MR analyses and to obtain the associations of genetic variants on IS, risk factors and AF, respectively. Beta coefficients (logOR) and standard errors were obtained for the per allele association of each SNP with all exposures and outcomes from these data sources. The basic characters of these data is briefly presented in Table 1 and Additional file 2: Tables S1-S7 for details. There is no sample overlap between ISGC Consortium and AFGen Consortium. To minimize the bias caused by population stratification, populations of the GWAS summary statistics data were mainly from European ancestry, and some of the GWAS summary statistics were adjusted for the population stratification by principal component analysis (PCA) (Price et al. 2006).
Table 1.
Summary statistics data sources
| Trait | Consortium | Data sources | Total no. or case/control | Ancestry |
|---|---|---|---|---|
| IS | ISGC | Malik; Neurology, 2016 | 10,307/19326 | Caucasians |
| DBP | UKBiobank | Neale; Neale Lab, 2017 | 317,756 | Europeans |
| SBP | UKBiobank | Neale; Neale Lab, 2017 | 317,754 | Europeans |
| FastingInsulin | MAGIC | Horikoshi; PLOS Genet, 2015 | 24,245 | Europeans |
| BMI | GIANT | Locke; Nature, 2015 | 322,154 | Europeans |
| WC | GIANT | Shungin D; Natrue, 2015 | 224,459 | Europeans |
| Height | GIANT | Wood; Nature, 2014 | 253,288 | Europeans |
| WBC | UK Biobank + INTERVAL + UK BiLEVE | Astle W; Cell, 2016 | 172,435 | Europeans |
| PCT | MRC-IEU | Ben Elsworth; MRC-IEU, 2018 | 3701/459309 | Europeans |
| AF | AFGen | Carolina; Nature, 2018 | 65,446/522744 | 99.2% European, 0.8% African American |
SNP selection
We used 103 IS-associated single-nucleotide polymorphisms (SNP) (explaining 6.1% of its variance) identified by Malik et al. as genetic instruments. This was conducted by first extracting the effect sizes for SNP associated (P ≤ 0.0005) with IS from the summary statistics for AF and its risk factors. As the extracted SNPs for IS might be correlated with each other, we pruned the variants by linkage disequilibrium (LD) (r2 = 0.001, clumping window = 500 kbp). We then organized these SNPs by quantifying the heterogeneity and the proportion of variance explained by the genetic instruments (R2, estimated from the summary statistics using R package gtx in R Version 3.5.3). The method assumes that all valid instrumental variables (IV) should yield the same causal estimate. The associations of each SNP with the outcome should be proportional to their association with IS. Presence of any substantial heterogeneity would be suggestive evidence of pleiotropic effects of the SNPs.
The proportion of variance (R2) of the trait explained by the genetic instruments will rise with the addition of more SNPs. However, the improvement beyond the optimum number of SNPs in the instrument will come increasingly as a result of heterogeneity. In order to determine the final tally of SNPs for inclusion in genetic instruments for each trait, the process is described as follows (White et al. 2016):
Step 1 Choose the SNP that explains the largest proportion of variance (R2) of the trait as the initial element of the IV set.
Step 2 Add a SNP from the remaining SNPs to IV set so that the IV set explains the largest proportion of variance (R2) of the trait and without heterogeneity.
Step 3 Repeat the step 2 until there is heterogeneity.
After the above three steps, the homogeneity assumption is ensured to be satisfied, which means instrumental variables-exposure, instrumental variables-outcome and exposure-outcome relationships with no effect heterogeneity. To some extent, it reduces the occurrence of pleiotropic. In terms of the rule of thumb proposed by Staiger and Stock (1997), F statistics of the 103 SNPs we selected are all greater than 10, which means the first core assumption of MR is satisfied and avoids the bias caused by weak instruments (Burgess & Thompson, 2011). Finally, we matched SNPs across the data sources by aligning them to the same effect allele, which were checked for concordance. The details are presented in Additional file 1 and Additional file 2: Tables S8-S11.
Mendelian randomization
Mendelian randomization (MR) can be used to assess the causal effect of an exposure on an outcome using genetic variants as IVs (Davey Smith &Hemani, 2014; Didelez & Sheehan, 2007; Zheng et al, 2017). Three assumptions of Mendelian Randomization should be satisfied: 1) the genetic variants are associated with the exposure; 2) the genetic variants are not associated with any confounders of the exposure and the outcome; 3) the genetic variants are conditionally independent of the outcome given the exposure and confounders (Burgess et al. 2015b). To perform a two-sample MR analyses using summary statistics (Burgess et al, 2015a), we constructed IVs using multiple genetic variants with an inverse variance weighted method to estimate the causal effect sizes (White et al. 2016).
