Abstract
Background
In 2011, Benin’s National Malaria Control Programme (NMCP) organized a nationwide mass distribution campaign of LLINs throughout the country. Following this intervention, it was important to assess whether the level of susceptibility of malaria vectors to insecticides had remained the same as compared to the pre-intervention period. The current study investigated this.
Methods
Larval collections were conducted in Ifangni, Sakété, Pobè and Kétou districts located in Plateau department, Southeastern Benin before (2009) and after (2012–2013) LLIN distribution. Anopheles gambiae sensu lato (s.l.) larvae from the 4 study districts were reared to adulthood and WHO susceptibility tests were conducted. The insecticides tested were deltamethrin (0.05%), permethrin (0.75%), bendiocarb (0.1%) and DDT (4%). Molecular species identification as well as, the characterization of the kdr L1014F mutation were also performed in the An. gambiae s.l. complex using PCR method.
Results
Overall, a significant decrease in mortality rates of An. gambiae s.l. to deltamethrin (0.05%), permethrin (0.75%) and DDT (4%) was observed post-LLIN distribution, respectively: (100% vs 80.9%, p < 0.0001), (77.5% vs 70%, p = 0.01) and, (47.8% vs 4.4%, p < 0.0001). By contrast, susceptibility of vectors to bendiocarb (0.1%) remained the same (100% mortality in the WHO susceptibility tube tests) pre- and post-intervention. An increase in the kdr L1014F frequency was observed post-LLIN distribution [F(kdr) = 0.91)] compared to the pre-intervention period [F(kdr) = 0.56], p < 0.0001. Anopheles coluzzii and An. gambiae were the two molecular species identified in the study area.
Conclusion
The decrease susceptibility to pyrethroids and DDT as well as, the increase in the frequency of the kdr L1014F mutation after the intervention stressed at the time, the need for the development and implementation of effective insecticide resistance management strategies. At present, an update of the vectors resistance status in the area is also necessary for decision-making.
Keywords: MIILDs, Efficacy, Resistance, Anopheles gambiae sensu lato
Background
In sub-Saharan Africa, the major vector of Plasmodium falciparum, the parasite responsible for the most severe form of human malaria, is Anopheles gambiae sensu lato (s.l.) [1]. This highly anthropophilic mosquito comprises 8 sub-species among which An. gambiae sensu stricto, Anopheles coluzzii and Anopheles. arabiensis are the major malaria vectors in Africa [2]. The vector control strategy of Benin’s National Malaria Control Programme (NMCP) relies mainly on the distribution of LLINs and indoor residual spraying (IRS). With the financial support of the President’s Malaria Initiative (PMI) of the US government and the World Bank, the NMCP launched a national mass LLIN distribution campaign in 2011 to ensure universal coverage of the population (1 LLIN for every 1.8 people) [3]. LLINs are an excellent means of providing personal and community protection from malaria [4, 5]. Until very recently, pyrethroids were the only insecticide class used for impregnation of LLINs, owing to their rapid action, excito-repellent effects, effectiveness at low doses and low toxicity to humans [6]. Unfortunately, pyrethroid resistance in malaria vectors has emerged and spread rapidly in several parts of Africa, including Benin [7–11], Burkina Faso [12], Cameroon [13], Côte d’Ivoire [14, 15] and, Kenya [16]. In Benin, kdr L1014F mutation as well as metabolic enzymes such as CYP450s, CYP6P3 and CYP6M2 [10, 11, 17, 18] are implicated in malaria vector resistance to pyrethroids.
Distribution of Olyset® nets, a polyethylene 150D LLIN impregnated with permethrin (2%), was carried out by the NMCP in Benin in 2011 with the aims of achieving universal coverage of populations-at risk. It is possible that this expansion of pyrethroid-based vector control may have eliminated susceptible mosquitoes in favour of resistant ones, thus increasing levels of pyrethroid resistance in malaria vector populations [19]. Considering this, the aim of the current study was to monitor changes in insecticide susceptibility and the frequency of the kdr L1014F mutation in the natural populations of An. gambiae s.l. before (2009) and after (2012–2013) the distribution of LLINs in 4 districts of the Plateau department, South-East Benin.
