Abstract
Platinum‐based chemotherapy is commonly associated with toxic sensory neuropathies, but also, although rarely, with Guillain‐Barré syndrome (GBS). We describe five patients who developed GBS while receiving platinum‐based chemotherapy for a solid tumor and report the five cases published so far. Most patients had received cumulative platinum doses below known neurotoxic levels, and all of them had an optimal outcome after platinum discontinuation, associated in most cases with administration of intravenous immunoglobulin. Clinical presentation, electroneuromyography, and cerebrospinal fluid analysis help clinicians to differentiate GBS from toxic neuropathy. Platinum compounds are the only chemotherapeutic agents used for solid tumors that have been associated to GBS. Thus, we propose that GBS may constitute a non–dose‐dependent side effect of platinum drugs and that awareness needs to be raised among oncologists on this rare but potentially life‐threatening complication of platinum chemotherapy.
Implications for Practice
Many patients on platinum‐based chemotherapy for solid tumors develop sensory neuropathy, a common dose‐dependent side effect. The authors propose that Guillain‐Barré syndrome may constitute an immune‐mediated, non‐dose‐related side effect of platinum‐based chemotherapy. Prompt diagnosis of Guillain‐Barré syndrome and distinction from classical toxic neuropathy are crucial for optimal treatment. Platinum discontinuation, associated if needed to intravenous immunoglobulin administration, radically changes the course of the disease and minimizes neurological sequelae.
Short abstract
To increase awareness of a potentially life‐threatening complication of platinum chemotherapy, this brief communication describes five patients who developed GBS while receiving platinum‐based chemotherapy for a solid tumor and reports the results of a review of the literature on this topic.
Introduction
Platinum‐based chemotherapy is commonly associated with sensory neuropathies related to a dose‐dependent damage of dorsal root ganglia 1, 2. However, although rarely, peripheral neuropathies resembling Guillain‐Barré syndrome (GBS) have been reported in patients receiving platinum compounds, suggesting an immune‐mediated process.
GBS is an immune‐mediated polyradiculoneuropathy that typically occurs after infection or vaccination and presents with progressive muscle weakness, sensory impairment, and decreased tendon reflexes. GBS can be a potentially life‐threatening condition when associated with bulbar muscle involvement, respiratory failure and dysautonomia. Nonetheless, treatment with intravenous immunoglobulin (IVIG) or plasma exchange can lead to a favorable outcome.
Here we describe five patients who developed GBS while receiving platinum‐based chemotherapy for a solid tumor and report the results of a literature review on this topic. The aim of this study is to increase the awareness of oncologists on this rare but potentially life‐threatening complication of platinum chemotherapy.
Subjects, Materials, and Methods
We performed a retrospective research in the OncoNeuroTox database (French network for patients with neurological complications from oncologic treatments) for patients diagnosed with polyradiculoneuropathy while receiving chemotherapy for a solid tumor between January 2012 and January 2019. Out of 449 patients diagnosed with peripheral neuropathies during the period of interest, five patients presented with acute polyradiculoneuropathy. All patients met the Brighton criteria for the diagnosis of GBS (level 1 of diagnostic certainty: three patients; level 2 of diagnostic certainty: two patients) 3. The clinical, electrophysiological, and biological data of these five patients were collected and reviewed in detail. We then conducted a comprehensive literature review in search for similar cases published in the PubMed, Embase, and Cochrane databases up to December 2018.
The study was approved by the French Region Committees on Health Research Ethics and the French Data Protection Agency. Data were collected and stored anonymized according to the guidelines of the French Data Protection Agency.