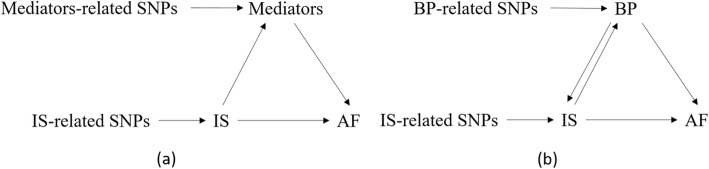
We performed network MR to explore the causal pathway from IS to AF. The framework of the network MR analysis (Zhan et al, 2017) is described in Fig. 1a. It consists of three different MR tests that are all described below (I–III) (Burgess et al. 2015b).
-
I.
The causal effect of genetically determined IS on AF is estimated.
-
II.
The causal effects of genetically determined IS on the risk factors for AF are estimated.
-
III.
The causal effects of the possible mediators on AF are estimated.
Fig. 1.
a Network Mendelian randomization analysis framework; b Graphical diagram of network relationship among IS, AF and BP
If causal associations are evaluated in all three steps, the conclusion can be drawn that the specific risk factor is a mediator.
For the first step (I), 103 SNPs of genome-wide significance with IS in the GWAS from the International Stroke Genetics Consortium (ISGC) were used to estimate the causal effect of genetically determined IS on AF using the summary statistics from the AFGen consortium. The second step (II) used the same IVs for IS as described in the first step and estimated the causal effect of genetically determined IS on fasting insulin, body mass index, waist circumference, height, systolic and diastolic blood pressure from the respective GWAS summary statistics. Finally, the third MR analyses (III) were performed for risk factors on AF if IS was shown to have a causal effect on the risk factors in the second test (II). The association between IS and AF was mediated by DBP and SBP were tested in an additional analysis after DBP and SBP were identified as potential mediators. The detailed description of Mendelian Randomization analysis can be referred to the Additional file 1.
Sensitive analysis
Sensitivity analyses were performed to test the sensitivity of the disproportionate effects of variants and the pleiotropy in MR analysis. These issues were analyzed by leave-one-out validation and MR-Egger regression (Bowden et al. 2015), respectively.
To test the sensitivity of variants, we designed a leave-one-out validation measure. Each SNP was removed from the 103 SNPs to carry out IVW point estimate and then evaluated the influence of each SNP on the results. The fluctuation of the results before and after removing each SNP reflects the sensitivity of this SNP.
For MR-Egger method, we performed weighted linear regression with the intercept unconstrained. The intercept represents the average pleiotropic effect across the genetic variants (the average direct effect of a variant with the outcome). If the intercept differs from zero (the MR-Egger test), then there is the evidence of directional pleiotropy. The Instrument Strength Independent of Direct Effect (InSIDE) assumption needs to be satisfied, which means the effect of genetic variants on the exposure is independent of the direct effects of the genetic variants on the outcome.
Additionally, bidirectional MR (Chung et al. 2001) analyses were performed to examine whether the mediators (systolic and diastolic blood pressure) could causally affect IS by exchanging IS and mediator and using the mediator-associated SNPs as the IV. All statistical analyses were performed using R (version 3.5.3) and R package TwoSampleMR. Statistical power was calculated in http://cnsgenomics.com/shiny/mRnd/.
Results
Causal association between genetically determined IS and AF
The causal estimate using 103 SNPs as IVs showed that IS will increase the risk of developing AF (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.01--1.22; P = 0.019). The intercept of MR-Egger regression for these 103 SNPs was not statistically significant (P = 0.397) so there is no evidence of directional pleiotropy. However, the results of leave-one-out indicated the SNP rs12646447 had strong influence on the estimation of causal association (Additional file 1: Figure S6). After removing rs12646447, we found that IS increased the risk of developing AF (odds ratio [OR], 1.05; 95% confidence interval [CI], 1.02--1.07; P = 1.3 × 10−5). Details are presented in Table 3 and Additional file 1: Figure S4.
Table 3.