Methods
Study area
The study was performed in Ifangni, Sakété, Pobè and Kétou, 4 districts of the Plateau department, Southeastern Benin (Fig. 1). As of 2013, this department had an area of 3264 km2 and a total population of 624,146 inhabitants [20]. The climate is of the Guinean type with two rainy (March–July and, September–November) and, two dry (August and, December–February) seasons occurring annually. There are two cropping periods during the year that coincide with the rainy seasons. Agriculture is the main activity of populations in the Plateau department.
Fig. 1.
Map showing the study area
Collecting mosquito larvae
Field visits were organized between May and July in search of the breeding sites of An. gambiae s.l. in 2009 and 2012–2013 respectively before and after the LLIN distribution which occurred in July 2011. In each district, the sampling was performed in 2–3 villages that were the same pre- and post-intervention. Once breeding sites were identified, larvae and pupae were collected from the surface of the water using a larval dipper. The harvested larvae were then reared to adulthood at CREC insectary.
WHO susceptibility testing
Susceptibility of 3–5 day old female An. gambiae s.l. were assessed through the World Health Organization (WHO) tube test method [21]. All tests were performed with papers impregnated at the diagnostic dose recommended by the WHO: deltamethrin (0.05%), permethrin (0.75%), DDT (4%) and bendiocarb (0.1%). For each insecticide, mosquitoes were introduced into four tubes lined with insecticide-impregnated paper in batches of 25 and exposed for 1 h. A fifth batch of mosquitoes was exposed to a tube lined with untreated paper which served as a control. During exposure to the insecticide, the number of mosquitoes’ knocked down by the insecticide was noted after 5, 10, 20, 30, 40, 45, 50 and 60 min. Mosquitoes of the susceptible reference strain of An. gambiae (Kisumu) were also subjected to the impregnated papers to ensure their quality. After 60 min of exposure, the mosquitoes were transferred to the observation tubes and provided a 10% honey solution and kept under observation for 24 h. After the tests, dead and live mosquitoes were kept separately in Eppendorf tubes containing silica gel and cotton and stored at − 20 °C for molecular characterization of resistance mechanisms and species.
Molecular analysis
Female mosquitoes from susceptibility tube testing were analysed by PCR according to the protocols described by Scott et al. [22] and Favia et al. [23] for species and molecular forms identification respectively. The kdr L1014F mutation was also detected in the dead and live mosquitoes [24]. Only, a subset of mosquitoes (24–45 specimens randomly selected per district per year) was screened for molecular analyses.
Statistical analysis
Data from this study was collected in two periods (Pre-intervention: in 2009 and, post-intervention: from 2012 to 2013). Mortality of An. gambiae s.l. following exposure to insecticides as well as the frequencies of the kdr L1014F mutation frequencies recorded during the two periods (2009 versus 2012–2013) were compared using a Chi square test. The same test was also used to compare kdr L1014F frequencies between dead and live mosquitoes exposed to deltamethrin, to investigate whether other mechanisms were implicated in resistance. A logistic regression followed by a maximum likelihood test was performed to assess whether the kdr L1014F mutation and, molecular species had an effect on the resistance status (dead or alive) of mosquitoes after susceptibility testing.
The association between Mmortality and kdr L1014F frequency was tested by calculating the risk ratio (RR), using the unconditional maximum likelihood estimation (Wald), and small sample adjustment (small). The RR Confidence intervals were determined using the normal approximation (Wald), and normal approximation with small sample adjustment (small), and bootstrap method (boot). Statistical analyses were performed with the R-2.15.2 software [25].
Results
Susceptibility of An. gambiae to insecticides pre- and post-intervention
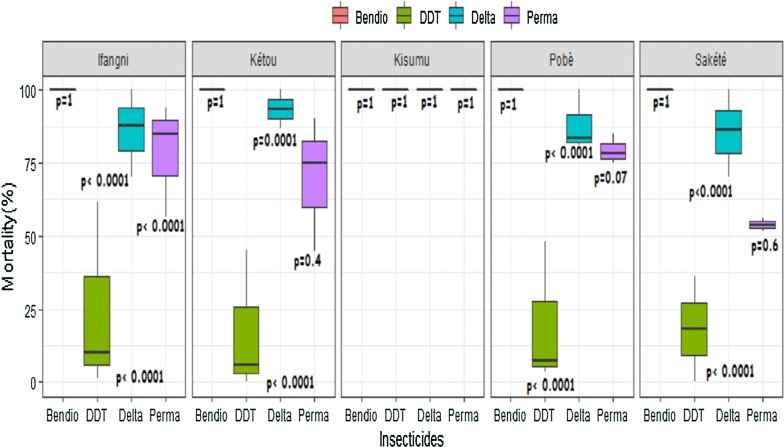
Throughout the study, the Kisumu reference strain was susceptible to all tested insecticides [deltamethrin (0.05%), permethrin (0.75%), bendiocarb (0.1%) and DDT (4%)] (Fig. 2). In all study districts, wild populations of An. gambiae s.l. were fully susceptible to bendiocarb with a 100% mortality rate recorded regardless of the period (pre- and post-intervention) (Fig. 2).
