Abstract
Background
Exercise during chemotherapy is suggested to provide clinical benefits, including improved chemotherapy completion. Despite this, few randomized controlled exercise trials have reported on such clinical endpoints. From the OptiTrain trial we previously showed positive effects on physiological and health‐related outcomes after 16 weeks of supervised exercise in patients with breast cancer undergoing chemotherapy. Here, we examined the effects of exercise on rates of chemotherapy completion and hospitalization, as well as on blood cell concentrations during chemotherapy.
Patients and Methods
Two hundred forty women scheduled for chemotherapy were randomized to 16 weeks of resistance and high‐intensity interval training (RT‐HIIT), moderate‐intensity aerobic and high‐intensity interval training (AT‐HIIT), or usual care (UC). Outcomes included chemotherapy completion, hospitalization, hemoglobin, lymphocyte, thrombocyte, and neutrophil concentrations during chemotherapy.
Results
No significant between‐groups differences were found in the proportion of participants who required dose reductions (RT‐HIIT vs. UC: odds ratio [OR], 1.08; AT‐HIIT vs. UC: OR, 1.39), or average relative dose intensity of chemotherapy between groups (RT‐HIIT vs. UC: effect size [ES], 0.08; AT‐HIIT vs. UC: ES, −0.07). A significantly lower proportion of participants in the RT‐HIIT group (3%) were hospitalized during chemotherapy compared with UC (15%; OR, 0.20). A significantly lower incidence of thrombocytopenia was found for both RT‐HIIT (11%) and AT‐HIIT (10%) versus UC (30%; OR, 0.27; OR, 0.27).
Conclusion
No beneficial effects of either RT‐HIIT or AT‐HIIT on chemotherapy completion rates were found. However, combined resistance training and high‐intensity interval training were effective to reduce hospitalization rates, and both exercise groups had a positive effect on thrombocytopenia. These are important findings with potential positive implications for the health of women with breast cancer and costs associated with treatment‐related complications.
Implications for Practice
Completing the prescribed chemotherapy regimen is strongly associated with a good prognosis for patients with primary breast cancer. Despite this, treatment‐induced side effects make it necessary to reduce or alter the treatment regimen and can also lead to hospitalization. Exercise during chemotherapy is suggested to provide clinical benefits, including improved chemotherapy completion. This study showed that combined resistance and high‐intensity interval training during chemotherapy resulted in lower hospitalization rates and a lower incidence of thrombocytopenia in women with breast cancer undergoing chemotherapy. However, no beneficial effects of either exercise program on chemotherapy completion rates were found, which is in contrast to previous findings in this population. The findings reported in the current article have positive implications for the health of women with breast cancer and costs associated with treatment‐related complications.
Keywords: Chemotherapy completion, Hospitalization, High intensity interval training, Breast cancer
Short abstract
The positive effect of supervised exercise in patients with breast cancer undergoing chemotherapy has previously been shown. This article focuses on the effects of exercise on chemotherapy completion and hospitalization rates, as well as blood cell concentrations during chemotherapy.
Introduction
Completing the prescribed chemotherapy regimen is strongly associated with a good prognosis for patients with primary breast cancer 1. Despite this, treatment‐induced side effects such as febrile neutropenia, neuropathies, and fatigue make it necessary to reduce or alter the treatment regimen and are also associated with increased hospitalization rates. For patients receiving chemotherapy for early‐stage breast cancer, relative dose intensities (RDIs) below 85% have been reported to be 26% for conventional chemotherapy regimens and 32% for those receiving dose‐dense chemotherapy regimens 2. Given the large number of women affected by the disease and that up to one third of women are unable to complete the recommended dose of adjuvant treatment, strategies to improve completion rates of chemotherapy need to be identified.
Few randomized controlled exercise trials have reported on clinical outcomes such as chemotherapy completion 3, hospitalization rates 4, and blood concentrations over the course of chemotherapy. Two previous trials that included patients with early‐stage breast cancer are available and showed that supervised resistance training 5 and supervised combined resistance and aerobic training 6 had beneficial effects on chemotherapy completion rates. A few trials in other cancer populations did, however, fail to find beneficial effects of exercise on chemotherapy completion rates 3. In patients undergoing allogeneic hematopoietic stem cell transplantation, exercise has been found to reduce the duration of hospitalization and the extent of neutropenia and thrombocytopenia 4. Moreover, muscle strength has been shown to be negatively associated with days of having fever 7. In healthy individuals, both moderate‐intensity as well as high‐intensity interval aerobic training has been shown to lead to an improved neutrophil function 8, and recent findings show that resistance training can increase the number of neutrophils and lymphocytes after exercise 9.
Low skeletal muscle mass concomitant with excess adiposity has been shown to be present in over one third of newly diagnosed patients with early‐stage breast cancer 10 and has clinical implications such as poorer treatment completion rates, leading to a poorer prognosis 11. In other cancer populations, greater muscle mass has been associated with higher chemotherapy completion rates 12, 13.
Previous findings from the OptiTrain trial showed that 16 weeks of supervised combined resistance and high‐intensity aerobic interval training (RT‐HIIT) improved cancer‐related fatigue, muscle strength 14, muscle function, and muscle mass as indicated by a larger muscle fiber cross‐sectional area 15. This finding and findings from others led to the hypothesis that participants in the RT‐HIIT group, through preservation of muscle mass and muscle function, would show improved treatment tolerance compared with the usual care group. Moreover, it was hypothesized that both exercise groups would have a lower incidence of hospitalization during chemotherapy because of an improved immune response by counteracting neutropenia and thrombocytopenia compared with the control group. The aim was to examine and compare the effects of combined resistance and high‐intensity aerobic interval training (RT‐HIIT) and moderate‐intensity continuous aerobic and high‐intensity aerobic interval training (AT‐HIIT) with those of usual care (UC) on chemotherapy completion and hospitalization rates, as well as blood cell concentrations during chemotherapy in patients with early‐stage breast cancer. Moreover, differences in characteristics and blood cell concentrations of patients were assessed comparing (A) those who completed chemotherapy with those who did not and (B) patients who were hospitalized with those who were not.