Causal estimates for the association between IS and AF and mediators
| Exposure | Outcome | Causal estimate | |||||
|---|---|---|---|---|---|---|---|
| Num of IVs | R2 | Beta/OR | 95% CI | PValue | Power | ||
| IS | AF | 103 | 6.1% | 1.05 | 1.02–1.07 | 1.3 × 10−5 | 0.84 |
| IS | DBP | 103 | 6.1% | 0.02 | 0.001–0.034 | 2.9 × 10−2 | – |
| DBP | AF | 91 | 1.5% | 1.18 | 1.03–1.35 | 1.2 × 10−2 | 1 |
| IS | SBP | 103 | 6.1% | 0.02 | 0.006–0.034 | 3.1 × 10−3 | – |
| SBP | AF | 80 | 1.7% | 1.18 | 1.01–1.38 | 4.0 × 10−2 | 1 |
| DBP | IS | 91 | 1.5% | 1.65 | 1.27–2.14 | 1.7 × 10−4 | 1 |
| SBP | IS | 80 | 1.7% | 1.48 | 1.17–1.87 | 9.3 × 10−4 | 0.99 |
| AF | IS | 103 | 1.1% | 1.18 | 1.08–1.28 | 1.3 × 10−4 | 1 |
Causal association between genetically determined IS and risk factors
The causal estimates between genetically determined IS and risk factors, including fasting insulin, body mass index, waist circumference, height, systolic and diastolic blood pressure are listed in Table 2. The MR analyses showed that IS was associated with body mass index, systolic and diastolic blood pressure. The intercept of MR-Egger regression for these 102 SNPs in each trait was not statistically significant except BMI. Because of pleiotropic effects of 8 SNPs (rs17696736, rs3184504, rs11250077, rs2074314, rs7123414, rs10172342, rs1483968, rs1483968), they were then excluded from the analysis for BMI. The MR-Egger test for testing the remaining SNPs was not statistically significant (P = 0.08). Analysis of the remaining SNPs yielded an effect size of IS on BMI was − 0.00248 (95% CI, − 0.013358 to 0.008398; P = 0.654). As a result, the MR analyses showed that IS had causal association with systolic and diastolic blood pressure. Details are presented in Table 2 and Additional file 1: Figure S5.
Table 2.
Causal estimates for the association between IS and risk factors for AF
| Trait | Causal estimate | ||
|---|---|---|---|
| Beta | SE | PValue | |
| BMI | −0.00248 | 0.00555 | 0.654 |
| height | −0.00767 | 0.00706 | 0.276 |
| WC | −0.00612 | 0.00660 | 0.354 |
| FastingInsulin | 0.00104 | 0.00560 | 0.851 |
| DBP | 0.01866 | 0.00853 | 0.029 |
| SBP | 0.02094 | 0.00708 | 0.003 |
| WBC | 0.02446 | 0.01300 | 0.060 |
| PCT | 0.00035 | 0.00026 | 0.177 |
Causal association between genetically determined systolic and diastolic blood pressure and AF
Based on the first two steps of Network Mendelian randomization analysis, systolic and diastolic blood pressure were suspected to be potential mediators from higher DBP and SBP to increased risk of AF. Further, we evaluated whether DBP and SBP were associated with AF using MR analysis. We used 131 SNPs and 105 SNPs associated with DBP and SBP as the IVs, respectively. Higher DBP and SBP levels were associated with increased risk of AF (DBP: OR, 1.18; 95% CI, 1.03--1.35; P = 0.012. SBP: OR, 1.18; 95% CI, 1.01--1.38; P = 0.04). The intercept of MR-Egger regression for these SNPs was not statistically significant (DBP: P = 0.461; SBP: P = 0.80), which means there is no evidence of directional pleiotropy. Therefore, DBP and SBP might act as mediators in the causal pathway from IS to AF and account for 6.7 and 7.4% of the total effect of IS on AF, respectively.
The bidirectional causality between blood pressure and IS
Additionally, bidirectional MR analyses were performed to examine whether there existed the bidirectional causality between blood pressure and IS. We used 91 SNPs and 80 SNPs that were reported by the Neale Lab (Churchhouse & Neale, 2017) to be associated with DBP and SBP as the IVs, respectively, and found that increased DBP and SBP levels were associated with higher risk of IS (DBP: OR, 1.65; 95% CI, 1.27–2.14; P = 1.73 × 10−4. SBP: OR, 1.48; 95% CI, 1.17--1.87; P = 0.001). Therefore, the bidirectional causality between blood pressure and IS was verified. In other words, the causal association between blood pressure (DBP, SBP) and AF was simultaneously mediated by IS (Fig. 1b). Finally, we obtain that the mediated proportion of IS in the causal pathway from blood pressure (DBP, SBP) to AF were 13.9 and 14.1%, respectively.
The bidirectional causality between AF and IS
We additionally performed MR of AF on IS to examine the bidirectional causality between AF and IS. The causal estimate using 103 SNPs (explaining 1.1% of exposure’s variance) as IVs showed that AF will increase the risk of developing IS (odds ratio [OR], 1.18; 95% confidence interval [CI], 1.08–1.28; P = 0.001). The intercept of MR-Egger regression for these 103 SNPs was not statistically significant (P = 0.737) so there is no evidence of directional pleiotropy. Thus, there are bidirectional causality between AF and IS and they are risk factors for each other. Details are briefly presented in Table 3 and the results of bidirectional MR analyses are listed in Additional file 2: Table S11-S12 and Additional file 1: Figure S7. There was > 80% power to detect all the causal associations and details were listed in Table 3.