Fig. 2.
Variation in mortality rates of wild females An. gambiae s.l. and, of lab susceptible strain (An. gambiae Kisumu) to insecticides pre- and post-intervention. Bendio: Bendiocarb; Delta: Deltamethrin; Perm: Permethrin; DDT: Dichlorodiphenyltrichloroethane. The two lines outside the boxes are the whiskers
With deltamethrin, the mortality rate pre-intervention was 100% in all districts. However, this rate decreased to 78.9% (95% CI 73.3–84.5%) in Ifangni (p < 0.0001), 86.8% (95% CI 80.6–93%) in Kétou (p = 0.0001), 82.1% (95% CI 76.7–87.6%) in Pobè (p < 0.0001) and 78.1% (95% CI 72.2–84.1%) in Sakété (p < 0.0001) post-intervention (Fig. 2). Combining the data from the 4 districts, mortality rate of mosquitoes to deltamethrin decreased from 100% before intervention to 80.90% (95% CI 76.9–83.3%) after (p < 0.0001).
A similar trend was observed with permethrin in Ifangni where significant reduction in mortality rate was observed post-intervention [94% (95% CI 89.3–98.7%) vs 70.1% (95% CI 63.5–76.7%), p < 0.0001]. However, in the other three districts, although a slight decrease in susceptibility to permethrin was observed post-intervention, this effect was not significant [Kétou: 75% (95% CI 66.5–83.5%) vs 70.2% (95% CI 63.3–77.2%), p = 0.4], [Sakété: 56% (95% CI 46.3–65.7%) vs 51.7% (95% CI 39–64.3%), p = 0.6] and, [Pobè: 85% (95% CI 78–92%) vs 75.8% (95% CI 69.7–82%), p = 0.07] (Fig. 2). Combined data from the 4 districts show a significant decrease of mortality rate to permethrin post-intervention [77.5% (95% CI 73.4–81.6%) vs 70.1% (95% CI 67–73.2%), p = 0.01].
An increase in phenotypic resistance to DDT post-intervention was also observed in Ifangni (5.9%, 95% CI 2.3–9.4%), Sakété (0%), Pobè (4.4%, 95% CI 1.2–7.6%) and, Ketou (3.9%, 95% CI 0.5–7.3%) compared to the pre-intervention period where mortality rates were 62% (95% CI 52.5–71.5%, p < 0.0001), 36% (95% CI 26.6–45.4%, p < 0.0001), 48% (95% CI 38.2–57.8%, p < 0.0001) and, 45% (95% CI 35.2–54.8%, p < 0.0001), respectively (Fig. 2). Similarly, the combined data of the 4 districts show a decrease in the susceptibility of An. gambiae s.l. to DDT post-intervention [47.8% (95% CI 42.9–52.6%) vs 4.4% (95% CI 2.6–6.2%), p < 0.001].
Frequency of the molecular species of the An. gambiae complex in the four study districts pre- and post-intervention
Table 1 shows the frequency of molecular species of the An. gambiae s.l. complex in the 4 study districts pre- and post-intervention. In total, 356 female An. gambiae s.l. collected over the study were analysed (Table 1). Pre-intervention (2009), only An. coluzzii had been detected in the four districts. The post-intervention (2012–2013) results revealed a proportion of 29.65% of An. gambiae and 70.35% of An. coluzzii after cumulating data of all 4 districts. The frequency of An. gambiae was of 7.14% in Ifangni, 20.83% in Sakété, 28% in Pobè and 65.52% in Ketou (Table 1).
Table 1.
