Abstract
Background
Patients with advanced lung neuroendocrine neoplasms (NENs) have few treatment options. Capecitabine and temozolomide have recently showed significant activity in patients with pancreatic neuroendocrine tumors (NETs), but data in lung NETs are limited.
Methods
We retrospectively reviewed the records of patients treated at a large referral center to identify patients seen between January 2008 and September 2018 with metastatic lung NENs who received treatment with capecitabine and temozolomide (CAPTEM). Patients with small cell lung cancer were excluded. The primary endpoint was overall response rate per RECIST 1.1. Secondary endpoints included progression‐free survival, overall survival, and toxicity.
Results
Twenty patients were identified who received treatment with capecitabine and temozolomide. Fourteen (70%) had typical lung NETs, five had (25%) atypical carcinoids, and one (5%) had disease defined as a large‐cell neuroendocrine carcinoma. Nineteen patients were evaluable for response. Six (30%) patients exhibited a best response of partial response per RECIST 1.1 criteria, 11 (55%) stable disease, and 2 (10%) progressive disease; objective response rate was 30%, and disease control rate was 85%. Eleven patients eventually progressed, only six of whom exhibited progression per RECIST 1.1 criteria. Median progression‐free survival was 13 months (95% confidence interval [CI], 4.4–21.6 months). Median overall survival was 68 months (95% CI, 35.3–100.7 months). Toxicity profile was mild with mainly grade 1, expected toxicities. Six patients required dose reduction because of toxicity.
Conclusion
The CAPTEM regimen is associated with a high response rate and a relatively tolerable toxicity profile in lung NENs. This regimen warrants further exploration in a prospective clinical trial.
Implications for Practice
Patients with advanced lung neuroendocrine neoplasms have very few systemic treatment options. The capecitabine and temozolomide regimen has previously shown significant activity in patients with pancreatic neuroendocrine tumors (NETs) but has not been explored in metastatic lung NETs. This study showed that this regimen is associated with a high response rate (30%) and a relatively tolerable toxicity profile in this population.
Keywords: Neuroendocrine tumor, Lung, Typical carcinoid, Atypical carcinoid, Neuroendocrine neoplasm, Capecitabine, Temozolomide, Bronchial
Short abstract
Patients with advanced lung neuroendocrine tumors (NETs) have limited treatment options. Everolimus is the only FDA‐approved systemic therapy. This study explored a regimen of capecitabine and temozolomide in patients with lung NETs.
Introduction
Lung neuroendocrine neoplasms (NENs) are a heterogenous group of tumors that comprise approximately 20% of all lung cancers and approximately 25% of neuroendocrine tumors. Lung NENs are comprised of four subtypes: typical carcinoids, atypical carcinoids, large‐cell neuroendocrine carcinomas (LCNECs), and small cell lung carcinomas (SCLCs) 1, 2. Tumor grade and mitotic count are important prognostic factors. Typical carcinoid tumors, defined as low‐grade neuroendocrine tumors (NETs) with a mitotic count of fewer than two per ten high power fields (HPFs) and no necrosis, are generally slow growing. Atypical carcinoid tumors (intermediate‐grade lung NETs) are rarer and more aggressive than typical carcinoids, with a mitotic count of two to ten per HPF and focal necrosis. LCNECs are much more aggressive NENs with a mitotic count of more than ten mitoses per HPF and cytologic features of a large‐cell carcinoma. SCLCs, the most aggressive of the four subgroups, make up approximately 13% of all lung cancers and generally have a very high mitotic rate as well as a Ki‐67 proliferation index of more than 70% 2.
Although the therapeutic landscape for gastroenteropancreatic neuroendocrine tumors (GEP‐NETs) has significantly improved over the past decade, patients with advanced lung NENs have few treatment options. Everolimus is approved for metastatic typical and atypical carcinoid tumors, and platinum‐based chemotherapy (generally either cisplatin or carboplatin with etoposide) is used for SCLC and LCNEC. Somatostatin analogs are often used in somatostatin‐receptor positive typical and atypical carcinoid tumors, but randomized prospective studies are lacking in this population. Although peptide receptor radiotherapy with 177Lu‐dotatate was approved for GEP‐NETs, the U.S. Food and Drug Administration approval did not encompass bronchopulmonary NENs 3.
