Abstract
Background
Trifluridine and tipiracil (FTD + TPI) and regorafenib (REG) are approved treatments for the treatment of refractory metastatic colorectal cancer (mCRC). This study assesses adherence and duration of therapy with FTD + TPI versus REG and explores the effect of sequencing on adherence.
Materials and Methods
Adults diagnosed with mCRC were identified in the IQVIA Real‐World Data Adjudicated Claims: U.S. database (October 2014–July 2017). The observation period spanned from the index date (first dispensing of FTD + TPI or REG) to the earliest of a switch to another mCRC agent, the end of continuous enrollment, or the end of data availability. Medication possession ratio (MPR), proportion of days covered (PDC), and persistence and time to discontinuation (gap ≥45 days) were compared between FTD + TPI and REG users and among switchers (FTD + TPI‐to‐REG vs. REG‐to‐FTD + TPI).
Results
A total of 469 FTD + TPI and 311 REG users were identified. FTD + TPI users had higher compliance with an MPR ≥80% (odds ratio [OR], 2.47; p < .001) and PDC ≥80% (OR, 2.77; p < .001). FTD + TPI users had better persistence (82.8% vs. 68.0%; p < .001) and lower risk of discontinuation (hazard ratio [HR], 0.76; p = .006). Among switchers (96 FTD + TPI‐to‐REG; 83 REG‐to‐FTD + TPI), those switching from FTD + TPI to REG were more likely to have an MPR ≥80% (OR, 2.91; p < .001) and PDC ≥80% (OR, 4.60; p < .001) compared with REG‐to‐FTD + TPI switchers while treated with these drugs. Additionally, FTD + TPI‐to‐REG switchers had a lower risk of first treatment discontinuation (HR, 0.66; p = .009).
Conclusion
FTD + TPI users had significantly higher adherence and persistence, and patients who were treated with FTD + TPI before switching to REG also had higher adherence and persistence outcomes.
Implications for Practice
Trifluridine plus tipiracil (FTD + TPI) and regorafenib (REG) prolong survival in refractory metastatic colorectal cancer (mCRC) but have different tolerability profiles. This study assessed real‐world adherence to treatment with FTD + TPI versus REG and compared outcomes among patients who switched from FTD + TPI to REG and vice versa. FTD + TPI was associated with significantly higher medication adherence and longer time to discontinuation than REG. Patients treated with FTD + TPI prior to switching to REG also showed higher adherence outcomes. Findings could help inform decision making regarding the choice and sequencing of treatment with FTD + TPI versus REG in patients with mCRC.
Keywords: Metastatic colorectal cancer, Adherence, Trifluridine/tipiracil, Regorafenib
Short abstract
This article assesses real‐world treatment patterns and adherence of patients with metastatic colorectal cancer treated with trifluridine plus tipiracil (FTD/TPI) versus regorafenib (REG) and explores the effect of the treatment sequence on adherence among patients who switched from FTD + TPI to REG and vice versa.
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer‐related death in the U.S. 1. In 2018, there were an estimated 140,250 new cases and 50,630 related deaths in the U.S. 1, 2. Approximately 20% of patients with CRC have metastatic disease at diagnosis and between 50% and 60% of patients develop metastases over their treatment course. Metastatic CRC (mCRC) is associated with a poor prognosis, with a 5‐year survival rate of about 14% 1. However, this represents successive improvements over the last decade, with the incorporation of novel treatment agents, predictive biomarkers, and a more strategic approach to the delivery of systemic therapies. Currently, the median overall survival for patients with mCRC being treated both in phase III trials and in large observational series or registries is approximately 30 months—more than double that of 20 years ago 3, 4, 5.
Trifluridine plus tipiracil (FTD + TPI; Lonsurf; Taiho Oncology Inc., Princeton, NJ) and regorafenib (REG; Stivarga; Bayer, Whippany, NJ) are two agents approved by the U.S. Food and Drug Administration for the treatment of refractory mCRC 6, 7. FTD + TPI is an oral thymidine‐based nucleoside analog, and REG is a multikinase inhibitor involved in multiple pathways, including those driving angiogenesis and oncogenesis. In their respective phase III registration trials, the RECOURSE and CORRECT studies, FTD + TPI and REG both demonstrated significant improvements in overall survival compared with placebo for patients with mCRC that had progressed on multiple lines of therapy 8, 9. Although there have been no head‐to‐head trials of these two drugs, they are often compared in clinical practice as they are both oral agents with identical indications that were approved within a few years of each other.
An important factor affecting patient outcomes with oral therapies is medication adherence, defined as the extent to which patients take medications as prescribed 10, 11. In treating cancer, previous studies have shown that survival and disease progression are directly impacted by adherence 12, 13, 14. Although patients show a strong preference for oral treatments over intravenous treatments 15, adherence to oral chemotherapy treatments among patients with cancer is low. The objective of the current study was to assess real‐world treatment patterns and adherence of patients with mCRC treated with FTD + TPI versus REG and to explore the effect of the treatment sequencing on adherence among patients who switched from FTD + TPI to REG and vice versa.
Materials and Methods
Data Source
The present study used claims data from the IQVIA Real‐World Data Adjudicated Claims, U.S. database (IQVIA database). The database is the largest non‐payer‐owned integrated claims database of commercial insurers as well as Medicare‐eligible retirees with employer‐provided Medicare Supplemental plans covered by the health benefit programs of large employers. The IQVIA database includes medical and pharmacy claims (e.g., retail and mail order) for more than 80 million members from more than 100 health plans across all 50 states of the U.S. These claims are representative of the national commercially insured population and include historical information on patient demographics and inpatient, outpatient, and pharmacy claims. The database is fully compliant with the Health Insurance Portability and Accountability Act of 1996 and its implementing regulations.
Study Design and Patient Selection
A retrospective longitudinal cohort design was used to conduct this study. Patients were included in the study if they (a) were diagnosed with CRC (International Classification of Diseases, 9th Revision, Clinical Modification, codes 153.x, 154.0x, 154.1x, 154.8x; International Classification of Diseases, 10th Revision, Clinical Modification: C18.x, C19.x, C20.x, C21.8); (b) received either FTD + TPI or REG (the date of the first dispensing was termed as the index date if after FTD + TPI approval [i.e., October 2015]) between October 2015 and July 2017; (c) were 18 years or older as of the index date; and (d) had at least 3 months of continuous eligibility before and after the index date. The baseline period was defined as the 3‐month period prior to the index date. The observation period spanned from the index date until the earliest date between end of data availability, end of insurance coverage, or the day before a switch to another mCRC therapy. Patients were categorized into two study cohorts based on the treatment they received at the index date: FTD + TPI and REG cohorts. The treatment period was defined as the period from the index date to the last day of supply of the last dispensing over the observation period.