Materials and Methods
Participants and Study Design
The methods of the OptiTrain randomized controlled trial (NCT02522260, Optimal Training Women with Breast Cancer [OptiTrain], http://www.clinicaltrials.gov) have been reported elsewhere 16, 17. In brief, recruitment took place at two different oncology clinics in Stockholm, Sweden, from March 2013 to July 2016. Eligibility criteria were (A) women aged 18–70 years, (B) diagnosed with I–IIIa stage breast cancer, (C) who planned to receive adjuvant chemotherapy (consisting of anthracyclines, taxanes, or a combination of the two). Ethical approval was obtained from the Regional Ethical Review Board in Stockholm Sweden (Dnr 2012/1347‐31/1, 2012/1347‐31/2, 2013/7632‐32, 2014/408‐32) and was conducted in accordance with the Helsinki Declaration. All participants signed informed consent.
Participants were randomized to 16 weeks of resistance training combined with high‐intensity interval training (RT‐HIIT), moderate‐intensity aerobic training combined with high‐intensity interval training (AT‐HIIT), or usual care (UC). The intervention groups (RT‐HIIT and AT‐HIIT) commenced the exercise training 3 days after the second chemotherapy session and ended the intervention 3 weeks after the last chemotherapy session. The participant flow during and following the exercise trial is shown in Figure 1, which has been published previously 16.
Figure 1.
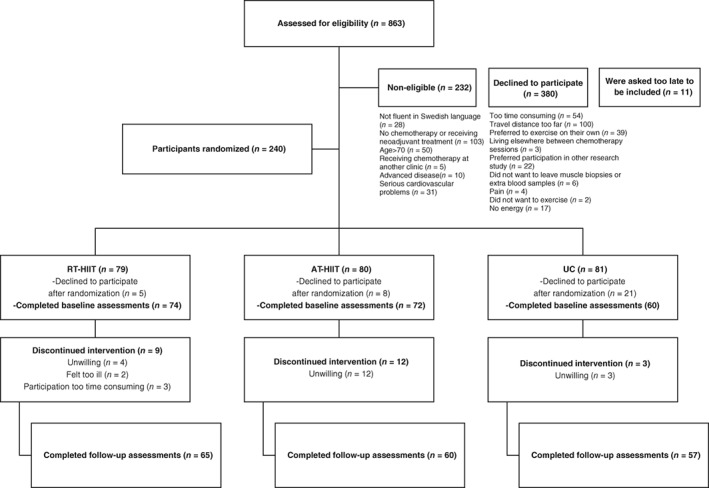
CONSORT flow diagram.
Abbreviations: AT‐HIIT, moderate‐intensity aerobic and high‐intensity interval training group; RT‐HIIT, resistance and high‐intensity interval training group; UC, usual care group.
Exercise Training Interventions
The OptiTrain exercise protocol has been described previously 16, 17. The exercise groups undertook the supervised exercise sessions in an in‐hospital exercise clinic twice per week for 16 weeks, and the session duration was approximately 60 minutes. High‐intensity interval training was combined either with resistance training (RT‐HIIT) or incorporated as part of an overall endurance training session (AT‐HIIT) in order to obtain similar exercise durations in both groups. In brief, the RT‐HIIT group performed high‐load resistance exercises targeting the major muscle groups consisting of two to three sets of 8–12 repetitions at an initial intensity of 70% of their estimated one repetition maximum (1‐RM), progressing to 80% of 1‐RM. The RT‐HIIT sessions concluded with three 3‐minute bouts of high‐intensity interval exercise at a rating of perceived exertion (RPE) of 16 to 18 on a cycle ergometer interspersed with one minute of recovery. The AT‐HIIT group initiated each session with 20 minutes of moderate‐intensity continuous aerobic exercise at an RPE of 13 to 15, followed by the same high‐intensity interval exercise as in RT‐HIIT. The UC group was given written information about physical activity at the initiation of the intervention period about exercise recommendations for patients with cancer according to American College of Sports Medicine guidelines 18.
Outcomes and Procedures
In the current study, the secondary outcomes of chemotherapy completion, hospitalization, and change in blood cell concentrations over the course of chemotherapy were reported, which were extracted from the patients’ medical records. The chemotherapy completion rate is reported as the mean RDI in mg × m−2 × wk−1, which represents the actual received dose intensity as a fraction of the originally planned dose intensity of the chemotherapy regimen by dividing the dose of chemotherapy per square meter in each cycle by the number of weeks in a cycle 19. A chemotherapy completer was defined as having an RDI of 100%, while someone with an RDI below 100% was considered a noncompleter. An in‐patient hospital stay for at least one night was considered as being hospitalized. Hemoglobin, neutrophil, lymphocyte, and thrombocyte concentrations were measured 1 to 3 days prior to each chemotherapy session (six time points) in the clinical laboratory at the Karolinska University Hospital.
Objectively measured activity patterns were assessed only at baseline by an accelerometer (GT3X; ActiGraph, Pensacola, FL). A more detailed description regarding methods and analysis has been published elsewhere 16. Cancer and general medical history and participant demographics were recorded by questionnaires. Attendance was calculated as the mean of the individual percentages (attended exercise sessions divided by the total number of sessions).