Frequency of molecular species by district according to the study period (pre and post intervention) in Benin
| Districts | Periods | Ag s.l. | Molecular species | |||
|---|---|---|---|---|---|---|
| Ac | % Ac | Ag | % Ag | |||
| Ifangni | Pre-intervention | 40 | 40 | 100 | 0 | 0 |
| Post-intervention | 70 | 65 | 92.86 | 5 | 7.14 | |
| Sakété | Pre-intervention | 30 | 30 | 100 | 0 | 0 |
| Post-intervention | 48 | 38 | 79.17 | 10 | 20.83 | |
| Pobè | Pre-intervention | 30 | 30 | 100 | 0 | 0 |
| Post-intervention | 50 | 36 | 72 | 14 | 28 | |
| Kétou | Pre-intervention | 30 | 30 | 100 | 0 | 0 |
| Post-intervention | 58 | 20 | 34.48 | 38 | 65.52 | |
| Total | Pre-intervention | 130 | 130 | 100 | 0 | 0 |
| Post-intervention | 226 | 159 | 70.35 | 67 | 29.65 | |
Ag s.l.: Anopheles gambiae s.l., Ac: Anopheles coluzzii, Ag: Anopheles gambiae
Frequencies of the kdr L1014F mutation in the 4 study districts pre- and post-intervention
In the pre-intervention period, the kdr L1014F frequencies were relatively low in Ifangni [f(kdr L1014F) = 0.03] and Pobè [f (kdr L1014F) = 0.58] whereas they were very high in Sakété [f (kdr L1014F) = 0.90] and Ketou [f (kdr L1014F) = 0.92] (Table 2).
Table 2.
Frequencies of the Kdr L1014F mutation in the 4 study districts pre- and post intervention in Benin
| Districts | Periods | Total | Genotypes Kdr L1014F | F(Kdr L1014F) | x2-value | df | p value | ||
|---|---|---|---|---|---|---|---|---|---|
| RR | RS | SS | |||||||
| Ifangni | Pre-intervention | 40 | 0 | 2 | 38 | 0.03a | 186.36 | 1 | < 0.0001 |
| Post-intervention | 73 | 67 | 5 | 1 | 0.95b | ||||
| Sakété | Pre-intervention | 30 | 24 | 6 | 0 | 0.90a | 0.406 | 1 | 0.523 |
| Post-intervention | 79 | 69 | 10 | 0 | 0.94a | ||||
| Pobè | Pre-intervention | 30 | 11 | 13 | 6 | 0.58a | 10.28 | 1 | 0.0013 |
| Post-intervention | 80 | 55 | 19 | 6 | 0.81b | ||||
| Kétou | Pre-intervention | 30 | 25 | 5 | 0 | 0.92a | 0.0105 | 1 | 0.918 |
| Post-intervention | 88 | 77 | 10 | 1 | 0.93a | ||||
| Total | Pre-intervention | 130 | 60 | 26 | 44 | 0.56a | 138.67 | 1 | < 0.0001 |
| Post-intervention | 320 | 268 | 44 | 8 | 0.91b | ||||
a,bValues with different superscripts pre and post intervention within a same district are significantly different (p < 0.05)
Post-intervention, these frequencies were 0.94 in Sakété and 0.93 in Kétou and did not differ significantly compared to the pre-intervention period (p > 0.05) (Table 2). By contrast, a significant increase in the frequencies of the kdr L1014F mutation was observed post-intervention, in Ifangni [f (kdr L1014F) = 0.95, p < 0.0001] and Pobè [f (kdr L1014F) = 0.81, p = 0.0013] (Table 2). The evolution of this frequency was much more marked in Ifangni (0.03 pre-intervention vs. 0.95 post-intervention, p < 0.0001). Overall, by aggregating data from the 4 districts, the kdr L1014F frequency increased from 0.56 pre-intervention to 0.96 post-intervention (p < 0.0001) (Table 2).
Frequencies of the kdr L1014F mutation in dead and live mosquitoes to deltamethrin in 2013
To evaluate the involvement of other mechanisms of pyrethroid resistance in An. gambiae s.l. from Plateau department, the kdr L1014F frequencies were compared between dead and live mosquitoes from susceptibility tests carried out with deltamethrin in 2013. In all districts, the results show that the kdr L1014F frequencies of dead mosquitoes were similar to those of live ones (p > 0.05) (Table 3). This result suggests that the kdr L1014F mutation is not the only mechanism involved in vector resistance to pyrethroids. However, the small numbers of mosquitoes tested in some cases did not allow for a representative estimate of the frequency of the kdr L1014F mutation.
Table 3.