A similar design was used for assessing the effect of treatment sequencing on adherence analysis for those patients that received both FTD + TPI and REG (“switcher analysis”). Patients were categorized into two cohorts based on the sequence of treatment they received: FTD + TPI‐to‐REG and REG‐to‐FTD + TPI cohorts. The observation period spanned from the index date until the earliest date between end of data availability, end of insurance coverage, or the day before a switch to another mCRC therapy (excluding FTD + TPI and REG). The treatment period was defined as the period from the index date to the last day of supply of the last dispensing of the second therapy.
Study Outcomes
Medication adherence was assessed using medication possession ratio (MPR) and proportion of days covered (PDC). MPR was calculated by dividing total number of days of medication supplied by total number of days between the first prescription and the last day of supply of the last prescription among patients with at least two prescriptions. PDC was defined as the number of unique days with medication divided by the length of a fixed time interval. For both MPR and PDC, patients with a value >0.80 were considered adherent to their therapy. Persistence was defined as continuous use of the index therapy over a fixed time interval, with a specified allowable gap (i.e., 45 or 60 days) between two consecutive prescriptions or in the period between the last day of supply of the last prescription and the end of the observation period. Time to discontinuation (TTD) was assessed over the entire observation period using the same two allowable gap thresholds (i.e., 45 or 60 days).
Statistical Analysis
Baseline demographic and clinical characteristics were described and compared between the two cohorts (FTD + TPI vs. REG users). Differences in the baseline characteristics between the two cohorts were compared using chi‐square tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
Multivariable linear regression models were used to estimate mean differences in adherence between the two cohorts. In addition, multivariable logistic regression models were used to compare the proportion of adherent patients (MPR and PDC >.80) between the two cohorts and estimate the odds ratios (ORs) and their corresponding 95% confidence intervals (CIs). Multivariable Cox proportional hazards models were used to compare TTD between the two cohorts and estimate hazard ratios (HRs) and their corresponding 95% CIs. The baseline covariates adjusted for in the regression models included age, gender, region, insurance plan, year of index date, Quan‐Charlson comorbidity index (Quan‐CCI) score, all‐cause baseline drug costs, and all‐cause baseline medical costs.
Results
Baseline Characteristics
A total of 1,452 patients treated with FTD + TPI or REG were identified from the IQVIA database. After applying all inclusion and exclusion criteria, the final study sample comprised 469 FTD + TPI users and 311 REG users who were initiated on therapy after FTD + TPI approval (i.e., October 2015; Fig. 1). Table 1 summarizes the baseline demographic and clinical characteristics of each cohort. The age, gender, and insurance coverage of patients in both cohorts were similar. In both cohorts, the largest group of patients treated were from the South; however, the FTD + TPI cohort did contain a higher percentage of patients from the Northeast (18.6% vs. 10.3%; p = .002). The mean Quan‐CCI score was similar between the two cohorts (6.3 vs. 6.3; p = .853), and the most common comorbidities identified during the 3‐month baseline period were hypertension, venous thromboembolism, and coronary artery disease. The two most commonly used mCRC antineoplastic therapies prior to the index date were 5‐fluouracil and irinotecan. Patients treated with FTD + TPI had been prescribed these agents less frequently compared with patients treated with REG (5‐fluouracil: 46.5% vs. 53.7%, p = .048; irinotecan: 46.1% vs. 53.4%, p = .045). Of note, baseline characteristics of REG patients who were initiated on REG prior to FTD + TPI approval date were also evaluated, and no differences were found between these study populations.
Figure 1.
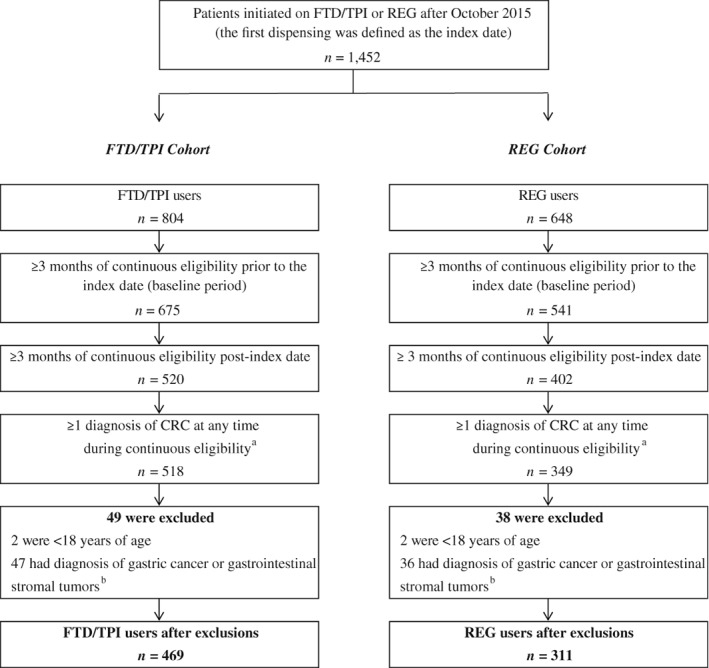
Patient disposition. aColorectal cancer (CRC) was identified using the International Classification of Diseases (ICD)‐9‐Clinical Modification (CM) codes 153.x, 154.0, 154.1, and 154.8 and ICD‐10 codes C18.x, C19, C20, and C21.8. bGastric cancer was identified using the ICD‐9‐CM code 151.x and the ICD‐10‐CM codes C16.8 and C16.9. Gastrointestinal stromal tumor was identified using the ICD‐9‐CM codes 171.5, 215.5, and 238.1 and ICD‐10 codes C49.4, D21.4, and D48.1.
Abbreviations: FTD + TPI, trifluridine+tipiracil; REG, regorafenib.
Table 1.