Statistical Analysis
The power calculation for the OptiTrain trial is based on the primary outcome cancer‐related fatigue and has been described previously 16. Non‐normally distributed data were log‐transformed prior to the main analysis. Independent t tests (continuous data) and χ2 or Fisher’s exact tests (categorical data) were used to assess differences at baseline between intervention groups and between the following subgroups: chemotherapy completers versus noncompleters; hospitalized and nonhospitalized patients. To compare the proportions of patients who were or were not hospitalized (binary outcome: hospitalization), and to compare those who required or did not require chemotherapy dose adjustments (binary outcome: chemotherapy completion), χ2 or Fisher’s exact test was used. The dose intensities of the actual received intensity and the originally planned dose intensity of the chemotherapy regimen were calculated as total milligrams of chemotherapy administered divided by the product of the body surface area and total number of weeks of treatment. The RDI was finally based on the actual dose intensity divided by the projected dose intensity as described previously 19. RDI was analyzed using a one‐way analysis of variance (ANOVA). To assess if there were differences between the groups in changes in hemoglobin, neutrophil, lymphocyte, and thrombocyte concentrations at the six time points over the course of chemotherapy, a linear mixed model was used adjusted for baseline values of the outcome. Analyses were performed on participants with at least one measurement and only for those who had at least a baseline measurement. The model accounts for missing data by taking the individual time trends and the observed group means at each time point into consideration to provide a more accurate estimate of the population mean at each time point. Between‐groups differences in the incidence of neutropenia (neutrophil concentration < 1.0 × 109/L), thrombocytopenia (thrombocyte concentration < 150 × 109/L), lymphocytopenia (lymphocyte concentration < 1.0 × 109/L), and anemia (hemoglobin concentration < 120 g/L) were assessed by a χ2 test. Bonferroni’s post hoc corrections were applied to both the one‐way ANOVA and the linear mixed model for multiple comparisons. For the binary outcomes (chemotherapy completion, hospitalization, and incidence of neutropenia, thrombocytopenia, and lymphocytopenia) corresponding odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. The mean differences and 95% CIs for the differences were calculated with corresponding effect sizes (ESs), in which between‐group differences of pre‐ and postintervention means were divided by the pooled baseline SD. Pooling only the pretest SD has been shown to provide an unbiased estimate of the effect size 20. According to Cohen, an ES between 0.2 and 0.5 indicates small differences, an ES between 0.5 and 0.8 indicates medium differences, and an ES larger than 0.8 indicates large differences 21. A two‐sided p < .05 was considered statistically significant for all analyses.
Results
The time period for baseline and 16 weeks postintervention ranged from March 2013 to July 2016. Baseline characteristics of the participants by intervention group are shown in Table 1. No significant differences in baseline characteristics were found between groups. Attendance of the exercise intervention for participants in the RT‐HIIT and AT‐HIIT groups were 68% and 63%, respectively, whereas adherence to intensity was 83% in the RT‐HIIT group and 75% in the AT‐HIIT group.
Table 1.
Participant baseline characteristics by group
| Participant characteristics | RT‐HIIT (n = 74) | AT‐HIIT (n = 72) | UC (n = 60) | p value |
|---|---|---|---|---|
| Age, mean ± SD, yr | 52.7 ± 10.3 | 54.4 ± 10.3 | 52.6 ± 10.2 | .482a |
| Body mass, mean ± SD, kg | 68.7 ± 11.3 | 67.7 ± 13.0 | 69.1 ± 11.0 | .778a |
| Body mass index, mean ± SD, kg/m2 | 25.1 ± 4.3 | 24.8 ± 4.4 | 25.0 ± 4.2 | .896a |
| SED, mean ± SD, min/day | 530.0 ± 70.0 | 544.0 ± 60.0 | 548.0 ± 67.0 | .219a |
| MVPA, mean ± SD min/day | 79.0 ± 25.0 | 71.0 ± 31.0 | 68.0 ± 31.0 | .112a |
| Married or partnered, % | 60.6 | 59.7 | 69.5 | .459b |
| University completed, % | 67.6 | 64.7 | 66.0 | .937b |
| Postmenopausal women, % | 51.4 | 63.9 | 61.7 | .350b |
| Comorbidities, % | 29.2 | 25.0 | 42.4 | .094b |
| Tumor receptor status, % | .153b | |||
| Triple negative | 14.9 | 11.0 | 16.7 | |
| HER2+, ER+/− | 21.6 | 30.2 | 20.0 | |
| HER2−, ER+ | 40.6 | 37.0 | 41.7 | |
| HER2−, ER− | 1.4 | 0.0 | 1.7 | |
| Chemotherapy received at baseline, % | .836b | |||
| Taxane or taxane + anthracycline based therapy | 59.4 | 63.0 | 58.3 | |
| Anthracycline based therapy | 40.6 | 37.0 | 41.7 | |
| G‐CSF treatment | 83.3 | 77.0 | 75.0 | .492b |
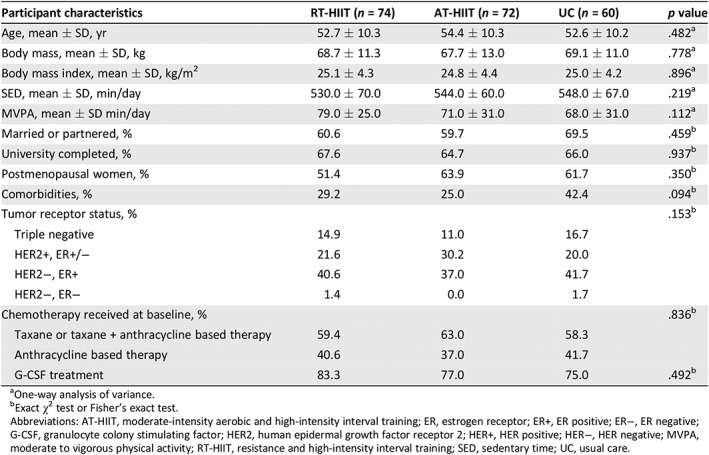
One‐way analysis of variance.
Exact χ2 test or Fisher's exact test.
Abbreviations: AT‐HIIT, moderate‐intensity aerobic and high‐intensity interval training; ER, estrogen receptor; ER+, ER positive; ER−, ER negative; G‐CSF, granulocyte colony stimulating factor; HER2, human epidermal growth factor receptor 2; HER+, HER positive; HER−, HER negative; MVPA, moderate to vigorous physical activity; RT‐HIIT, resistance and high‐intensity interval training; SED, sedentary time; UC, usual care.