Frequencies of the Kdr L1014F mutation in dead and live mosquitoes to deltamethrin in Benin
| Districts | Mosquito status | Total | Genotypes Kdr L1014F | F(kdr) | X2-value | df | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| RR | RS | SS | |||||||
| Ifangni | Dead | 20 | 18 | 1 | 1 | 92.5a | 1.38 | 1 | 0.239 |
| Live | 20 | 20 | 0 | 0 | 100a | ||||
| Sakété | Dead | 20 | 20 | 0 | 0 | 100a | n/a | 1 | 1 |
| Live | 12 | 12 | 0 | 0 | 100a | ||||
| Pobè | Dead | 20 | 19 | 1 | 0 | 97.5a | < 0.0001 | 1 | 1 |
| Live | 2 | 2 | 0 | 0 | 100a | ||||
| Kétou | Dead | 20 | 20 | 0 | 0 | 100a | n/a | 1 | 1 |
| Live | 9 | 9 | 0 | 0 | 100a | ||||
| Total | Dead | 80 | 77 | 2 | 1 | 97.5a | 1.91 | 1 | 0.166 |
| Live | 43 | 43 | 0 | 0 | 100a | ||||
n/a no data
aValues with the same superscripts pre and post intervention within a same district are statistically similar (p > 0.05)
Assessment of the effect of kdr L1014F mutation and molecular species on the mortality of An. gambiae to deltamethrin in 2013
Table 4 shows the result of the logistic regression performed, to assess the effect of the kdr L1014F mutation and molecular species on the mosquito mortality to deltamethrin. Thus, no effect of kdr L1014F mutation (p = 0.273) and molecular species (p = 0.072) on the mosquito mortality is observed.
Table 4.
Effect of Kdr L1014F mutation and molecular species on the mosquito mortality to deltamethrin in 2013 in Benin
| Explanatory variables | OR (95% CI) | p (Wald’s test) | p (LR-test) |
|---|---|---|---|
|
Kdr L1014F: RR |
1 | – | 0.273 |
| RS | 8649436.23 (0, Inf) | 0.992 | |
| SS | 8649436.23 (0, Inf) | 0.995 | |
|
Molecular species: An. gambiae vs An. coluzzii |
2.03 (0.93, 4.44) | 0.076 | 0.072 |
Evaluating the association between kdr L1014F and mortality rate in An. coluzzii and An. gambiae
In An. coluzzii, the kdr L1014F frequency was positively associated to the mortality rate (RR > 1) but, the association is not significant (p > 0.05) (Table 5). In An. gambiae, it was not possible to test the association as no RS or SS genotype was observed (Table 5).
Table 5.
Association between the Kdr L1014F mutation and mortality to deltamethrin in 2013 in Benin
| Molecular species | Genotypes (Kdr L1014F) |
N (dead) | N tested | Mortality (%) | RR | CI-95% (RR) | p-value (Fisher test) |
|---|---|---|---|---|---|---|---|
| An. coluzzii | RR | 37 | 65 | 56.9 | 1 | – | – |
| RS | 2 | 2 | 100 | 1.8 | 1.4–2.2 | 0.5 | |
| SS | 1 | 1 | 100 | 1.8 | 1.4–2.2 | 1 | |
| An. gambiae | RR | 40 | 54 | 74.1 | 1 | – | – |
| RS | 0 | 0 | ND | ND | ND | 1 | |
| SS | 0 | 0 | ND | ND | ND | 1 |
RR risk ratio, CI confidence interval, ND: no data
Discussion
The current study evaluated the evolution of insecticide resistance of malaria vectors in the Plateau department following mass distribution of Olyset nets in 2011 by the NMCP. Overall, An. gambiae s.l. was the main malaria vector in the Plateau department as previously showed by Padonou et al. [19] in Ouémé, a bordering department of Plateau. The molecular characterization revealed the simultaneous presence of An. gambiae and An. coluzzii post-intervention, whereas pre-intervention, only An. coluzzii was found. The detection of An. coluzzii only, over the pre-intervention period could presumably be due to the fact that, the data collection covered a shorter period as compared to the post-intervention period which spanned 2 years. This fully justified the low number of vector specimens sampled for PCR analysis pre-intervention. Thus, it is possible that An. gambiae were present at a very low frequency during the pre-intervention period and that a greater sample size of vectors may have demonstrated their presence. Moreover, the post-intervention data might have been generated with adult mosquitoes having emerged from larvae collected in highly diverse breeding sites as compared to the pre-intervention period. Hence, data collected during the post-intervention period may have provided a more representative capture of vector diversity and the species present in the study sites.