Discussion
Although the underlying mechanisms between IS and AF have been discussed in previous studies (Rizos et al. 2015), causal association between IS and AF has never been reported. In this study, we used publicly available summary statistics from several genetic consortia to verify the causal association between IS and AF. We further explored pathway that might be involved in the association from IS to AF by a network MR analysis. We concluded that IS was associated with higher concentrations of SBP and DBP, which could further increase AF risk. In addition, we also found, the causal association from blood pressure to AF may be mediated by IS under similar Network Mendelian randomization framework above.
The risk of stroke in patients with AF is 3–5 times higher than in patients with non-AF (Wolf et al. 1978). AF has been consistently associated with IS in different cohorts (Sposato et al, 2015; Rizos et al, 2016; Manolio et al. 1996). Intuitively, uncoordinated myocyte activity can explain the impaired atrial contraction in AF, and according to Virchow’s triad, the resulting stasis of blood should increase the risk of thromboembolic. AF is a common cause of stroke, which is biologically plausible and is proven in our study. And what we are most interested in is the causal pathway from IS to AF. The brain exerts the greatest control over heart rhythm through the autonomic nervous pathway, which may be affected by cerebral lesions (i.e., IS) or systemic inflammation (Kamel et al, 2016). In patients with insular or other cortical acute ischemic stroke, sudden loss of autonomic or even central regulation can cause the arrhythmia stimulus in the intrinsic system, which in turn triggers focal discharges in the pulmonary veins and non-pulmonary veins. Ultimately paroxysmal atrial fibrillation occurred. Inflammatory mediators with elevated plasma concentrations during the acute phase of ischemic stroke may affect the intrinsic system, leading to the development of focal firing and subsequent AF in acute stroke patients. Other factors different from autonomic dysfunction and inflammation should be acknowledged as possibly implicated in the pathophysiology of poststroke AF (Sposato et al. 2014).
Our study demonstrates that blood pressure is a mediator from IS to AF. Biologically, blood pressure is related to the mechanism of AF’s occurrence. The autonomic nervous system and its sympathetic arm play important roles in the regulation of blood pressure. Their role in the short-term regulation of blood pressure, especially in responses to transient changes in arterial pressure, via baroreflex mechanisms is well known (Joyner et al. 2010). Besides, inflammation is associated with elevated blood pressure in the general population. A prospective cohort study shows that C-reactive protein (CRP) levels are associated with future development of hypertension, which suggests that hypertension is in part an inflammatory disorder (Sesso et al. 2004). However, how the autonomic nervous system and inflammation act on blood pressure to cause AF is unclear. Therefore, the role of autonomic nervous system, inflammation and blood pressure in the causal pathway from IS to AF and how their interaction in this pathway to cause AF needs further attention. Lattanzi et al. (2013) mentioned that raised blood pressure is common after acute stroke, whether of ischaemic or haemorrhagic type. Other studies suggest that elevated blood pressure is present during rehabilitation and training for stroke patients (Odden et al, 2015). In addition, Roetker et al. (2014) founded that plus pressure emerged as a significant independent risk factor for AF in a Multi-Ethnic Study of Atherosclerosis. Chen et al. (2014) indicated that central nervous system injuries often affect the autonomic nervous system, which plays an important role in the pathogenesis of AF. Necrotic cell death from stroke activates a systemic inflammatory response, which also plays a role in the origin of AF. Clinical observations support the hypothesis that stroke may trigger AF. Therefore, there are reasons believe that the causal association from IS to AF may be mediated by blood pressure.
Castillo et al. (2004) and Zhang et al. (2006) have reported positive and reverse association between elevated blood pressure on stroke severity, respectively. In this study, the bidirectional causality between blood pressure and IS was verified by directional MR analysis. Hypertension is the most important risk factor for all types of stroke, especially in China. Zhang X F et al. found that for each increase of 10 mmHg in systolic blood pressure, there was a 1.44-fold risk for IS in Chinese hypertensive patients. In addition, elevated blood pressure is present during rehabilitation and training for stroke patients. Our study provided a strong evidence that raised blood pressure in stroke patients increases the risk of atrial fibrillation and active acute blood pressure lowering can improve outcome in ischemic stroke patients (Carlberg et al. 1993).