Chemotherapy Completion Rates
For the binary outcome chemotherapy completion, no significant differences were found between groups for the proportion of participants who required dose reductions (RT‐HIIT vs. UC: OR, 1.08; 95% CI, 0.48–2.46; AT‐HIIT vs. UC: OR, 1.39; 95% CI, 0.63–3.07; Fig. 2A), and no differences were found when assessing RDI of chemotherapy (RT‐HIIT vs. UC: ES, 0.08; 95% CI, −0.05 to 0.06 mg × m−2 × wk−1; AT‐HIIT vs. UC: ES, −0.07; 95% CI, −0.07 to 0.04 mg × m−2 × wk−1; Fig. 2B). Among those who required dose reductions, the average RDI was 70% for the UC group, 75% for the RT‐HIIT group (RT‐HIIT vs. UC: ES, 0.37; 95% CI, −0.08 to 0.17 mg × m−2 × wk−1), and 73% for the AT‐HIIT group (AT‐HIIT vs. UC: ES, 0.24; 95% CI, −0.09 to 0.15 mg × m−2 × wk−1; Fig. 2C). Moreover, no significant between‐group differences were found for the percentage of participants who received more than 85% of their planned RDI, which was 86.7% in the UC group compared with 86.5% in the RT‐HIIT group (OR, 1.01; 95% CI, 0.61–1.65) and 77.8% in the AT‐HIIT group (OR, 0.90; 95% CI, 0.54–1.49). The most common reasons for an altered chemotherapy regimen are shown in supplemental online Figure 1. The most common reasons for dose adjustments varied by group, with neuropathy as the most common reason for the RT‐HIIT group, gastrointestinal (GI) side effects for the AT‐HIIT group, and neutropenia for the UC group.
Figure 2.
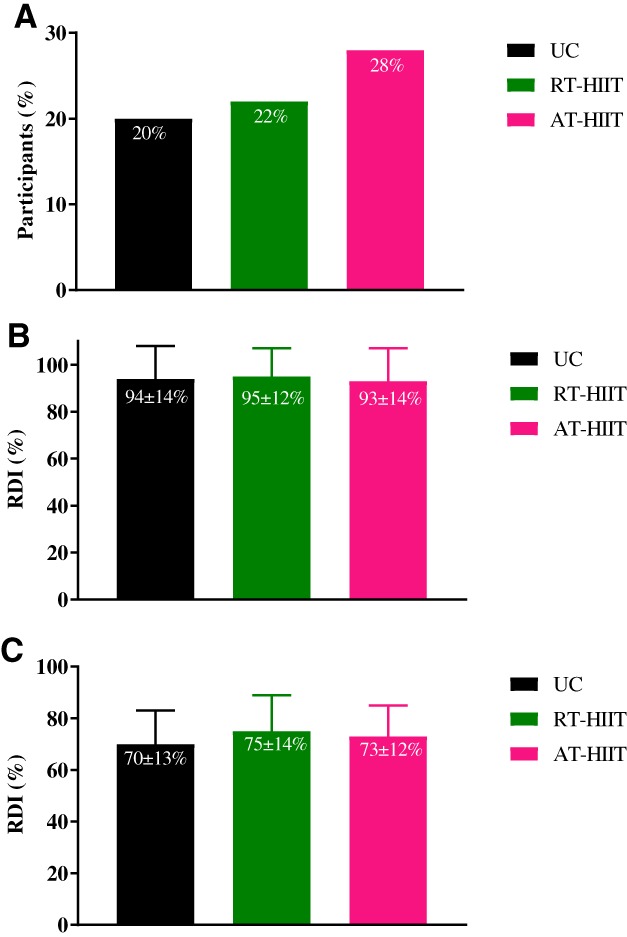
Effects of RT‐HIIT, AT‐HIIT, and UC on the (A): Proportion of participants requiring dose reductions for each group. (B): RDI of chemotherapy for all participants presented as means ± SD. (C): RDI of chemotherapy among those that required dose reductions presented as means ± SD.
Abbreviations: AT‐HIIT, moderate‐intensity aerobic and high‐intensity interval training; RDI, relative dose intensity; RT‐HIIT, resistance and high‐intensity interval training; UC, usual care.
A subgroup analysis comparing chemotherapy completers versus noncompleters showed that a significantly higher proportion of those who did not complete the planned dose intensity of chemotherapy also did not have a partner or were unmarried (p = .04) and received granulocyte colony stimulating factor (G‐CSF) prophylaxis compared with those who completed chemotherapy (p = .03) (supplemental online Table 1).
Hospitalization Rates
Hospitalization rates are shown in Figure 3. None of the hospitalized patients required a longer duration of hospital stay than one night, and the only reason for being hospitalized was febrile neutropenia (febrile neutropenia is defined as having a neutrophil count of less than 1.0 × 109/L and a temperature of 38°C or above on one occasion) 22. For the binary outcome hospitalization, a significantly lower proportion of participants in the RT‐HIIT group were hospitalized over the course of chemotherapy compared with UC (OR, 0.2; 95% CI, 0.04–0.99, p = .02).
Figure 3.
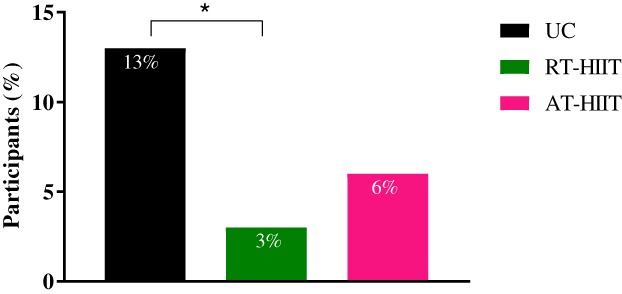
Percentage of each group being hospitalized in the RT‐HIIT, AT‐HIIT, and UC groups. * indicates p < .05 between groups.
Abbreviations: AT‐HIIT, moderate‐intensity aerobic and high‐intensity interval training; RT‐HIIT, resistance and high‐intensity interval training; UC, usual care.
A subgroup analysis comparing those who were hospitalized and not hospitalized showed that a significantly higher proportion of those who were hospitalized had comorbidities at baseline (p = .03) and were receiving taxane‐based chemotherapy compared with nonhospitalized patients (p = .04) (supplemental online Table 1).