Baseline demographic and clinical characteristics
| Characteristicsa | FTD + TPI users (n = 469) | REG users (n = 311) | p valueb |
|---|---|---|---|
| Age at treatment initiation, mean ± SD (median) | 55.7 ± 9.6 (56) | 57.0 ± 8.5 (58) | .072 |
| Female, n (%) | 203 (43.3) | 143 (46.0) | .458 |
| Year of treatment initiation, n (%) | |||
| 2015 | 83 (17.7) | 64 (20.6) | .314 |
| 2016 | 296 (63.1) | 199 (64.0) | .804 |
| 2017 | 90 (19.2) | 48 (15.4) | .178 |
| Region, n (%) | |||
| South | 193 (41.2) | 145 (46.6) | .131 |
| Midwest | 117 (24.9) | 86 (27.7) | .399 |
| Northeast | 87 (18.6) | 32 (10.3) | .002c |
| West | 65 (13.9) | 45 (14.5) | .811 |
| Unknown | 7 (1.5) | 3 (1.0) | .748 |
| Insurance plan at treatment initiation, n (%) | |||
| PPO | 362 (77.2) | 244 (78.5) | .676 |
| HMO | 73 (15.6) | 43 (13.8) | .504 |
| POS | 20 (4.3) | 17 (5.5) | .439 |
| Indemnity/traditional | 12 (2.6) | 6 (1.9) | .567 |
| Quan‐CCI,d mean ± SD (median) | 6.3 ± 1.5 (6) | 6.3 ± 1.5 (6) | .853 |
| Selected comorbidities,d n (%) | |||
| Hypertension | 161 (34.3) | 118 (37.9) | .303 |
| Venous thromboembolism | 27 (5.8) | 13 (4.2) | .328 |
| Coronary artery disease | 17 (3.6) | 11 (3.5) | .949 |
| Other ischemic heart disease | 16 (3.4) | 11 (3.5) | .925 |
| Cardiac dysrhythmia | 11 (2.3) | 8 (2.6) | .840 |
| Congestive heart failure | 5 (1.1) | 4 (1.3) | .747 |
| Arterial thromboembolism | 3 (0.6) | 0 (0.0) | .280 |
| Acute myocardial infarction | 2 (0.4) | 0 (0.0) | .520 |
| Stroke | 2 (0.4) | 2 (0.6) | .653 |
| mCRC antineoplastic therapy use,d n (%) | |||
| 5‐fluorouracil | 218 (46.5) | 167 (53.7) | .048c |
| Irinotecan | 216 (46.1) | 166 (53.4) | .045c |
| Bevacizumab | 159 (33.9) | 112 (36.0) | .544 |
| Leucovorin | 156 (33.3) | 120 (38.6) | .128 |
| Oxaliplatin | 66 (14.1) | 45 (14.5) | .877 |
| Cetuximab | 62 (13.2) | 42 (13.5) | .909 |
| Capecitabine | 56 (11.9) | 34 (10.9) | .666 |
| Panitumumab | 49 (10.4) | 27 (8.7) | .415 |
| Ramucirumab | 11 (2.3) | 7 (2.3) | .931 |
| Ziv‐aflibercept | 1 (0.2) | 0 (0.0) | >.999 |
Evaluated at the index date.
Chi‐square tests were used for categorical variables and Wilcoxon tests were used for continuous variables.
p value < .05.
Evaluated during the 3‐month baseline period.
Abbreviations: FTD + TPI, trifluridine + tipiracil; HMO, health maintenance organization; mCRC, metastatic colorectal cancer; POS, point of service; PPO, preferred provider organization; Quan‐CCI, Quan‐Charlson comorbidity index; REG, regorafenib; SD, standard deviation.
Treatment Adherence
Table 2 presents the comparison of treatment patterns between FTD + TPI users and REG users during the observation period (mean length of observation period: 168 days vs. 144 days; p = .006). The mean MPR was significantly higher for FTD + TPI users compared with REG users (0.93 vs. 0.86; p < .001). Similarly, the proportions of patients with MPR ≥0.80 and ≥ 0.90 were also significantly higher for FTD + TPI users compared with REG users (MPR ≥0.80: 87.1% vs. 72.6%; MPR ≥0.90: 74.6% vs. 54.3%; both p < .001). The mean PDCs at 3 and 6 months were significantly higher for FTD + TPI users compared with REG users (mean PDC at 3 months, 0.72 vs. 0.60; p < 0.001; mean PDC at 6 months, 0.56 vs. 0.48; p = .020). The proportion of patients considered adherent (i.e., PDC ≥0.80) was also significantly higher in the FTD + TPI cohort compared with the REG cohort at 3 months of follow‐up (50.8% vs. 28.0%; p < .001) but did not reach statistical significance at 6 months of follow‐up (21.4% vs. 16.0%; p = .325).
Table 2.
Treatment patterns, adherence, and persistence postindex date
| Outcomes | FTD + TPI users (n = 469) | REG users (n = 311) | p valuea |
|---|---|---|---|
| Length of the observation periodb | |||
| Mean ± SD (median), daysd | 167.8 ± 107.2 (138) | 143.5 ± 82.5 (124) | .006c |
| Patients followed for ≥3 mo, n (%) | 396 (84.4) | 250 (80.4) | .142 |
| Patients followed for ≥6 mo, n (%) | 159 (33.9) | 81 (26.0) | .020c |
| Treatment patterns | |||
| Duration of treatment,d mean ± SD (median)d | 94.2 ± 73.3 (81) | 81.4 ± 69.8 (60) | <.001c |
| MPR | |||
| Patients with ≥2 claims, n (%) | 402 (85.7) | 208 (66.9) | <.001c |
| MPR, Mean ± SD (median) | 0.93 ± 0.12 (1.0) | 0.86 ± 0.16 (0.9) | <.001c |
| MPR ≥0.80, n (%) | 350 (87.1) | 151 (72.6) | <.001c |
| MPR ≥0.90, n (%) | 300 (74.6) | 113 (54.3) | <.001c |
| PDC | |||
| At 3 mo | 396 (84.4) | 250 (80.4) | |
| Mean PDC ± SD (median) | 0.72 ± 0.24 (0.8) | 0.60 ± 0.24 (0.6) | <.001c |
| PDC ≥0.80, n (%) | 201 (50.8) | 70 (28.0) | <.001c |
| PDC ≥0.90, n (%) | 137 (34.6) | 34 (13.6) | <.001c |
| At 6 mo | 159 (33.9) | 81 (26.0) | |
| Mean PDC ± SD (median) | 0.56 ± 0.25 (0.6) | 0.48 ± 0.25 (0.5) | .020c |
| PDC ≥0.80, n (%) | 34 (21.4) | 13 (16.0) | .325 |
| PDC ≥0.90, n (%) | 19 (11.9) | 2 (2.5) | .014c |
| Persistence,e n (%) | |||
| At 3 mo | 396 (84.4) | 250 (80.4) | |
| No gap ≥45 d | 328 (82.8) | 170 (68.0) | <.001c |
| No gap ≥60 d | 334 (84.3) | 176 (70.4) | <.001c |
| At 6 mo | 159 (33.9) | 81 (26.0) | |
| No gap ≥45 d | 62 (39.0) | 26 (32.1) | .295 |
| No gap ≥60 d | 71 (44.7) | 33 (40.7) | .563 |
| Time to discontinuation, mean ± SD (median) | |||
| No gap ≥45 d | 94.8 ± 67.1 (84) | 78.0 ± 61.4 (62) | <.001c |
| No gap ≥60 d | 99.3 ± 71.1 (91) | 86.5 ± 68.1 (73) | <.001c |
Chi‐square tests were used for categorical variables and Wilcoxon tests were used for continuous variables.