Post-intervention, the decrease in susceptibility to permethrin, deltamethrin and DDT combined with the significant increase in kdr L1014F frequency could be due to increased use of pyrethroid LLINs following the mass distribution by the NMCP in 2011. Similar observations have been made in Kenya and Niger, respectively by Stump et al. [26] and Czeher et al. [27]. Indeed LLINs might have killed susceptible mosquitoes within natural populations, thus selecting for resistant ones that will mate and produce more resistant offspring. Domestic use of aerosol insecticides [28] as well as the uncontrolled use of insecticides in agriculture [29] observed in Southern Benin, might have also been causal factors of increased pyrethroid resistance levels.
The decrease susceptibility to pyrethroid insecticides as well as the continued susceptibility to bendiocarb observed post-intervention in An. gambiae s.l. suggest that IRS with carbamate insecticides could effectively control An. gambiae s.l. in the Plateau Department. A combined intervention of pyrethroid LLINs and IRS with bendiocarb could be particularly effective in improving the impact of control whilst delaying the onset of resistance. However, the emergence of carbamate resistance in Atacora, a department in Northern Benin [30], emphasizes the importance of judicious insecticide application. Rotational use of IRS insecticides such as bendiocarb, pirimiphos-methyl and clothianidin, could prevent the establishment of resistance and preserve the effectiveness of the non-pyrethroid insecticide classes.
The logistic regression performed reveals that the kdr L1014F mutation as well as the molecular species were non-significantly correlated with the mortality rate to deltamethrin, which suggests that if they had an impact, it was in a very low way. The results are similar to those from Reimer et al. [31] in Cameroon. Thus, apart from the kdr L1014F mutation, a combination of other resistance mechanisms might explained the pyrethroid resistance observed in An. gambiae s.l. This is confirmed by the similarity of the kdr L1014F frequency in dead and live mosquitoes of the 4 surveyed districts. A non significant association between the kdr L1014F mutation and the mortality rate to deltamethrin was also observed in An. coluzzii while, the opposite result was obtained by Ibrahim et al. [32] with lambacyhalothrin.
The presence of mono-oxygenase mediated pyrethroid resistance has been demonstrated in An. gambiae s.l. collected in Missérété, a neighbouring site of the Plateau Department [33]. In addition, the presence of the N1575Y mutation was demonstrated in the natural populations of An. gambiae s.l. in Covè, another neighbouring district of the Plateau department [18]. It is, therefore, possible that the detoxification enzymes as well as the N1575Y mutation are also implicated in the resistance of the vectors to pyrethroids in the Plateau department.
Conclusion
The data of the current study provide important information on vector resistance to insecticides in the Plateau Department, following mass deployment of LLINs. The decrease of the susceptibility of An. gambiae s.l. to pyrethroids and DDT, as well as the increase of the frequency of the kdr L1014F mutation constituted an alert to the NMCP which should at the time, consider development and implementation of an effective resistance management strategy. At the molecular level, it would have been of interest to perform insecticide resistance intensity tests, and Taqman PCR assays to evaluate the contribution of metabolic enzymes and N1575Y mutation to vector resistance to pyrethroids. At present, the effectiveness of a strategy for combatting malaria vectors in the area requires an update of their resistance status.
Acknowledgements
We thank the Centre de Recherche Entomologique de Cotonou (CREC) which provided funding for this study. We are also grateful to Virgile Gnanguenon who drawn the map of the study area and, the technicians for their huge contribution during field and lab work.
Abbreviations
- NMCP
National Malaria Control Programme
- LLIN
long-lasting insecticidal net
- Kdr
knock down resistance
- IRS
indoor residual spraying
- CREC
Centre de Recherche Entomologique de Cotonou
- WHO
World Health Organization
- DDT
dichlorodiphenyltrichloroethane
- PCR
polymerase chain reaction
Authors’ contributions
AS and MCA designed the study. AS, ASS, RA, CZK, UCN carried out the surveys. FOA analysed the data. AS, RG, RO, GGP and CZK drafted the manuscript. RA, GGP, RG, TS, FT and MCA critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This study has been financially supported by Centre de Recherche Entomologique de Cotonou.
Availability of data and materials
All data generated or analysed during this study are included in this article and are available from the corresponding author.