We additionally performed MR for AF and IS subtypes (cardioembolic stroke (CE), large vessel disease (LVD), small vessel disease (SVD)) and results showed only a strong causal relationship from AF to cardiogenic stroke (Additional file 2: Table S12). Several large prospective epidemiological investigations suggested that other markers of left atrial dysfunction such as elevated N-terminal pro-Brain Natriuretic Peptide (NT pro-BNP) (Folsom et al. 2013), p-wave terminal force in lead V1 (PTFV1) of a 12-lead electrocardiogram (Lattanzi et al. 2017) and left atrial enlargement (Yaghi et al. 2015) are associated with cardioembolic stroke. Various studies have proved that NT-proBNP is increased in AF and proposed mechanisms are high frequency of atrial myocyte contraction and local atrial inflammation (Jayachandran and Johnson 2009). Goda T et al. found that PTFV1 on admission ECG is a strong, independent predictor of PAF in patients with acute ischemic stroke (Goda et al. 2017). Once AF occurs, LA dilation might progress due to either progressive heart disease, loss of the atrial systole, or both factors (Andersen et al. 1991). Therefore, the markers of left atrial dysfunction may play an important role in the causal mechanism between IS and AF. And further studies need to attention is the causal association among these markers, cardioembolic stroke and AF.
The main strength of our study is the large sample size accrued from the GWAS summary statistics, enabling us to examine the causal relationship among IS, risk factors, including DBP and SBP, and AF. In addition, the IVs that explained the largest variance of exposure without heterogeneity were selected by an iterative algorithm, which promises the effect of IS on traits is not a violation of the third assumption about pleiotropy. And we further valid the first assumption by the rule of thumb proposed by Staiger and Stock (1997) and test the third assumption by MR-Egger regression in the sensitive analysis. The random assortment of alleles at birth should rule out confounding factors in the association among IS, DBP and SBP, and AF. Another obvious major strength of using GWAS summary statistics with two sample MR is the increased statistical power, particularly when the outcome is a binary trait like AF. Besides, the bidirectional causality between blood pressure and IS was verified.
The limitations mainly concern the assumptions for two sample MR analyses. There is no sample overlap between the cohort of exposure ISGC (IS) and the outcome AFGen (AF), but there may still be individuals participating in multiple surveys that we cannot ascertain with available summary-level GWAS statistics. The test of InSIDE assumption in MR-Egger might be a problem. InSIDE assumption is that the effect of genetic variants on the exposure is independent of the direct effects of the genetic variants on the outcome, which is difficult to evaluate. Therefore, it is recommended to use individual data in one sample as much as possible to perform Mendelian randomization studies, so as to obtain higher accuracy and power. In addition, no matter in one-sample or two-sample MR analysis, potential violation of the second core assumption cannot be ruled out because of possible unmeasured confounders. While instrument strength can be verified by the calculation of F-statistics and pleiotropy can be tested by MR-Egger.
Conclusion
In summary, we provided a causal diagram among blood pressure, IS and AF. We found that IS could increase the risk of AF and the effect of genetically determined IS on AF was partially mediated by blood pressure. Additionally, due to the bidirectional causality between blood pressure and IS, IS might also play a mediating role on the pathway between blood pressure and AF. Thus, raised blood pressure in stroke patients increases the risk of AF and active acute blood pressure lowering can improve outcome in IS patients, which is of significance in clinical application. Further validation of these findings is warranted based on large-scale longitudinal studies that repeat the measurements of IS, blood pressure, and AF.
Supplementary information
Additional file 1. Methods details including Inverse-variance weighted method, MR-Egger method, Weak Instruments and Mediation analysis. Leave-one-out plots in the sensitive analysis.
Additional file 2. The basic characters of summary data used in this study. Details of instrumental invariables selected for each traits.
Acknowledgements
Not applicable.
Abbreviations
- AF
Atrial fibrillation
- AFGen
Atrial Fibrillation Consortium
- BMI
Body mass index
- DBP
Diastolic blood pressure
- GIANT
Genetic investigation of anthropometric traits
- GWAS
Genome-wide association study
- IS
Ischemic stroke
- ISGC
International Stroke Genetics Consortium
- IV
Instrument variable
- MAGIC
Meta-analyses of glucose and insulin-related traits consortium
- MR
Mendelian randomization
- MRC-IEU
MRC Integrative Epidemiology Unit
- PCT
Procalcitonin
- SBP
Systolic blood pressure
- SNP
Single-nucleotide polymorphisms
- WBC
White blood cell count
- WC
Waist circumference
Authors’ contributions
FX, HL and MX conducted the research, and reviewed and edited the manuscript. LH designed the study, performed analysis, analyzed the data and wrote the manuscript. YY, XS, XL, LL, YL, TY and WL conducted the research and contributed to the discussion. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant 81773547).