The observation period was defined as the period from the index date to the earliest date between the day before a switch to a metastatic CRC treatment, end of continuous insurance coverage, or end of data availability.
p value < .05.
The treatment period was defined as the period from the index date to the last day of supply of the last dispensing over the observation period.
Persistence was defined as continuous treatment without a gap longer than a permissible duration within a fixed time interval. The gap was calculated as time between the end of a dispensing and the beginning of next dispensing or time between the last day of the last dispensing and the end of the assessment period.
Abbreviations: FTD + TPI, trifluridine + tipiracil; MPR, medication possession ratio; PDC, proportion of days covered; REG, regorafenib; SD, standard deviation.
Consistently, the FTD + TPI cohort had significantly higher mean MPR, PDC, and proportion of patients considered adherent compared with the REG cohort after adjusting for baseline covariates (Table 3). More specifically, FTD + TPI users were more likely to have an MPR ≥0.80 compared with REG users (OR, 2.47; p < .001) and to have a PDC ≥0.80 at 3 months (OR, 2.77; p < .001). The FTD + TPI users were more likely to have a PDC ≥0.80 at 6 months, but the results were not statistically significant (OR, 1.43; p = .351).
Table 3.
Comparison of persistence and adherence
| Outcomes | Mean differencea (95% CI), FTD + TPI vs. REG | p value | Odds ratiob (95% CI), FTD + TPI vs. REG | p value | Hazard ratioc (95% CI), FTD + TPI vs. REG | p value |
|---|---|---|---|---|---|---|
| MPR | ||||||
| MPR (%) | 6.33 (4.03–8.64) | <.001d | ||||
| MPR ≥80% | 2.47 (1.60–3.79) | <.001d | ||||
| PDC | ||||||
| PDC (%), at 3 mo | 11.88 (8.17–15.59) | <.001d | ||||
| PDC (%), at 6 mo | 6.73 (0.22–13.23) | .043d | ||||
| PDC ≥80%, at 3 mo | 2.77 (1.95–3.94) | <.001d | ||||
| PDC ≥80%, at 6 mo | 1.43 (0.68–3.02) | .351 | ||||
| Time to discontinuation | ||||||
| No gap ≥45 d | .76 (0.63–0.93) | .006d | ||||
| No gap ≥60 d | .91 (0.73–1.12) | .374 |
Mean differences were estimated using multivariate linear models adjusted for demographic covariates (age, gender, region, insurance plan, year of index date), Quan‐Charlson comorbidity index, all‐cause baseline drug costs, all‐cause baseline medical costs.
Odds ratios were estimated using logit binomial models adjusted for demographic covariates (age, gender, region, insurance plan, year of index date), Quan‐Charlson comorbidity index, all‐cause baseline drug costs, all‐cause baseline medical costs.
Hazard ratio were estimated using Cox proportional hazards models adjusted for demographic covariates (i.e., age, gender, region, insurance plan, year of index date), Quan‐Charlson comorbidity index, all‐cause baseline drug costs, all‐cause baseline medical costs.
p value < .05.
Abbreviations: CI, confidence interval; FTD + TPI, trifluridine + tipiracil; MPR, medication possession ratio; PDC, proportion of days covered; REG, regorafenib.
Treatment Persistence and Time to Discontinuation
Patients treated with FTD + TPI had a significantly longer treatment duration compared with patients treated with REG (mean length of treatment: 94 days vs. 81 days; p < .001). Persistence at 3 months, whether defined with a permissible gap of 45 or 60 days between dispensings, was higher in the FTD + TPI cohort than the REG cohort (45‐day gap: 82.8% vs. 68.0%; p < .001; 60‐day gap: 84.3% vs. 70.4%; p < .001), but the difference was not statistically significant at 6 months (45‐day gap: 39.0% vs. 32.1%; p = .295; 60‐day gap: 44.7% vs. 40.7%; p = .563; Table 2).
The mean TTD was significantly longer for FTD + TPI users compared with REG users, with discontinuation defined either as a gap in treatment of 45 or 60 days (45‐day gap: 94.8 vs. 78.0 days; p < .001; 60‐day gap: 99.3 vs. 86.5 days; p < .001). After adjusting for the baseline covariates, FTD + TPI users had significantly lower risk of discontinuation than REG users (HR, 0.76; p = .006) when an allowable gap of 45 day was used. With an allowable gap of 60 days, the risk of discontinuation was not significantly different between the two cohorts (Table 3).