Blood Cell Concentrations
For the outcome of blood cell concentrations over the course of chemotherapy (six time points), significantly higher thrombocyte concentrations were found for the RT‐HIIT group compared with UC prior to the third chemotherapy session (ES, 0.44; 95% CI, 0.84 to 54.47 × 109/L; p = .04) and compared with both UC (ES, 0.51; 95% CI, 3.78 to 57.62 × 109/L; p = .019) and AT‐HIIT (ES, 0.42; 95% CI, 0.09 to 52.95 × 109/L; p = .05) prior to the fifth chemotherapy session. No between‐group differences were found for hemoglobin, lymphocyte, or neutrophil concentrations over the course of chemotherapy (Fig. 4). For the binary outcomes of neutropenia, lymphocytopenia, thrombocytopenia, and anemia, both RT‐HIIT (11%) and AT‐HIIT (10%) groups had a significantly lower incidence of thrombocytopenia compared with UC (30%; RT‐HIIT vs. UC: OR, 0.27; p = .03; AT‐HIIT vs. UC: OR, 0.24; p = .01). There were no significant differences in the incidence of neutropenia, and no incidences of lymphocytopenia or anemia were found over the course of chemotherapy.
Figure 4.
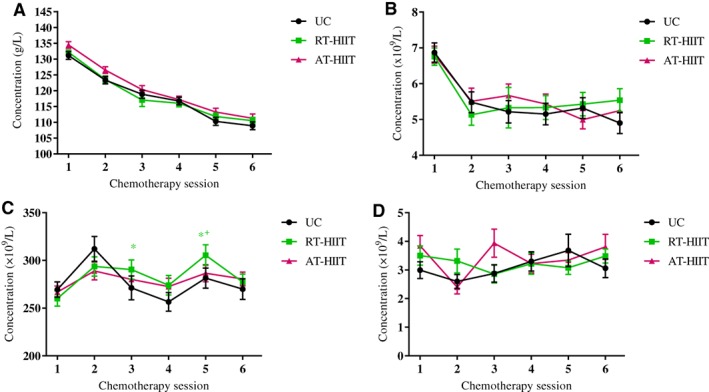
Effect of RT‐HIIT, AT‐HIIT, and UC on blood cell concentrations, measured prior to chemotherapy sessions. (A): Hemoglobin. (B): Lymphocytes. (C): Thrombocytes. (D): Neutrophils. Data are presented as means and SEM. *indicates p < .05 between RT‐HIIT and UC; † indicates p < .05 between RT‐HIIT and AT‐HIIT.
Abbreviations: AT‐HIIT, moderate‐intensity aerobic and high‐intensity interval training; RT‐HIIT, resistance and high‐intensity interval training; UC, usual care.
When assessing differences in blood cell concentrations between chemotherapy completers and noncompleters, noncompleters had higher lymphocyte concentrations before the planned third (ES, 0.49; 95% CI, 0.43 to 2.73 × 109/L; p = .007), fourth (ES, 0.21; 95% CI, 0.14 to 1.82 × 109/L; p = .02), and sixth (ES, 0.30; 95% CI, 0.29 to 2.02 × 109/L; p = .009) chemotherapy sessions and higher neutrophil concentrations before the planned fourth chemotherapy session (ES, 0.56; 95% CI, 0.21 to 2.05 × 109/L; p = .02; Fig. 5).
Figure 5.
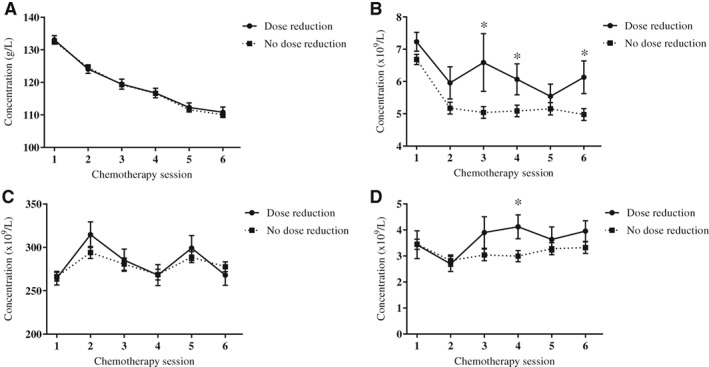
Blood cell concentrations, in the chemotherapy completers and noncompleters subgroups, measured prior to six chemotherapy sessions. (A): Hemoglobin. (B): Lymphocytes. (C): Thrombocytes. (D): Neutrophils. * indicates p < .05 between groups.
When assessing differences in blood cell concentrations between those patients who were hospitalized compared with those who were not hospitalized, hemoglobin concentrations were significantly higher for those patients who were hospitalized before the planned first (ES, 0.61; 95% CI, 0.53 to 9.98 g/L; p = .03) and second (ES, 0.04; 95% CI, 0.78 to 10.38 g/L; p = .02) chemotherapy sessions, whereas neutrophil concentrations were significantly lower for those patients who were hospitalized before the planned third chemotherapy session (ES, −0.56; 95% CI, −3.01 to −0.09 × 109/L; p = .04). No significant differences were found between the hospitalized and nonhospitalized patients for thrombocyte or lymphocyte concentrations at any time point (Fig. 6).
Figure 6.
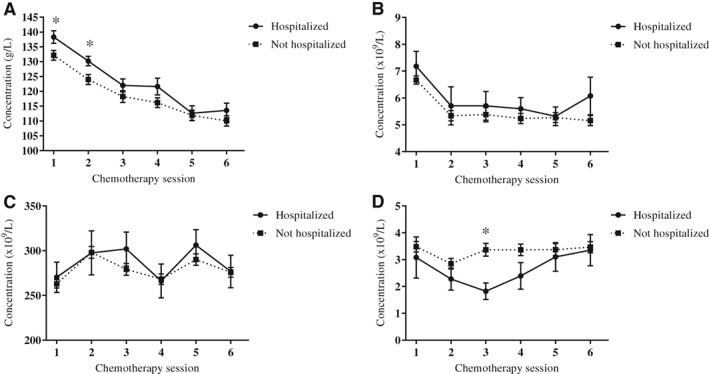
Blood cell concentrations, in the hospitalized and not hospitalized subgroups, measured prior to six chemotherapy sessions. (A): Hemoglobin. (B): Lymphocytes. (C): Thrombocytes. (D): Neutrophils. * indicates p < .05 between groups.