Availability of data and materials
The GWAS summary statistics datasets we used in this study were from the ISGC Consortium (https://strokegenetics.org/) for ischemic stroke; MAGIC Consortium (http://www.magicinvestigators.org/) for FastingInsulin; GIANT Consortium (https://www.broadinstitute.org/collaboration/giant/index) for body mass index, waist circumference and height; Neale Lab (http://www.nealelab.is/) for systolic and diastolic blood pressure; Astle W for white blood cell count; MRC-IEU Consortium (http://www.bristol.ac.uk/integrative-epidemiology/) for procalcitonin; AFGen Consortium (https://www.afgen.org/) for atrial fibrillation.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hongkai Li, Email: 15253114451@163.com.
Fuzhong Xue, Email: xuefzh@sdu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s10020-019-0133-y.
References
- Andersen JS, Egeblad H, Abildgaard U, et al. Atrial fibrillation and left atrial enlargement: cause or effect? J Intern Med. 1991;229(3):253–256. doi: 10.1111/j.1365-2796.1991.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Astle WJ, Elding H, Jiang T, et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell. 2016;167(5):1415–1429.e19. doi: 10.1016/j.cell.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune D, Sen A, Schlesinger S, Norat T, Janszky I, Romundstad P, Tonstad S, Riboli E, Vatten LJ. Body mass index, abdominal fatness, fat mass and the risk of atrial fibrillation: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32:181–192. doi: 10.1007/s10654-017-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Daniel RM, Butterworth AS, Thompson SG, EPIC-InterAct Consortium Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. 2015;44:484–495. doi: 10.1093/ije/dyu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Scott RA, Timpson NJ, Davey SG, Thompson SG. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–552. doi: 10.1007/s10654-015-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–764. doi: 10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- Carlberg B, Asplund K, Hagg E. The prognostic value of admission blood pressure in patients with acute stroke. Stroke. 1993;24(9):1372–1375. doi: 10.1161/01.STR.24.9.1372. [DOI] [PubMed] [Google Scholar]
- Castillo J, Leira R, García MM, et al. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35(2):520–526. doi: 10.1161/01.STR.0000109769.22917.B0. [DOI] [PubMed] [Google Scholar]
- Cerasuolo JO, Cipriano LE, Sposato LA, et al. The complexity of atrial fibrillation newly diagnosed after ischemic stroke and TIA: advances and uncertainties. Curr Opin Neurol. 2017;30:28–37. doi: 10.1097/WCO.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasuolo JO, Montero-Odasso M, Ibañez A, et al. Decision-making interventions to stop the global atrial fibrillation-related stroke tsunami. Int J Stroke. 2017;12:222–228. doi: 10.1177/1747493016687579. [DOI] [PubMed] [Google Scholar]
- Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Huang QF, Sheng CS, Lei L, Xu SK, Zhang W, Shao S, Wang D, Cheng YB, Wang Y, Guo QH, Zhang DY, Li Y, Li Y, Freedman SB, Wang JG. Cross-sectional association between blood pressure status and atrial fibrillation in an elderly Chinese population. Am J Hypertens. 2019;32:777–785. doi: 10.1093/ajh/hpz060. [DOI] [PubMed] [Google Scholar]
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- Churchhouse C, Neale B. Rapid GWAS of thousands of phenotypes for 337,000 samples in the UK Biobank. Neale Lab. 2017; Available at: http://www.nealelab.is/blog/2017/7/19/rapid-gwas-of-thousands-of-phenotypes-for-337000-samples-in-the-uk-biobank. Accessed 14 Dec 2017.