Switchers Analysis
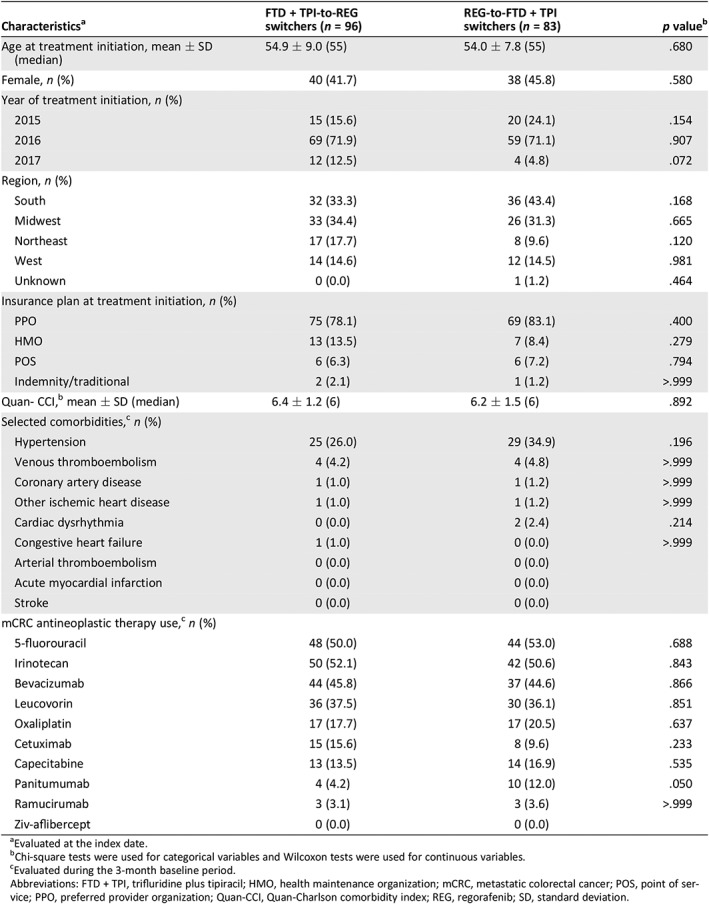
A total of 96 FTD + TPI‐to‐REG switchers and 83 REG‐to‐FTD + TPI switchers were identified in the subgroup analysis of switchers. Baseline characteristics and previously ordered treatments were similar between both cohorts. The mean Quan‐CCI score was also similar between both cohorts (6.4 vs. 6.2; p = .892), and the most common comorbidities seen were the same as those in the FTD + TPI and REG cohorts in the primary analysis (Table 4).
Table 4.
Baseline demographic and clinical characteristics: Subgroup analysis among switchers
| Characteristicsa | FTD + TPI‐to‐REG switchers (n = 96) | REG‐to‐FTD + TPI switchers (n = 83) | p valueb |
|---|---|---|---|
| Age at treatment initiation, mean ± SD (median) | 54.9 ± 9.0 (55) | 54.0 ± 7.8 (55) | .680 |
| Female, n (%) | 40 (41.7) | 38 (45.8) | .580 |
| Year of treatment initiation, n (%) | |||
| 2015 | 15 (15.6) | 20 (24.1) | .154 |
| 2016 | 69 (71.9) | 59 (71.1) | .907 |
| 2017 | 12 (12.5) | 4 (4.8) | .072 |
| Region, n (%) | |||
| South | 32 (33.3) | 36 (43.4) | .168 |
| Midwest | 33 (34.4) | 26 (31.3) | .665 |
| Northeast | 17 (17.7) | 8 (9.6) | .120 |
| West | 14 (14.6) | 12 (14.5) | .981 |
| Unknown | 0 (0.0) | 1 (1.2) | .464 |
| Insurance plan at treatment initiation, n (%) | |||
| PPO | 75 (78.1) | 69 (83.1) | .400 |
| HMO | 13 (13.5) | 7 (8.4) | .279 |
| POS | 6 (6.3) | 6 (7.2) | .794 |
| Indemnity/traditional | 2 (2.1) | 1 (1.2) | >.999 |
| Quan‐ CCI,b mean ± SD (median) | 6.4 ± 1.2 (6) | 6.2 ± 1.5 (6) | .892 |
| Selected comorbidities,c n (%) | |||
| Hypertension | 25 (26.0) | 29 (34.9) | .196 |
| Venous thromboembolism | 4 (4.2) | 4 (4.8) | >.999 |
| Coronary artery disease | 1 (1.0) | 1 (1.2) | >.999 |
| Other ischemic heart disease | 1 (1.0) | 1 (1.2) | >.999 |
| Cardiac dysrhythmia | 0 (0.0) | 2 (2.4) | .214 |
| Congestive heart failure | 1 (1.0) | 0 (0.0) | >.999 |
| Arterial thromboembolism | 0 (0.0) | 0 (0.0) | |
| Acute myocardial infarction | 0 (0.0) | 0 (0.0) | |
| Stroke | 0 (0.0) | 0 (0.0) | |
| mCRC antineoplastic therapy use,c n (%) | |||
| 5‐fluorouracil | 48 (50.0) | 44 (53.0) | .688 |
| Irinotecan | 50 (52.1) | 42 (50.6) | .843 |
| Bevacizumab | 44 (45.8) | 37 (44.6) | .866 |
| Leucovorin | 36 (37.5) | 30 (36.1) | .851 |
| Oxaliplatin | 17 (17.7) | 17 (20.5) | .637 |
| Cetuximab | 15 (15.6) | 8 (9.6) | .233 |
| Capecitabine | 13 (13.5) | 14 (16.9) | .535 |
| Panitumumab | 4 (4.2) | 10 (12.0) | .050 |
| Ramucirumab | 3 (3.1) | 3 (3.6) | >.999 |
| Ziv‐aflibercept | 0 (0.0) | 0 (0.0) |
Evaluated at the index date.
Chi‐square tests were used for categorical variables and Wilcoxon tests were used for continuous variables.
Evaluated during the 3‐month baseline period.
Abbreviations: FTD + TPI, trifluridine plus tipiracil; HMO, health maintenance organization; mCRC, metastatic colorectal cancer; POS, point of service; PPO, preferred provider organization; Quan‐CCI, Quan‐Charlson comorbidity index; REG, regorafenib; SD, standard deviation.