Ethical consideration and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. The technical basis for coordinated action against insecticide resistance. Preserving the effectiveness of modern malaria vector control, meeting report, 4–6 May 2010. Geneva: World Health Organization; 2011.
- 2.Coetzee M, Hunt H, Wilkerson R, Della Tore A, Coulibali MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–274. doi: 10.11646/zootaxa.3619.3.2. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Achieving and maintaining universal coverage with long-lasting insecticidal nets for malaria control. Geneva: World Health Organization; 2017. [Google Scholar]
- 4.Damien GB, Djènontin A, Chaffa E, Yamadjako S, Drame PM, Ndille EE, et al. Effectiveness of insecticidal nets on uncomplicated clinical malaria: a case-control study for operational evaluation. Malar J. 2016;15:102. doi: 10.1186/s12936-016-1156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hounkonnou C, Djènontin A, Egbinola S, Houngbegnon P, Bouraima A, Soares C, et al. Impact of the use and efficacy of long lasting insecticidal net on malaria infection during the first trimester of pregnancy—a pre-conceptional cohort study in southern Benin. BMC Public Health. 2018;18:683. doi: 10.1186/s12889-018-5595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaim M, Guillet P. Alternative insecticides: an urgent need. Trends Parasitol. 2002;18:161–163. doi: 10.1016/S1471-4922(01)02220-6. [DOI] [PubMed] [Google Scholar]
- 7.Akogbéto M, Yacoubou S. Resistance of malaria vectors to pyrethroids used for impregnated bednets, Benin, West Africa. Bull Soc Path Exot. 1999;92:123–130. [PubMed] [Google Scholar]
- 8.Corbel V, N’Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007;101:207–216. doi: 10.1016/j.actatropica.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Sovi A, Djegbe I, Soumanou L, Tokponnon F, Gnanguenon V, Azondekon R, et al. Microdistribution of the resistance of malaria vectors to deltamethrin in the region of Plateau (southeastern Benin) in preparation for an assessment of the impact of resistance on the effectiveness of long-lasting insecticidal nets (LLINs) BMC Infect Dis. 2014;14:103. doi: 10.1186/1471-2334-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aïkpon R, Sèzonlin M, Ossè R, Akogbéto M. Evidence of multiple mechanisms providing carbamate and organophosphate resistance in field An. gambiae population from Atacora in Benin. Parasit Vectors. 2014;7:568. doi: 10.1186/s13071-014-0568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salako AS, Ahogni I, Aïkpon R, Sidick A, Dagnon F, Sovi A, et al. Insecticide resistance status, frequency of L1014F Kdr and G119S Ace-1 mutations, and expression of detoxification enzymes in Anopheles gambiae (s.l.) in two regions of northern Benin in preparation for indoor residual spraying. Parasit Vectors. 2018;11:618. doi: 10.1186/s13071-018-3180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabaté A, Baldet T, Chandre F, Guiguemde RT, Brengues C, Guillet P, et al. First report of the kdr mutation in An. gambiae M form from Burkina Faso, West Africa. Parassitologia. 2002;44:157–158. [PubMed] [Google Scholar]
- 13.Etang J, Fonjo E, Chandre F, Morlais I, Brengues C, Nwane P, et al. First report of knockdown mutations in the malaria vector Anopheles gambiae from Cameroon. Am J Trop Med Hyg. 2006;74:795–797. doi: 10.4269/ajtmh.2006.74.795. [DOI] [PubMed] [Google Scholar]
- 14.Chandre F, Darriet F, Duchon S, Finot L, Manguin S, Carnevale P, et al. Modifications of pyrethroid effect associated with kdr mutation in Anopheles gambiae. Med Vet Entomol. 2000;14:81–88. doi: 10.1046/j.1365-2915.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 15.Camara S, Koffi AA, Ahoua Alou LP, Koffi K, Kabran JK, Koné A, et al. Mapping insecticide resistance in Anopheles gambiae (s.l.) from Côte d’Ivoire. Parasit Vectors. 2018;11:19. doi: 10.1186/s13071-017-2546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vulule JM, Beach RF, Atieli FK, Roberts JM, Mount DL, Mwangi RW. Reduced susceptibility of An. gambiae to permethrin associated with the use of permethrin impregnated bed nets and curtains in Kenya. Med Vet Entomol. 1994;8:71–75. doi: 10.1111/j.1365-2915.1994.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 17.Djouaka RF, Bakare AA, Coulibaly ON, Akogbeto MC, Ranson H, Hemingway J, et al. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genomics. 2008;9:538. doi: 10.1186/1471-2164-9-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngufor C, N’Guessan R, Fagbohoun J, Subramaniam K, Odjo A, Fongnikin A, et al. Insecticide resistance profile of Anopheles gambiae from a phase II field station in Cové, southern Benin: implications for the evaluation of novel vector control products. Malar J. 2015;14:464. doi: 10.1186/s12936-015-0981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padonou GG, Sezonlin M, Ossé R, Aizoun N, Oké-Agbo F, Oussou O, et al. Impact of three years of large scale Indoor Residual Spraying (IRS) and Insecticide Treated Nets (ITNs) interventions on insecticide resistance in Anopheles gambiae s.l. in Benin. Parasit Vectors. 2012;5:72. doi: 10.1186/1756-3305-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.INSAE, RGPH4. Cahier des villages et quartiers de ville du département du Plateau; 2016.