Discussion
This randomized controlled trial provides important insights about the role of exercise training during chemotherapy for patients with breast cancer on the clinical outcomes chemotherapy completion and hospitalization rates, as well as blood cell concentrations over the course of chemotherapy. In contrast to our hypothesis and to previous findings in studies of women with early‐stage breast cancer, there were no significant effects of exercise training during chemotherapy on chemotherapy completion rates in this study. However, the combined resistance and high‐intensity interval training group had lower hospitalization rates than the usual care group and lower thrombocyte concentrations compared with both the AT‐HIIT and the usual care group, whereas both exercise groups had a lower incidence of thrombocytopenia over the course of chemotherapy.
The importance of sustaining full dose‐intensity in adjuvant chemotherapy for early‐stage breast cancer have been shown in clinical trials with evidence of a threshold effect of approximately 85% of the RDI. Observational data suggest that an increase in muscle mass is associated with a higher treatment tolerance 11. Previous findings from the OptiTrain trial showed beneficial effects of exercise during chemotherapy on several outcomes, including muscle strength and muscle mass as indicated by a larger muscle fiber cross‐sectional area, in comparison with detrimental effects in the UC group 14, 15, 16. However, in contrast to our hypothesis, the findings from the current study do not mirror previous findings of the potential of exercise to improve the RDI of chemotherapy in women with primary breast cancer 5, 6, but are in line with findings from other cancer populations showing no beneficial effects of exercise on chemotherapy completion 3. In the current trial, the percentage of participants able to sustain an RDI greater than 85% was 86.7% in the UC group compared with 86.5% in the RT‐HIIT group and 77.8% in the AT‐HIIT group. These numbers indicate that most of the participants, regardless of group, were able to sustain high relative dose intensities over the course of chemotherapy, in contrast to findings by Courneya et al. 5 who showed that 78% of the participants in their resistance training group had an RDI above 85% compared with 65.9% in their control group. More recently, the CARE trial 23, which compared the effect of three different exercise programs on chemotherapy completion rates, reported comparable RDI >85% rates ranging between 82.2% and 87.5%.
The reason for the discrepant findings and overall higher RDI of chemotherapy in the current study is unknown, considering that the baseline data of the participants are comparable across the START 5 and PACES 6 trials. However, since the START trial in 2007, medications to support chemotherapy tolerance such as antiemetics and G‐CSF are more widely prescribed, which partially may explain the discrepant findings. A few additional exercise trials in other cancer populations, including patients with colorectal 24 and esophageal 25 cancer, found no beneficial effects of exercise on chemotherapy completion; however, unlike the current study, these studies reported an overall low adherence to chemotherapy regimens. In the START trial 5, the authors speculated that an exercise‐induced acute upregulation of neutrophils may alter chemotherapy delivery decisions; however, findings from the current study indicated no exercise effects on neutrophil concentrations prior to any chemotherapy session. Moreover, patients who required a dose reduction had higher neutrophil and lymphocyte concentrations compared with those who completed their planned chemotherapy regimen, likely as a consequence of a reduced chemotherapy dose.
Of note, marital status was associated with chemotherapy completion, which is in line with a recent meta‐analysis 26. One possible underlying etiology for the benefits of marriage for a better adherence to chemotherapy could be social support. The distress and anxiety associated with finding out about a cancer diagnosis could potentially be relieved by having a supportive spouse who can share the emotional and physical burden and provide motivational support to undergo the planned treatment regimen and live with treatment‐related adverse effects.
Interestingly, RT‐HIIT had lower hospitalization rates compared with UC. Although 8%–13% of patients with early‐stage breast cancer are affected by febrile neutropenia, leading to hospitalization 27, no previous exercise trials in patients with breast cancer have included this clinical endpoint. In patients undergoing allogeneic hematopoietic stem cell transplantation, aerobic exercise had a positive effect on the duration of hospitalization 4. In contrast, a pilot study including patients receiving chemotherapy for leukemia or lymphoma found no effects of an aerobic exercise program on time spent in hospital 28. Although no between‐group differences were found for neutrophil concentration levels in the current study, our findings indicate that the RT‐HIIT group was partly protected from febrile neutropenia that required hospitalization (i.e., having a neutrophil count of less than 1.0 × 109/L in combination with a temperature of 38°C or above on one occasion). The study by Dimeo et al. 4 showed that aerobic exercise shortened the duration of being neutropenic and thrombocytopenic and of hospitalization in patients with hematologic malignancies. In healthy individuals, both moderate‐intensity and high‐intensity interval aerobic training have been shown to lead to a mobilization of neutrophils into the peripheral blood 29 and to an improved neutrophil function 8 through enhanced chemotaxis and citrate synthase activity 30. Therefore, despite exercise not being able to counteract chemotherapy‐induced neutropenia, we speculate that exercise still has the potential to maintain or improve the function of the existing immune cell populations. Of note, the RT‐HIIT group showed increased thrombocyte concentrations prior to the third and fifth chemotherapy session, and both RT‐HIIT and AT‐HIIT resulted in a lower incidence of thrombocytopenia compared with UC. Evidence suggests that both moderate‐ and high‐intensity exercise training are associated with favorable effects on platelet count and activation 31, 32. However, because no patient reached a critically low platelet count to be at risk of bleeding (count <50 × 109/L), the clinical implication of this finding is not clear. However, beyond an important role in thrombosis, platelets assist and modulate inflammatory reactions and immune responses 33, which are important in order to withstand infections. This may be critical for patients during chemotherapy because of an increased susceptibility to infections.
A significantly higher proportion of participants who were hospitalized received taxane‐containing chemotherapy. In line with these findings, a previous study showed that chemotherapy regimens containing taxanes were associated with the highest risk of chemotherapy‐related hospitalization in patients with early‐stage breast cancer 34, placing this regimen in a high‐risk category for febrile neutropenia.
Although hospitalization was due to febrile neutropenia in all cases in the current study, having a greater number of chronic comorbidities at baseline was associated with higher hospitalization rates. This is in line with previous findings showing that patients with breast cancer receiving chemotherapy who had other comorbidities were at higher risk of febrile neutropenia 34, 35. Although moderate neutropenia is associated with a better survival in patients with early‐stage breast cancer 36, hospitalization due to febrile neutropenia can have detrimental consequences. Our findings indicate the role of RT‐HIIT to counteract febrile neutropenia, which may be particularly important for patients with existing additional comorbidities.