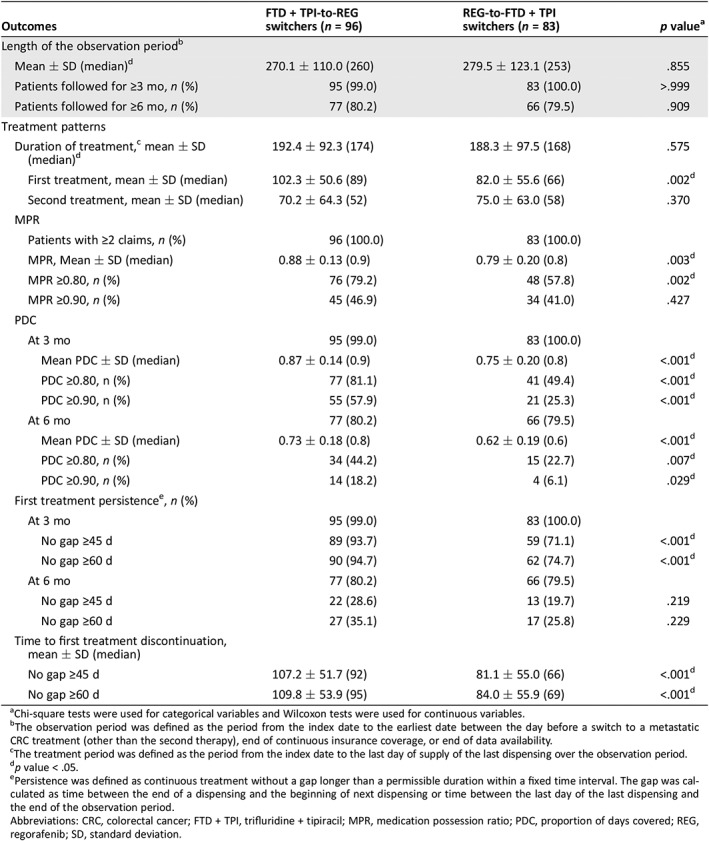
Table 5 presents the comparison of treatment patterns between FTD + TPI‐to‐REG switchers and REG‐to‐FTD + TPI switchers during the observation period (mean length of observation period, FTD + TPI‐to‐REG vs. REG‐to‐FTD + TPI: 270 days vs. 280 days; p = .855). The mean MPR was significantly higher for FTD + TPI‐to‐REG switchers compared with REG‐to‐FTD + TPI switchers (0.88 vs. 0.79; p = .003). The mean PDC values at 3 months and 6 months were significantly higher for FTD + TPI‐to‐REG switchers compared with REG‐to‐FTD + TPI switchers (mean PDC at 3 months, 0.87 vs. 0.75; p < .001; mean PDC at 6 months, 0.73 vs. 0.62; p < .001). The proportion of adherent patients (i.e., MPR ≥0.80) was significantly higher in the FTD + TPI‐to‐REG cohort compared with the REG‐to‐FTD + TPI cohort (79.2% vs. 57.8%; p = .002; OR, 2.91; p = .004). Consistently, the proportion of adherent patients (i.e., PDC ≥0.80) was also significantly higher in the FTD + TPI‐to‐REG cohort compared with the REG‐to‐FTD + TPI cohort at 3 months (81.1% vs. 49.4%, p < .001; OR, 4.60, p < .001) and 6 months (44.2% vs. 22.7%, p = .007; OR, 2.95, p = .011).
Table 5.
Comparison of persistence and adherence: Subgroup analysis among switchers
| Outcomes | FTD + TPI‐to‐REG switchers (n = 96) | REG‐to‐FTD + TPI switchers (n = 83) | p valuea |
|---|---|---|---|
| Length of the observation periodb | |||
| Mean ± SD (median)d | 270.1 ± 110.0 (260) | 279.5 ± 123.1 (253) | .855 |
| Patients followed for ≥3 mo, n (%) | 95 (99.0) | 83 (100.0) | >.999 |
| Patients followed for ≥6 mo, n (%) | 77 (80.2) | 66 (79.5) | .909 |
| Treatment patterns | |||
| Duration of treatment,c mean ± SD (median)d | 192.4 ± 92.3 (174) | 188.3 ± 97.5 (168) | .575 |
| First treatment, mean ± SD (median) | 102.3 ± 50.6 (89) | 82.0 ± 55.6 (66) | .002d |
| Second treatment, mean ± SD (median) | 70.2 ± 64.3 (52) | 75.0 ± 63.0 (58) | .370 |
| MPR | |||
| Patients with ≥2 claims, n (%) | 96 (100.0) | 83 (100.0) | |
| MPR, Mean ± SD (median) | 0.88 ± 0.13 (0.9) | 0.79 ± 0.20 (0.8) | .003d |
| MPR ≥0.80, n (%) | 76 (79.2) | 48 (57.8) | .002d |
| MPR ≥0.90, n (%) | 45 (46.9) | 34 (41.0) | .427 |
| PDC | |||
| At 3 mo | 95 (99.0) | 83 (100.0) | |
| Mean PDC ± SD (median) | 0.87 ± 0.14 (0.9) | 0.75 ± 0.20 (0.8) | <.001d |
| PDC ≥0.80, n (%) | 77 (81.1) | 41 (49.4) | <.001d |
| PDC ≥0.90, n (%) | 55 (57.9) | 21 (25.3) | <.001d |
| At 6 mo | 77 (80.2) | 66 (79.5) | |
| Mean PDC ± SD (median) | 0.73 ± 0.18 (0.8) | 0.62 ± 0.19 (0.6) | <.001d |
| PDC ≥0.80, n (%) | 34 (44.2) | 15 (22.7) | .007d |
| PDC ≥0.90, n (%) | 14 (18.2) | 4 (6.1) | .029d |
| First treatment persistencee, n (%) | |||
| At 3 mo | 95 (99.0) | 83 (100.0) | |
| No gap ≥45 d | 89 (93.7) | 59 (71.1) | <.001d |
| No gap ≥60 d | 90 (94.7) | 62 (74.7) | <.001d |
| At 6 mo | 77 (80.2) | 66 (79.5) | |
| No gap ≥45 d | 22 (28.6) | 13 (19.7) | .219 |
| No gap ≥60 d | 27 (35.1) | 17 (25.8) | .229 |
| Time to first treatment discontinuation, mean ± SD (median) | |||
| No gap ≥45 d | 107.2 ± 51.7 (92) | 81.1 ± 55.0 (66) | <.001d |
| No gap ≥60 d | 109.8 ± 53.9 (95) | 84.0 ± 55.9 (69) | <.001d |
Chi‐square tests were used for categorical variables and Wilcoxon tests were used for continuous variables.
The observation period was defined as the period from the index date to the earliest date between the day before a switch to a metastatic CRC treatment (other than the second therapy), end of continuous insurance coverage, or end of data availability.
The treatment period was defined as the period from the index date to the last day of supply of the last dispensing over the observation period.
p value < .05.