- Staiger Douglas, Stock James H. Instrumental Variables Regression with Weak Instruments. Econometrica. 1997;65(3):557. doi: 10.2307/2171753. [DOI] [Google Scholar]
- Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007;16:309–330. doi: 10.1177/0962280206077743. [DOI] [PubMed] [Google Scholar]
- Fauchier L, Clementy N, Bisson A, Stamboul K, Ivanes F, Angoulvant D, Babuty D, Lip GY. Prognosis in patients with atrial fibrillation and a presumed “temporary cause” in a community-based cohort study. Clin Res Cardiol. 2017;106(3):202–210. doi: 10.1007/s00392-016-1040-7. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Nambi V, Bell EJ, et al. Troponin T, NT-proBNP, and incidence of stroke: the atherosclerosis risk in communities (ARIC) study. Stroke. 2013;44(4):961. doi: 10.1161/STROKEAHA.111.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L, Benjamin EJ, Fenger-Grøn M, Pedersen A, Tjønneland A, Overvad K. Body fat, body fat distribution, lean body mass and atrial fibrillation and flutter. A Danish cohort study. Obesity. 2014;22:1546–1552. doi: 10.1002/oby.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda T, Sugiyama Y, Ohara N, et al. P-wave terminal force in lead V 1 predicts paroxysmal atrial fibrillation in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(9):1912. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.031. [DOI] [PubMed] [Google Scholar]
- Haeusler KG, Gröschel K, Köhrmann M, et al. Expert opinion paper on atrial fibrillation detection after ischemic stroke. Clin Res Cardiol. 2018;107:871. doi: 10.1007/s00392-018-1256-9. [DOI] [PubMed] [Google Scholar]
- Jayachandran T, Johnson F. N-terminal pro-brain natriuretic peptide and atrial fibrillation. Indian Pacing Electrophysiol J. 2009;9(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension. 2010;56(1):10–16. doi: 10.1161/HYPERTENSIONAHA.109.140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel H, Okin PM, Elkind MSV, et al. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47(3):895–900. doi: 10.1161/STROKEAHA.115.012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam BS, Chavez-Moreno A, Koh W, et al. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16(1):120. doi: 10.1186/s12933-017-0604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas MG, Yee LM, Biggs ML, Djousse L, Mukamal KJ, Ix JH, Zieman SJ, Siscovick DS, Gottdiener JS, Rosenberg MA, Kronmal RA, Heckbert SR, Kizer JR. Measures of body size and composition and risk of incident atrial fibrillation in older people: the cardiovascular health study. Am J Epidemiol. 2016;183:998–1007. doi: 10.1093/aje/kwv278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo Yoshihiro, Watanabe Makoto, Higashiyama Aya, Nakao Yoko M., Kobayashi Takashi, Watanabe Takuya, Okamura Tomonori, Okayama Akira, Miyamoto Yoshihiro. Interaction of Blood Pressure and Body Mass Index With Risk of Incident Atrial Fibrillation in a Japanese Urban Cohort: The Suita Study. American Journal of Hypertension. 2015;28(11):1355–1361. doi: 10.1093/ajh/hpv038. [DOI] [PubMed] [Google Scholar]
- Lattanzi S, Cagnetti C, Pulcini A, et al. The P-wave terminal force in embolic strokes of undetermined source. J Neurol Sci. 2017;375:175–178. doi: 10.1016/j.jns.2017.01.063. [DOI] [PubMed] [Google Scholar]
- Lattanzi S, Silvestrini M, Provinciali L. Elevated blood pressure in the acute phase of stroke and the role of angiotensin receptor blockers. Int J Hypertens. 2013;2013:941783. doi: 10.1155/2013/941783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Li H, Qin X, Liu B, Zhao J, Maihe G, Li Z, Wei Y. Increased risk of ischemic stroke associated with new-onset atrial fibrillation complicating acute coronary syndrome: a systematic review and meta-analysis. Int J Cardiol. 2018;265:125–131. doi: 10.1016/j.ijcard.2018.04.096. [DOI] [PubMed] [Google Scholar]
- Malik R, Traylor M, Pulit SL, et al. Low-frequency and common genetic variation in ischemic stroke: the METASTROKE collaboration. Neurology. 2016;86(13):1217–1226. doi: 10.1212/WNL.0000000000002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Kronmal RA, Burke GL, O’Leary DH, Price TR. Short-term predictors of incident stroke in older adults. The Cardiovascular Health Study. Stroke. 1996;27:1479–1486. doi: 10.1161/01.STR.27.9.1479. [DOI] [PubMed] [Google Scholar]
- Mitchell R, Elsworth B, Raistrick C, Paternoster L, Hemani G, Gaunt T. MRC IEU UK Biobank GWAS pipeline version 1. University of Bristol Research Data Repository. 2017. [Google Scholar]
- Nattel S. Atrial fibrillation and body composition: is it fat or lean that ultimately determines the risk? J Am Coll Cardiol. 2017;69:2498–2501. doi: 10.1016/j.jacc.2017.03.566. [DOI] [PubMed] [Google Scholar]
- Odden MC, Mcclure LA, Sawaya BP, et al. Achieved blood pressure and outcomes in the secondary prevention of small subcortical strokes trial. Hypertension. 2015;67(1):63–69. doi: 10.1161/HYPERTENSIONAHA.115.06480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet M, Cerasuolo JO, Thorburn V, Fridman S, Alsubaie R, Lopes RD, et al. Pathophysiology and risk of atrial fibrillation detected after ischemic stroke (PARADISE). A translational, integrated, and transdisciplinary approach. J Stroke Cerebrovasc Dis. 2018;27(3):606–619. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.038. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Rizos T, Horstmann S, Dittgen F, et al. Preexisting heart disease underlies newly diagnosed atrial fibrillation after acute ischemic stroke. Stroke. 2016;47(2):336–341. doi: 10.1161/STROKEAHA.115.011465. [DOI] [PubMed] [Google Scholar]