Treatment with capecitabine and temozolomide (CAPTEM) has shown significant activity in patients with pancreatic NETs with reported objective response rates ranging from 33% to 70% and median progression‐free survival ranging from 18 months to 22.7 months, respectively 4, 5, 6. Moreover, the combination of CAPTEM demonstrated superior progression‐free survival (PFS) as well as overall survival (OS) compared with temozolomide monotherapy in a randomized prospective study of patients with progressive pancreatic NETs, thereby establishing CAPTEM as a standard of care in this disease. Temozolomide monotherapy has shown modest activity in lung NENs. In a retrospective study of 31 lung NETs (including 14 typical and 15 atypical carcinoid tumors), a partial response (PR) was observed in three patients (14%), and the median PFS was 5.3 months 7. To our knowledge, there are no published data on use of CAPTEM in lung NENs.
Subjects, Materials, and Methods
We reviewed the records of patients seen between January 2008 and September 2018 with metastatic lung NENs who received treatment at Moffitt Cancer Center with CAPTEM. Patients with SCLC were excluded. Patients who were followed only at outside institutions after the initial chemotherapy prescription were also excluded. The study protocol was approved by the local institutional review board.
Data Collection
Demographic and pathological data were collected from each patient, including date and stage of initial diagnosis, histologic grade, Ki‐67 proliferation and mitotic count, primary disease site, oncologic therapy history, toxicity during treatment, dose reductions and modifications, most recent follow‐up date, vital status, and date of death. Dates of all interventions and therapies were also collected, along with dates of progression on said therapies. Scans were read independently by a radiologist (B.M.).
Statistical Analysis
PFS was defined as time from initiation of treatment to either clinical progression or radiographic progression per RECIST 1.1 (whichever shortest), or death because of any cause. OS was measured from date of initial diagnosis until death from any cause or last known follow‐up. Survival curves were estimated using the Kaplan‐Meier method and compared by the log‐rank test. Exact 95% confidence intervals (CIs) were calculated for each proportion of interest. All statistical analyses were performed using SPSS version 25.
Results
Patient and Tumor Characteristics
Table 1 presents the demographics and tumor characteristics of the 20 patients on the study. Overall, the majority of patients had either typical lung NETs (n = 14, 70%) or atypical lung NETs (n = 5, 25%). One patient was described as having a large‐cell neuroendocrine carcinoma. Ki‐67% proliferation index was reported in 14 cases and ranged from 1% to 41%, with two cases in the high‐grade range (Ki‐67 index >20%).
Table 1.
Patient demographic and tumor characteristics
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 10 (50) |
| Female | 10 (50) |
| Age, years | |
| 49–60 | 8 (40) |
| 61–75 | 8 (40) |
| 75+ | 4 (20) |
| Histologic subtype | |
| Typical NET | 14 (70) |
| Atypical NET | 5 (25) |
| LCNEC | 1 (5) |
| Ki‐67 proliferation index | |
| ≤2% | 5 (25) |
| 3–20% | 8 (40) |
| >20% | 1 (5) |
| Unknown | 6 (30) |
| Number of prior lines of systemic treatment | |
| 0 | 4 (20) |
| 1–3 | 14 (70) |
| 4–6 | 2 (10) |
| Number of prior local interventions | |
| 0 | 12 (60) |
| 1 | 6 (30) |
| 2 | 2 (10) |
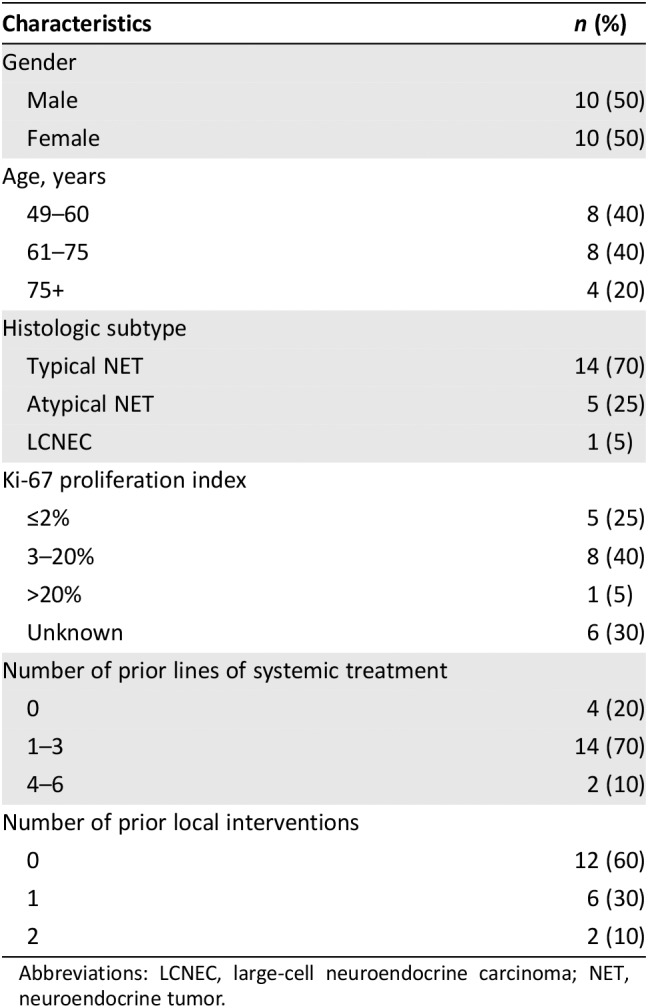
Abbreviations: LCNEC, large‐cell neuroendocrine carcinoma; NET, neuroendocrine tumor.