The reason for the potential positive findings on hospitalization, but not chemotherapy completion, may relate to the different reasons for different treatment‐related complications. There seemed to be some benefits of exercise to counteract the incidence of febrile neutropenia. Although the only reason for hospitalization was febrile neutropenia, different reasons were found for dose reduction between groups. Neutropenia was the most common reason for dose reduction in the UC group, whereas neuropathies and GI side effects were the most common reasons for dose reductions in the RT‐HIIT and AT‐HIIT groups, respectively. Similar findings were reported by van Waart et al. 6, showing that the main reason for dose reduction in their control group was febrile neutropenia, whereas neuropathies were the most common reason for dose reductions in their exercise groups. However, the reason for the high incidence of neuropathies in the RT‐HIIT group and GI side effects in the AT‐HIIT group is unknown. Some evidence indicates that strenuous aerobic exercise can have an acute negative impact on upper and lower GI symptoms because of reduced portal blood flow 37. The AT‐HIIT program may have led to a volume of aerobic exercise that compromised gastrointestinal health or exacerbated preexisting gastrointestinal health issues in some individuals. However, this warrants further investigation.
The incidence and severity of chemotherapy‐induced neutropenia, which may lead to both dose reductions of chemotherapy and hospitalization, may be reduced by the use of G‐CSF 38. Of note, a significantly higher proportion of those who required dose adjustments received G‐CSF (89.6%) compared with those not requiring any dose adjustments (74.7%), and a slightly higher proportion of those being hospitalized received G‐CSF (92.9%) compared with those not hospitalized (77.1%). This finding is likely due to a higher prescription of G‐CSF for those who received taxane‐based chemotherapy, especially high doses of taxanes 38, which were associated with a higher incidence of febrile neutropenia.
Strengths of the study include measurements of several clinical outcomes and blood cell concentrations measured at several time points over the course of chemotherapy. A limitation is that the current study may not be sufficiently powered for this type of analysis, and thus the results need to be interpreted with this in mind. Nevertheless, this study provides interesting and important findings that add to the current knowledge about the effects of exercise on clinically relevant endpoints and are hypothesis generating. Future sufficiently powered studies investigating the effect of exercise on clinical endpoints such as chemotherapy completion and hospitalization will be important to determine whether exercise during chemotherapy can improve prognosis through improved adherence to chemotherapy and will be able to address which subgroups of patients with breast cancer based on treatment regimen and other baseline characteristics will benefit most from the exercise intervention. Future studies should also aim to determine whether exercise interventions are cost effective for patients with breast cancer. Studying other populations of patients with cancer for whom adherence to chemotherapy is a challenge is also needed.
Conclusion
This study addressed the clinical outcomes of two exercise programs during chemotherapy for patients with early‐stage breast cancer. In the current study, we found no beneficial effects of either RT‐HIIT or AT‐HIIT on chemotherapy completion rates. However, combined resistance training and high‐intensity interval training were effective to reduce hospitalization rates and also had a positive effect on platelet concentrations. These are important findings that have positive implications for the health of women with breast cancer and on costs associated with treatment‐related complications.
Author Contributions
Conception/design: Sara Mijwel, Yvonne Wengström, Helene Rundqvist
Provision of study material or patients: Sara Mijwel, Theodoros Foukakis, Yvonne Wengström
Collection and/or assembly of data: Sara Mijwel, Jacob Gerrevall
Data analysis and interpretation: Sara Mijwel, Kate A. Bolam, Theodoros Foukakis, Jacob Gerrevall, Yvonne Wengström, Helene Rundqvist
Manuscript writing: Sara Mijwel, Kate A. Bolam, Helene Rundqvist
Final approval of manuscript: Sara Mijwel, Kate A. Bolam, Theodoros Foukakis, Jacob Gerrevall, Yvonne Wengström, Helene Rundqvist
Disclosures
Theodoros Foukakis: Pfizer, Roche (RF), Pfizer, Roche, Novartis, UpToDate (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Table
Acknowledgments
The authors would like to thank the participants in the OptiTrain trial, Malin Backman (Karolinska Institutet) for supervising the exercise sessions, and the staff at the oncological rehabilitation center (Karolinska University Hospital, Stockholm, Sweden) for the use of their exercise facilities.
Disclosures of potential conflicts of interest may be found at the end of this article.
Editor's Note: See the related commentary, “Supervised, Multimodal Exercise: The Chemotherapy Supportive Therapy That Almost Does It All,” by Amy A. Kirkham on https://dx.doi.org/10.1634/theoncologist.2019-0628 of this issue.