Persistence was defined as continuous treatment without a gap longer than a permissible duration within a fixed time interval. The gap was calculated as time between the end of a dispensing and the beginning of next dispensing or time between the last day of the last dispensing and the end of the observation period.
Abbreviations: CRC, colorectal cancer; FTD + TPI, trifluridine + tipiracil; MPR, medication possession ratio; PDC, proportion of days covered; REG, regorafenib; SD, standard deviation.
FTD + TPI‐to‐REG switchers had a longer duration of their first treatment compared with REG‐to‐FTD + TPI switchers (mean duration of first treatment: 102 vs. 82 days; p = .002). Persistence with first treatment evaluated at 3 months was higher in patients initiated on FTD + TPI first (allowable gap of 45 days: 93.7% vs. 71.1%; p < .001; allowable gap of 60 days: 94.7% vs. 74.7%; p < .001) but did not reach statistical significance at 6 months (allowable gap of 45 days: 28.6% vs. 19.7%; p = .219; allowable gap of 60 days: 35.1% vs. 25.8%; p = .229). The mean TTD of first treatment was significantly longer for FTD + TPI‐to‐REG switchers compared with REG‐to‐FTD + TPI switchers, regardless of whether discontinuation was defined as a gap in treatment of 45 days (107 vs. 81 days; p < .001) or 60 days (110 vs. 84 days; p < .001).
Discussion
In this study of real‐world treatment patterns among patients with mCRC treated with FTD + TPI and REG, FTD + TPI was associated with higher medication adherence than REG. FTD + TPI users were twice as likely to have an MPR ≥0.80 and significantly less likely to discontinue treatment. Moreover, patients who switched from FTD + TPI to REG showed higher adherence, higher compliance, and lower likelihood of discontinuing their first treatment compared with those who switched from REG to FTD + TPI.
To date, few studies have examined adherence to therapy in patients with mCRC using FTD + TPI or REG 16, 17, 18, 19. A single‐center study using self‐reported treatment diaries to evaluate adherence in patients using FTD + TPI reported an adherence rate of 95.0%–98.2% 17. These results are consistent with the mean MPR reported in the current study for FTD + TPI (mean MPR, 0.93). Another study found that adherence increased from 64.4% in the first cycle to 83.8% in the third cycle for patients treated with REG 19. Similarly, a study by Del Prete and colleagues reported an average adherence to treatment with REG of 82% during the first 4 months of treatment 18. These results corroborate our finding that mean MPR for REG users was 0.86 for a mean follow‐up of about 140 days.
More recently, we reported a comparative study of adherence to FTD + TPI versus REG in U.S. real‐world practice that found that patients with mCRC using FTD + TPI were 80% more likely to have an MPR ≥0.80 compared with patients using REG and more than twice as likely to have a PDC of ≥0.80 at 3 months. Furthermore, patients using FTD + TPI were 37% less likely to discontinue treatment (60‐day gap) than those using REG; only 40% of patients using FTD + TPI had discontinued treatment at 90 days after the initiation of therapy compared with 57% of patients using REG 16. That earlier study evaluated older patient data from limited supply channels. The results from this present study are consistent with those findings, showing significantly higher adherence and compliance rates as well as longer TTD for patient treated with FTD + TPI.
A retrospective single‐center study conducted in Japan showed that the safety profiles of FTD + TPI and REG significantly differ in many aspects 20, which may contribute to the observed difference in adherence. In that study, hand‐foot syndrome and liver dysfunction were much more common among patients treated with REG than those treated with FTD + TPI 20, and these two adverse events (AEs) are the most common causes of nonadherence to REG 19. Conversely, nausea and vomiting were more common among patients treated with FTD + TPI than those treated with REG, and these are among the most common factors associated with nonadherence to FTD + TPI 17. Therefore, these AEs appear likely to contribute to the difference in adherence observed in the current study, although this has not been formally evaluated. Further research is warranted to understand the factors underlying the difference in adherence between FTD + TPI and REG.
To the best of our knowledge, no studies have assessed the impact of administrating FTD + TPI and REG in sequence on adherence. This study's assessment of treatment adherence among patients who switched from FTD + TPI to REG and vice versa showed that FTD + TPI‐to‐REG switchers were twice more likely to have an MPR ≥0.80 and over four times more likely to have a PDC ≥0.80 compared with REG‐to‐FTD + TPI switchers. Notably, this increased adherence was demonstrated over the entire observation period, encompassing both lines of treatment. Moreover, the duration of the first treatment for FTD + TPI‐to‐REG switchers was significantly longer than that for REG‐to‐FTD + TPI switchers. In light of the present observation that adherence is higher in patients treated with FTD + TPI than those treated with REG, this suggests that at least part of the difference in adherence between FTD + TPI‐to‐REG versus REG‐to‐FTD + TPI switchers can be attributed to the longer time spent on FTD + TPI. These results extend the previous findings of increased adherence to FTD + TPI and suggest an advantage to initiating FTD + TPI before REG; this may be particularly relevant in this setting, where any treatment is generally associated with a higher risk of toxicities and limited potential for clinical benefits.
Ultimately, both FTD + TPI and REG represent active treatment options for patients with mCRC who have already progressed on multiple lines of therapy. The choice of treatment is a clinical decision that must be tailored to each patient's clinical and disease characteristics, with the aim to optimize treatment outcomes and reduce cumulative toxicities. Given that FTD + TPI and REG have demonstrated comparable survival benefits in this population, increased weight must be placed on other important considerations, including tolerability and schedule of administration 21, 22, 23. Greater information regarding the real‐world adherence to therapy and duration of treatment with these agents can provide additional insight useful in these treatment decisions.
Some limitations of this study should be noted. First, the study was subject to common limitations of studies based on health care claims data, such as possible billing inaccuracies or omissions in coded procedures, diagnoses, or pharmacy claims. However, potential inaccuracies or omissions are expected to affect all cohorts to a similar extent. Second, given that treatment patterns were derived from outpatient pharmacy claims, the presence of a dispensed medication does not indicate that the medication was consumed or that it was used as prescribed. In addition, medications received during an inpatient stay were not available in the database. Third, the observational design of the study is susceptible to additional potential biases such as information or classification bias (e.g., identification of false positive or false negative CRC diagnosis). Fourth, residual confounding may exist if potential confounders remained uncontrolled because of unavailability of data, limits of data collection, or potential inaccuracies in claims data. Finally, the generalizability of the study findings may be limited, because the IQVIA database was predominantly sourced from commercially insured plan members and may under‐represent Medicare beneficiaries.