Prior Treatments
The majority of patients (n = 16, 80%) had received at least one prior line of systemic treatment, with a median of two prior systemic treatments (range 0–6). Only four patients had not received any prior systemic therapy. Prior therapies included somatostatin analogs (n = 14, 70%), everolimus (n = 8, 40%), cytotoxic chemotherapy (n = 5, 25%), clinical trial drugs (n = 4, 20%), peptide receptor radiotherapy (n = 1, 5%), and sunitinib (n = 1, 5%). Nine patients (45%) had a baseline surgical resection, and 10 (50%) underwent subsequent locoregional treatment for thoracic disease or for treatment of liver metastases: radiation (n = 3, 15%), hepatic arterial embolization (n = 6, 30%), and microwave ablation (n = 1, 5%). All patients were actively progressing at time of treatment initiation.
Dosing
Patients were treated on a regimen consisting of capecitabine 750 mg/m2 b.i.d. days 1–14 and temozolomide 200 mg/m2 days 10–14 every 28 days (with ondansetron administered 30–60 minutes prior to temozolomide). Starting doses were generally rounded down to minimize the number of tablets and occasionally reduced at outset because of factors such as patient age or frailty. The average daily starting dose of capecitabine was 1,115 mg/m2 (or 558 mg/m2 b.i.d.), and the average starting dose of temozolomide was 170 mg/m2. The median daily starting dose of capecitabine was 1,250 mg/m2 in divided doses, and the median starting dose of temozolomide was 175 mg/m2. None of the patients required subsequent dose reductions greater than 25% because of treatment related toxicity. Patients remained on treatment for a median of 7.5 months (mean 9.15 months; range 0–30 months).
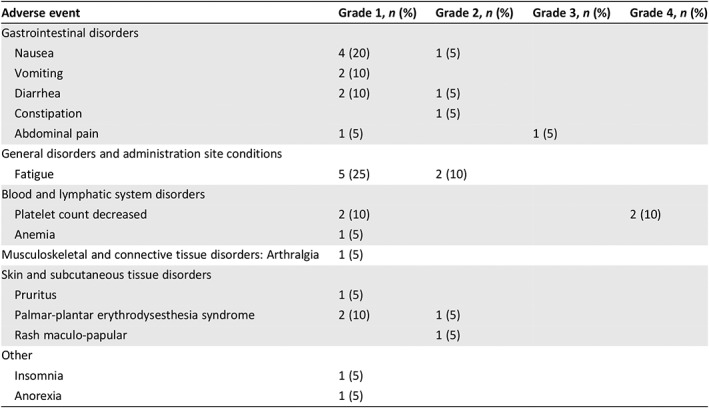
Toxicity and Discontinuation
Table 2 outlines the treatment related adverse events experienced by patients during treatment, graded per Common Terminology Criteria for Adverse Events, version 4.03. Overall, there were a total of 33 clinically significant drug related adverse events, the majority of which (n = 23, 70%) were expected and grade 1 in nature. Two patients (10%) experienced events of grade 4 thrombocytopenia that were corrected with blood transfusions followed by dose reductions and did not require permanent discontinuation of treatment. One patient had persistent grade 1 thrombocytopenia and anemia, as well as grade 2 fatigue and grade 1 nausea and arthralgia, which required a 20% dose reduction of temozolomide. Two patients (10%) required dose reduction because of events of grade 2 nausea and diarrhea. One (5%) patient's grade 2 hand‐foot syndrome required dose reduction; the patient was able to continue on treatment. One patient had a persistent grade 2 maculopapular rash with pruritus.
Table 2.