- Rizos T, Quilitzsch A, Busse O, et al. Diagnostic work-up for detection of paroxysmal atrial fibrillation after acute ischemic stroke: cross-sectional survey on German stroke units. Stroke. 2015;46(6):1693–1695. doi: 10.1161/STROKEAHA.115.009374. [DOI] [PubMed] [Google Scholar]
- Roberts JD. Thyroid function and the risk of atrial fibrillation: exploring potentially causal relationships through Mendelian randomization. JAMA Cardiol. 2019;4(2):97–99. doi: 10.1001/jamacardio.2018.4614. [DOI] [PubMed] [Google Scholar]
- Roetker NS, Chen LY, Heckbert SR, Nazarian S, Soliman EZ, Bluemke DA, Lima JA, Alonso A. Relation of systolic, diastolic, and pulse pressures and aortic distensibility with atrial fibrillation (from the multi-ethnic study of atherosclerosis) Am J Cardiol. 2014;114:587–592. doi: 10.1016/j.amjcard.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli C, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50(9):1225–1233. doi: 10.1038/s41588-018-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheitz JF, Erdur H, Haeusler KG, Audebert HJ, Roser M, Laufs U, Endres M, Nolte CH. Insular cortex lesions, cardiactroponin, and detection of previously unknown atrial fibrillation in acute ischemic stroke. Stroke. 2015;46(5):1196–1201. doi: 10.1161/STROKEAHA.115.008681. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Rifai N, et al. C-reactive protein and the risk of developing hypertension. ACC Curr J Rev. 2004;13(3):29. doi: 10.1016/j.accreview.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Magi R, Strawbridge R, Pers TH, Fischer K, Justice AE, Workalemahu T, Wu JM, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sposato LA, Cipriano LE, Saposnik G, et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14:377–387. doi: 10.1016/S1474-4422(15)70027-X. [DOI] [PubMed] [Google Scholar]
- Sposato LA, Riccio PM, Hachinski V. Poststroke atrial fibrillation: cause or consequence? Critical review of current views. Neurology. 2014;82(13):1180–1186. doi: 10.1212/WNL.0000000000000265. [DOI] [PubMed] [Google Scholar]
- Tikkanen E, Gustafsson S, Knowles JW, Perez M, Burgess S, Ingelsson E, et al. Body composition and atrial fibrillation: a Mendelian randomization study. Eur Heart J. 2019;40(16):1277–1282. doi: 10.1093/eurheartj/ehz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Swerdlow DI, Preiss D, et al. Lipid fractions and contrasting risks of coronary artery disease and diabetes. JAMA Cardiol. 2016;1(6):692. doi: 10.1001/jamacardio.2016.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf PA, Dawber TR, Thomas HE, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28:973–977. doi: 10.1212/WNL.28.10.973. [DOI] [PubMed] [Google Scholar]
- Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;11:1173–1186. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghi S, Moon YP, Mora-Mclaughlin C, et al. Left atrial enlargement and stroke recurrence: the northern Manhattan stroke study. Stroke. 2015;46(6):1488. doi: 10.1161/STROKEAHA.115.008711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Karlsson IK, Karlsson R, et al. Exploring the causal pathway from telomere length to coronary heart disease: a network mendelian randomization study. Circ Res. 2017;121:214–219. doi: 10.1161/CIRCRESAHA.116.310517. [DOI] [PubMed] [Google Scholar]
- Zhang X-F, Attia J, D’Este C, Ma X-Y. The relationship between higher blood pressure and ischaemic, haemorrhagic stroke among Chinese and Caucasians: meta-analysis. Eur J Cardiovasc Prev Rehabil. 2006;13:429–437. doi: 10.1097/01.hjr.0000214607.99113.b8. [DOI] [PubMed] [Google Scholar]
- Zheng J, Baird D, Borges MC, et al. Recent developments in Mendelian randomization studies. Curr Epidemiol Rep. 2017;4(4):330–345. doi: 10.1007/s40471-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Methods details including Inverse-variance weighted method, MR-Egger method, Weak Instruments and Mediation analysis. Leave-one-out plots in the sensitive analysis.
Additional file 2. The basic characters of summary data used in this study. Details of instrumental invariables selected for each traits.
Data Availability Statement
The GWAS summary statistics datasets we used in this study were from the ISGC Consortium (https://strokegenetics.org/) for ischemic stroke; MAGIC Consortium (http://www.magicinvestigators.org/) for FastingInsulin; GIANT Consortium (https://www.broadinstitute.org/collaboration/giant/index) for body mass index, waist circumference and height; Neale Lab (http://www.nealelab.is/) for systolic and diastolic blood pressure; Astle W for white blood cell count; MRC-IEU Consortium (http://www.bristol.ac.uk/integrative-epidemiology/) for procalcitonin; AFGen Consortium (https://www.afgen.org/) for atrial fibrillation.