Conclusion
This real‐world study of adherence and persistence in patients with mCRC indicates that treatment with FTD + TPI is associated with significantly higher medication adherence, persistence, and longer time to discontinuation than with REG. In those patients receiving both agents, patients treated with FTD + TPI prior to switching to REG also showed higher adherence, first‐line persistence, and duration of therapy. Moreover, this analysis demonstrates that claims data analysis can provide insight into oral chemotherapy adherence patterns in mCRC. These findings can help inform treatment decisions with respect to the choice and sequencing of treatment with FTD + TPI and REG in patients with mCRC.
Author Contributions
Conception/design: Anuj K. Patel, Victoria Barghout, Mihran A. Yenikomshian, Guillaume Germain, Philippe Jacques, François Laliberté, Mei S. Duh
Provision of study material or patients: Mihran A. Yenikomshian, Guillaume Germain, Philippe Jacques, François Laliberté, Mei S. Duh
Collection and/or assembly of data: Mihran A. Yenikomshian, Guillaume Germain, Philippe Jacques, François Laliberté, Mei S. Duh
Data analysis and interpretation: Anuj K. Patel, Victoria Barghout, Mihran A. Yenikomshian, Guillaume Germain, Philippe Jacques, François Laliberté, Mei S. Duh
Manuscript writing: Anuj K. Patel, Victoria Barghout, Mihran A. Yenikomshian, Guillaume Germain, Philippe Jacques, François Laliberté, Mei S. Duh
Final approval of manuscript: Anuj K. Patel, Victoria Barghout, Mihran A. Yenikomshian, Guillaume Germain, Philippe Jacques, François Laliberté, Mei S. Duh
Disclosures
Victoria Barghout: Taiho Oncology Inc. (C/A); Mihran A. Yenikomshian: Analysis Group Inc. (E), Taiho Oncology, iRhythm Technologies, AbbVie, Genentech (RF); Guillaume Germain: Groupe d'analyse, Ltée. (E); Philippe Jacques: Groupe d'analyse, Ltée. (E); François Laliberté: Analysis Group Inc. (E), Taiho Oncology (RF); Mei S. Duh: Analysis Group Inc. (E), Taiho Oncology (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The authors thank Sara Kaffashian, an employee of Groupe d'analyse, Ltée, for her assistance with writing services. This study is funded by Taiho Oncology Inc.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Cancer stat facts: Colon and rectum cancer . National Cancer Institute Surveillance Epidemiology and End Results Program website. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed May 17, 2018. [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490–1502. [DOI] [PubMed] [Google Scholar]
- 4. Price TJ, Segelov E, Burge M et al. Current opinion on optimal systemic treatment for metastatic colorectal cancer: Outcome of the ACTG/AGITG expert meeting ECCO 2013. Expert Rev Anticancer Ther 2014;14:1477–1493. [DOI] [PubMed] [Google Scholar]
- 5. Van Cutsem E, Cervantes A, Adam R et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–1422. [DOI] [PubMed] [Google Scholar]
- 6. Stivarga (regorafenib) Prescribing Information. Wayne, NJ: Bayer HealthCare Pharmaceuticals; 2012. [Google Scholar]
- 7. Lonsurf (trifluridine and tipiracil) Prescribing Information. Princeton, NJ: Taiho Pharmaceuticals Co, Ltd; 2015. [Google Scholar]
- 8. Grothey A, Van Cutsem E, Sobrero A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:303–312. [DOI] [PubMed] [Google Scholar]
- 9. Mayer RJ, Van Cutsem E, Falcone A et al. Randomized trial of TAS‐102 for refractory metastatic colorectal cancer. New Engl J Med 2015;372:1909–1919. [DOI] [PubMed] [Google Scholar]
- 10. Greer JA, Amoyal N, Nisotel L et al. A systematic review of adherence to oral antineoplastic therapies. The Oncologist 2016;21:354–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osterberg L, Blaschke T. Adherence to medication. New Engl J Med 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 12. Ganesan P, Sagar TG, Dubashi B et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 2011;86:471–474. [DOI] [PubMed] [Google Scholar]
- 13. Makubate B, Donnan PT, Dewar JA et al. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer 2013;108:1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marin D, Bazeos A, Mahon FX et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010;28:2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borner M, Scheithauer W, Twelves C et al. Answering patients’ needs: Oral alternatives to intravenous therapy. The Oncologist 2001;(suppl 4):12–16. [DOI] [PubMed] [Google Scholar]
- 16. Patel AK, Duh MS, Barghout V et al. Real‐world treatment patterns among patients with colorectal cancer treated with trifluridine/tipiracil and regorafenib. Clin Colorectal Cancer 2018;17:e531–539. [DOI] [PubMed] [Google Scholar]
- 17. Sugita K, Kawakami K, Yokokawa T et al. Self‐reported adherence to trifluridine and tipiracil hydrochloride for metastatic colorectal cancer: A retrospective cohort study. Oncology 2016;91:224–230. [DOI] [PubMed] [Google Scholar]
- 18. Del Prete S, Cennamo G, Leo L et al. Adherence and safety of regorafenib for patients with metastatic colorectal cancer: Observational real‐life study. Future Oncol 2017;13:415–423. [DOI] [PubMed] [Google Scholar]
- 19. Kawakami K, Suenaga M, Soejima A et al. Self‐reported adherence to regorafenib for metastatic colorectal cancer: A retrospective cohort study. J Clin Oncol 2017;35(suppl):783a. [Google Scholar]
- 20. Masuishi T, Taniguchi H, Hamauchi S et al. Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: A retrospective comparison. Clin Colorectal Cancer 2017;16:e15–e22. [DOI] [PubMed] [Google Scholar]
- 21. Kimura M, Usami E, Iwai M et al. Comparison of cost‐effectiveness of regorafenib and trifluridine/tipiracil combination tablet for treating advanced and recurrent colorectal cancer. Mol Clin Oncol 2016;5:635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin 2009;59:56–66. [DOI] [PubMed] [Google Scholar]
- 23. Weinberg BA, Marshall JL, Salem ME. Trifluridine/tipiracil and regorafenib: New weapons in the war against metastatic colorectal cancer. Clin Adv Hematol Oncol 2016;14:630–638. [PubMed] [Google Scholar]