Treatment‐related adverse events (per Common Terminology Criteria for Adverse Events, version 4.03)
| Adverse event | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|---|
| Gastrointestinal disorders | ||||
| Nausea | 4 (20) | 1 (5) | ||
| Vomiting | 2 (10) | |||
| Diarrhea | 2 (10) | 1 (5) | ||
| Constipation | 1 (5) | |||
| Abdominal pain | 1 (5) | 1 (5) | ||
| General disorders and administration site conditions | ||||
| Fatigue | 5 (25) | 2 (10) | ||
| Blood and lymphatic system disorders | ||||
| Platelet count decreased | 2 (10) | 2 (10) | ||
| Anemia | 1 (5) | |||
| Musculoskeletal and connective tissue disorders: Arthralgia | 1 (5) | |||
| Skin and subcutaneous tissue disorders | ||||
| Pruritus | 1 (5) | |||
| Palmar‐plantar erythrodysesthesia syndrome | 2 (10) | 1 (5) | ||
| Rash maculo‐papular | 1 (5) | |||
| Other | ||||
| Insomnia | 1 (5) | |||
| Anorexia | 1 (5) |
None of the patients discontinued treatment because of drug induced toxicity, and all were able to continue on treatment until maximal response (n = 2, 10%), progression (clinical or radiographic; n = 10, 50%), or completion of investigator defined duration of therapy (n = 2, 10%). One patient died soon after starting treatment from comorbidities unrelated to treatment, and one patient was lost to follow‐up after initiating treatment. Four patients remain on active treatment.
Response, Progression‐Free Survival, and Overall Survival
Six patients (30%), four with typical NETs, one with atypical NET, and one with LCNEC, exhibited a best response of PR per RECIST 1.1 criteria, three of whom eventually progressed (only one per RECIST 1.1; LCNEC). Eleven patients (55%), eight with typical and three with atypical NETs, exhibited a best response of stable disease, and six of those eventually progressed (four per RECIST). Two patients (10%) with typical NETs progressed per RECIST at the first restaging scan. One patient was unevaluable per RECIST. Disease control rate (DCR) was 85%.
All patients were evaluable for survival analysis. At the time of data cutoff, 12 patients had died, and 8 were alive. The median PFS was 13 months (95% CI, 4.4–21.6 months; Fig. 1). The median OS was 68 months (95% CI, 35.3–100.7 months; Fig. 2).
Figure 1.
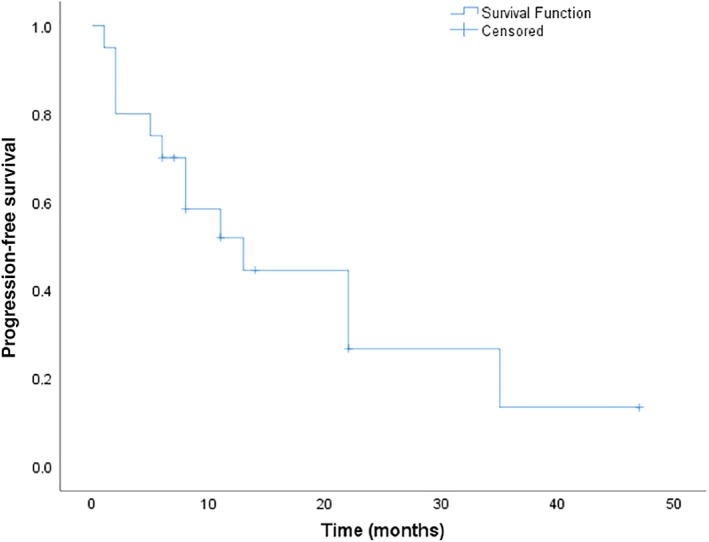
Progression‐free survival.
Figure 2.
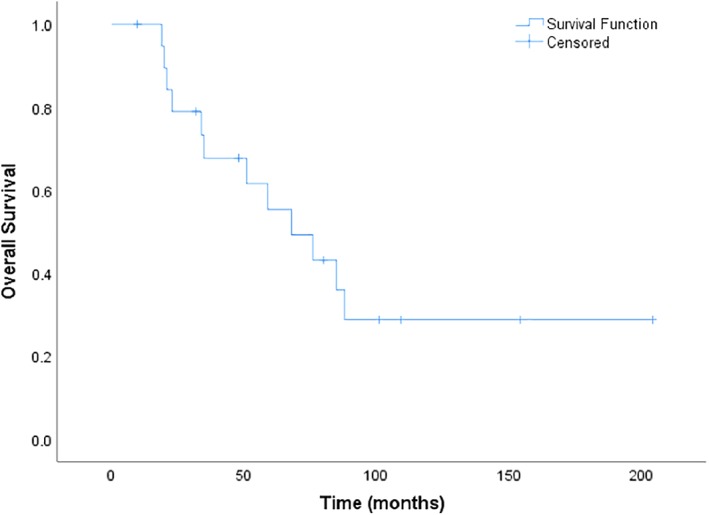
Overall survival.