- 21.WHO . Report of the informal consultation Test procedures for insecticide resistance monitoring in malaria vectors, bio efficacy and persistence of insecticides on treated surfaces. Geneva: World Health Organization; 1998. [Google Scholar]
- 22.Scott J, Brogdon W, Collins F. Identification of single specimens of the Anopheles gambiae complex by PCR. Am J Trop Med Hyg. 1993;14:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 23.Favia G, Della TA, Bagayoko M, Lanfrancotti A, Sagnon NF, Toure Y, et al. Molecular identification of sympatric chromosomal forms of Anopheles gambiae and futher evidence of their reproductive isolation. Insect Mol Biol. 1997;14:377–383. doi: 10.1046/j.1365-2583.1997.00189.x. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team . A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 26.Stump AD, Atieli FK, Vulule JM, Besansky NJ. Dynamics of the pyrethroid knockdown resistance allele in Western Kenya populations of Anopheles gambiae in responseto Insecticide-treated bed net trial. Am J Trop Med Hyg. 2004;70:591–596. doi: 10.4269/ajtmh.2004.70.591. [DOI] [PubMed] [Google Scholar]
- 27.Czeher C, Labbo R, Arzika I, Duchemin JB. Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar J. 2008;7:189. doi: 10.1186/1475-2875-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padonou G, Yadouléton A, Noukpo H, Akogbéto M, Gbédjissi G, Bankolé H. Studying physical and sociological environment of malaria to implement an indoor insecticide spraying campaign in Oueme region, Benin. J Public Health Epidemiol. 2011;3:622–631. doi: 10.5897/JPHE11.127. [DOI] [Google Scholar]
- 29.Yadouleton A, Martin T, Padonou G, Chandre F, Asidi A, Djogbenou L, et al. Cotton pest management practices and the selection of pyrethroid resistance in Anopheles gambiae population in northern Benin. Parasit Vectors. 2011;4:60. doi: 10.1186/1756-3305-4-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aïkpon R, Agossa F, Ossè R, Oussou O, Aïzoun N, Oké-Agbo F, et al. Bendiocarb resistance in Anopheles gambiae s.l. populations from Atacora department in Benin, West Africa: a threat for malaria vector control. Parasit Vectors. 2013;6:192. doi: 10.1186/1756-3305-6-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reimer L, Fondjo E, Patchoké S, Diallo B, Lee Y, Ng A, et al. Relationship between Kdr mutation and resistance to pyrethroid and DDT insecticides in natural populations of Anopheles gambiae. J Med Entomol. 2008;45:260–266. doi: 10.1093/jmedent/45.2.260. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahim SS, Manu YA, Tukur Z, Irving H, Wondji CS. High frequency of kdr L1014F is associated with pyrethroid resistance in Anopheles coluzzii in Sudan savannah of Northern Nigeria. BMC Infect Dis. 2014;14:441. doi: 10.1186/1471-2334-14-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aïzoun N, Aïkpon R, Padonou GG, Oussou O, Oké-Agbo F, Gnanguenon V, et al. Mixed-function oxidases and esterases associated with permethrin, deltamethrin and bendiocarb resistance in Anopheles gambiae s.l. in the south-north transect Benin, West Africa. Parasit Vectors. 2013;6:223. doi: 10.1186/1756-3305-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this article and are available from the corresponding author.