References
- 1. Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: An overview. BMC Med 2015;13:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weycker D, Barron R, Edelsberg J et al. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat 2012;133:301–310. [DOI] [PubMed] [Google Scholar]
- 3. Bland KA, Zadravec K, Landry T et al. Impact of exercise on chemotherapy completion rate: A systematic review of the evidence and recommendations for future exercise oncology research. Crit Rev Oncol Hematol 2019;136:79–85. [DOI] [PubMed] [Google Scholar]
- 4. Dimeo F, Fetscher S, Lange W et al. Effects of aerobic exercise on the physical performance and incidence of treatment‐related complications after high‐dose chemotherapy. Blood 1997;90:3390–3394. [PubMed] [Google Scholar]
- 5. Courneya KS, Segal RJ, Mackey JR et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol 2007;25:4396–4404. [DOI] [PubMed] [Google Scholar]
- 6. van Waart H, Stuiver MM, van Harten WH et al. Effect of low‐intensity physical activity and moderate‐ to high‐intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: Results of the PACES randomized clinical trial. J Clin Oncol 2015;33:1918–1927. [DOI] [PubMed] [Google Scholar]
- 7. Takekiyo T, Dozono K, Mitsuishi T et al. Effect of exercise therapy on muscle mass and physical functioning in patients undergoing allogeneic hematopoietic stem cell transplantation. Support Care Cancer 2015;23:985–992. [DOI] [PubMed] [Google Scholar]
- 8. Bartlett DB, Shepherd SO, Wilson OJ et al. Neutrophil and monocyte bactericidal responses to 10 weeks of low‐volume high‐intensity interval or moderate‐intensity continuous training in sedentary adults. Oxid Med Cell Longev 2017;2017:8148742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fortunato AK, Pontes WM, De Souza D et al. Strength training session induces important changes on physiological, immunological, and inflammatory biomarkers. J Immunol Res 2018;2018:9675216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caan BJ, Cespedes Feliciano EM, Prado CM et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 2018;4:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bozzetti F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 2017;28:2107–2118. [DOI] [PubMed] [Google Scholar]
- 12. Ida S, Watanabe M, Karashima R et al. Changes in body composition secondary to neoadjuvant chemotherapy for advanced esophageal cancer are related to the occurrence of postoperative complications after esophagectomy. Ann Surg Oncol 2014;21:3675–3679. [DOI] [PubMed] [Google Scholar]
- 13. Prado CM, Baracos VE, McCargar LJ et al. Body composition as an independent determinant of 5‐fluorouracil‐based chemotherapy toxicity. Clin Cancer Res 2007;13:3264–3268. [DOI] [PubMed] [Google Scholar]
- 14. Mijwel S, Backman M, Bolam KA et al. Highly favorable physiological responses to concurrent resistance and high‐intensity interval training during chemotherapy: The OptiTrain breast cancer trial. Breast Cancer Res Treat 2018;169:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mijwel S, Cardinale DA, Norrbom J et al. Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. FASEB J 2018;32:5495–5550. [DOI] [PubMed] [Google Scholar]
- 16. Mijwel S, Backman M, Bolam KA et al. Adding high‐intensity interval training to conventional training modalities: optimizing health‐related outcomes during chemotherapy for breast cancer: The OptiTrain randomized controlled trial. Breast Cancer Res Treat 2018;168:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wengström Y, Bolam KA, Mijwel S et al. Optitrain: A randomised controlled exercise trial for women with breast cancer undergoing chemotherapy. BMC Cancer 2017;17:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmitz KH, Courneya KS, Matthews C et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42:1409–1426. [DOI] [PubMed] [Google Scholar]
- 19. Longo DL, Duffey PL, DeVita VT Jr et al. The calculation of actual or received dose intensity: A comparison of published methods. J Clin Oncol 1991;9:2042–2051. [DOI] [PubMed] [Google Scholar]
- 20. Morris SB. Estimating effect sizes from pretest‐posttest‐control group designs. Organ Res Methods 2007;11:364–386. [Google Scholar]
- 21. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. London, U.K.: Routledge; 1988. [Google Scholar]
- 22. Freifeld AG, Bow EJ, Sepkowitz KA et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011;52:e56–e93. [DOI] [PubMed] [Google Scholar]
- 23. Courneya KS, McKenzie DC, Mackey JR et al. Effects of exercise dose and type during breast cancer chemotherapy: Multicenter randomized trial. J Natl Cancer Inst 2013;105:1821–1832. [DOI] [PubMed] [Google Scholar]
- 24. Van Vulpen JK, Velthuis MJ, Steins Bisschop CN et al. Effects of an exercise program in colon cancer patients undergoing chemotherapy. Med Sci Sports Exerc 2016;48:767–775. [DOI] [PubMed] [Google Scholar]
- 25. Xu YJ, Cheng JCH, Lee JM et al. A walk‐and‐eat intervention improves outcomes for patients with esophageal cancer undergoing neoadjuvant chemoradiotherapy. The Oncologist 2015;20:1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He X, Ye F, Zhao B et al. Risk factors for delay of adjuvant chemotherapy in non‐metastatic breast cancer patients: A systematic review and meta‐analysis involving 186982 patients. PLoS One 2017;12:e0173862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel K, Diergaarde B, Brufsky A et al. Incidence of febrile neutropenia with use of docetaxel plus cyclophosphamide (TC) for breast cancer. J Clin Oncol 2017;35(suppl 15):e12073A. [Google Scholar]
- 28. Baumann FT, Zimmer P, Finkenberg K et al. Influence of endurance exercise on the risk of pneumonia and fever in leukemia and lymphoma patients undergoing high dose chemotherapy. A pilot study. J Sports Sci Med 2012;11:638–642. [PMC free article] [PubMed] [Google Scholar]
- 29. Gustafson MP, DiCostanzo AC, Wheatley CM et al. A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J Immunother Cancer 2017;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Syu GD, Chen HI, Jen CJ. Differential effects of acute and chronic exercise on human neutrophil functions. Med Sci Sports Exerc 2012;44:1021–1027. [DOI] [PubMed] [Google Scholar]
- 31. Whittaker JP, Linden MD, Coffey VG. Effect of aerobic interval training and caffeine on blood platelet function. Med Sci Sports Exerc 2013;45:342–350. [DOI] [PubMed] [Google Scholar]
- 32. Lippi G, Salvagno GL, Danese E et al. Variation of red blood cell distribution width and mean platelet volume after moderate endurance exercise. Adv Hematol 2014;2014:192173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morrell CN, Aggrey AA, Chapman LM et al. Emerging roles for platelets as immune and inflammatory cells. Blood 2014;123:2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barcenas CH, Niu J, Zhang N et al. Risk of hospitalization according to chemotherapy regimen in early‐stage breast cancer. J Clin Oncol 2014;32:2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chia VM, Page JH, Rodriguez R et al. Chronic comorbid conditions associated with risk of febrile neutropenia in breast cancer patients treated with chemotherapy. Breast Cancer Res Treat 2013;138:621–631. [DOI] [PubMed] [Google Scholar]
- 36. Cameron DA, Massie C, Kerr G et al. Moderate neutropenia with adjuvant CMF confers improved survival in early breast cancer. Br J Cancer 2003;89:1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Costa RJS, Snipe RMJ, Kitic CM et al. Systematic review: Exercise‐induced gastrointestinal syndrome‐implications for health and intestinal disease. Aliment Pharmacol Ther 2017;46:246–265. [DOI] [PubMed] [Google Scholar]
- 38. Aapro MS, Bohlius J, Cameron DA et al. 2010 update of EORTC guidelines for the use of granulocyte‐colony stimulating factor to reduce the incidence of chemotherapy‐induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 2011;47:8–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Table