Discussion
Patients with advanced lung NENs have limited treatment options and are in need of new, less toxic systemic treatments. Everolimus is the only drug that has been studied in a randomized, controlled phase III clinical trial 8. In the lung NET cohort, which consisted of 90 patients, the median PFS was 9.2 months with everolimus (compared with 3.6 months with placebo), and there was only a single PR (1%) 9. The activity of cytotoxic chemotherapy has been tested primarily in small retrospective series. Platinum‐etoposide regimens have demonstrated modest response rates and short durations of PFS and OS in several small retrospective studies and are associated with significant toxicity 10, 11. Consequently, these regimens are not endorsed by guidelines for typical and atypical lung NETs 3. Temozolomide monotherapy, although well tolerated, is also associated with a modest response rate of 14%.
Our current retrospective analysis of 20 consecutively treated patients illustrates that the CAPTEM regimen likely has significant activity in lung NETs, albeit lower than in pancreatic NETs. There was no significant difference between responses based on NEN subtype. The toxicity profile was within expected side effects for the regimen, and none of the patients experienced long‐term, irreversible toxicity. No treatment discontinuations were due to toxicity. The median PFS of 13 months reported in this study is based on a conservative estimate in which evidence of clinical progression (e.g., change in treatment) was considered a progression event.
Limitations of this study include its retrospective nature, heterogeneity of enrolled patients, and small sample size. Nevertheless, the median PFS and particularly the OS observed in this study were encouraging. The response rate of 30% and DCR of 85% were also relatively high and indicate that this regimen can be used to cytoreduce tumors and palliate symptoms. These data support the need to further evaluate this regimen in prospective clinical studies focusing specifically on patients with lung NEN.
Conclusion
The CAPTEM regimen is associated with a high response rate and a relatively tolerable toxicity profile in lung NENs. This regimen warrants further exploration in a prospective clinical trial.
Author Contributions
Conception/design: Taymeyah Al‐Toubah, Brian Morse, Jonathan Strosberg
Provision of study material or patients: Jonathan Strosberg
Collection and/or assembly of data: Taymeyah Al‐Toubah, Brian Morse, Jonathan Strosberg
Data analysis and interpretation: Taymeyah Al‐Toubah, Brian Morse, Jonathan Strosberg
Manuscript writing: Taymeyah Al‐Toubah, Brian Morse, Jonathan Strosberg
Final approval of manuscript: Taymeyah Al‐Toubah, Brian Morse, Jonathan Strosberg
Disclosures
Jonathan Strosberg: Novartis (C/A), Ipsen, Lexicon (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Gustafsson BI, Kidd M, Chan A et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5–21. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Brambilla E, Nicholson AG et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243–1260. [DOI] [PubMed] [Google Scholar]
- 3. Shah MH, Goldner WS, Halfdanarson TR et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. J Natl Compr Canc Netw 2018;16:693–702. [DOI] [PubMed] [Google Scholar]
- 4. Strosberg JR, Fine RL, Choi J et al. First‐line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kunz PL, Catalano PJ, Nimeiri H et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG‐ACRIN Cancer Research Group (E2211). J Clin Oncol 2018;36(suppl 15):4004A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cives M, Ghayouri M, Morse B et al. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer 2016;23:759–767. [DOI] [PubMed] [Google Scholar]
- 7. Crona J, Fanola I, Lindholm DP et al. Effect of temozolomide in patients with metastatic bronchial carcinoids. Neuroendocrinology 2013;98:151–155. [DOI] [PubMed] [Google Scholar]
- 8. Yao JC, Fazio N, Singh S et al. Everolimus for the treatment of advanced, non‐functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT‐4): A randomised, placebo‐controlled, phase 3 study. Lancet 2016;387:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fazio N, Buzzoni R, Delle Fave G et al. Everolimus in advanced, progressive, well‐differentiated, non‐functional neuroendocrine tumors: RADIANT‐4 lung subgroup analysis. Cancer Sci 2018;109:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chong CR, Wirth LJ, Nishino M et al. Chemotherapy for locally advanced and metastatic pulmonary carcinoid tumors. Lung Cancer 2014;86:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wirth LJ, Carter MR, Janne PA et al. Outcome of patients with pulmonary carcinoid tumors receiving chemotherapy or chemoradiotherapy. Lung Cancer 2004;44:213–220. [DOI] [PubMed] [Google Scholar]