Results
Present Series
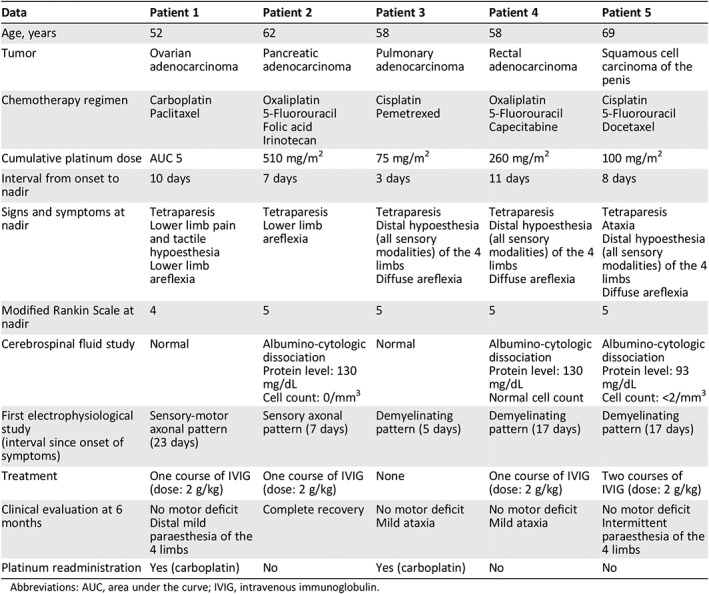
Table 1 summarizes clinical and paraclinical features, treatment, and outcome of the five patients included in the present series. All patients had received platinum compounds as part of their tumor treatment. Cumulative platinum doses were in all cases below the established threshold for neurological toxicity (i.e., less than 300–450 mg/m2 of cisplatin and less than 750–850 mg/m2 of oxaliplatin 1). Neurological symptoms appeared after a median delay of 4 days (range, 1–8 days) from last platinum administration; all patients experienced acute weakness of the four limbs and altered sensation of lower (or upper and lower) limbs. Electrophysiological findings were in all cases compatible with GBS, consisting of either demyelinating (patients 3–5) or axonal (patients 1–2) features. Cerebrospinal fluid analysis showed albumino‐cytologic dissociation (i.e., increased protein levels with normal cell count) in three out of the five patients. Screening for metabolic, infectious, and autoimmune causes of acquired peripheral neuropathy (e.g., vitamin B deficiencies, diabetes, thyroid disease, uremia, hepatitis B and C, human immunodeficiency virus, Lyme infection, or antiganglioside antibodies) was negative in all cases.
Table 1.
Our case series
| Data | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age, years | 52 | 62 | 58 | 58 | 69 |
| Tumor | Ovarian adenocarcinoma | Pancreatic adenocarcinoma | Pulmonary adenocarcinoma | Rectal adenocarcinoma | Squamous cell carcinoma of the penis |
| Chemotherapy regimen |
Carboplatin Paclitaxel |
Oxaliplatin 5‐Fluorouracil Folic acid Irinotecan |
Cisplatin Pemetrexed |
Oxaliplatin 5‐Fluorouracil Capecitabine |
Cisplatin 5‐Fluorouracil Docetaxel |
| Cumulative platinum dose | AUC 5 | 510 mg/m2 | 75 mg/m2 | 260 mg/m2 | 100 mg/m2 |
| Interval from onset to nadir | 10 days | 7 days | 3 days | 11 days | 8 days |
| Signs and symptoms at nadir |
Tetraparesis Lower limb pain and tactile hypoesthesia Lower limb areflexia |
Tetraparesis Lower limb areflexia |
Tetraparesis Distal hypoesthesia (all sensory modalities) of the 4 limbs Diffuse areflexia |
Tetraparesis Distal hypoesthesia (all sensory modalities) of the 4 limbs Diffuse areflexia |
Tetraparesis Ataxia Distal hypoesthesia (all sensory modalities) of the 4 limbs Diffuse areflexia |
| Modified Rankin Scale at nadir | 4 | 5 | 5 | 5 | 5 |
| Cerebrospinal fluid study | Normal |
Albumino‐cytologic dissociation Protein level: 130 mg/dL Cell count: 0/mm3 |
Normal |
Albumino‐cytologic dissociation Protein level: 130 mg/dL Normal cell count |
Albumino‐cytologic dissociation Protein level: 93 mg/dL Cell count: <2/mm3 |
| First electrophysiological study (interval since onset of symptoms) | Sensory‐motor axonal pattern (23 days) | Sensory axonal pattern (7 days) | Demyelinating pattern (5 days) | Demyelinating pattern (17 days) | Demyelinating pattern (17 days) |
| Treatment | One course of IVIG (dose: 2 g/kg) | One course of IVIG (dose: 2 g/kg) | None | One course of IVIG (dose: 2 g/kg) | Two courses of IVIG (dose: 2 g/kg) |
| Clinical evaluation at 6 months |
No motor deficit Distal mild paraesthesia of the 4 limbs |
Complete recovery |
No motor deficit Mild ataxia |
No motor deficit Mild ataxia |
No motor deficit Intermittent paraesthesia of the 4 limbs |
| Platinum readministration | Yes (carboplatin) | No | Yes (carboplatin) | No | No |
Abbreviations: AUC, area under the curve; IVIG, intravenous immunoglobulin.
Clinical deterioration was rapid, the median interval between onset and nadir of motor impairment being 8 days (range, 3–11 days). All patients presented with marked disability at the peak of disease severity: four patients were bedridden, and one patient needed support for walking. However, none of them experienced life‐threatening conditions or needed intensive care unit admission. Treatment consisted of platinum discontinuation and, in four out of the five cases, IVIG administration (2 g/kg over 3–5 days). Clinical recovery was optimal, and at 6‐month evaluation all patients were able to walk without support. Two patients underwent carboplatin readministration during follow‐up, without experiencing further neurological toxicity.
Literature Review
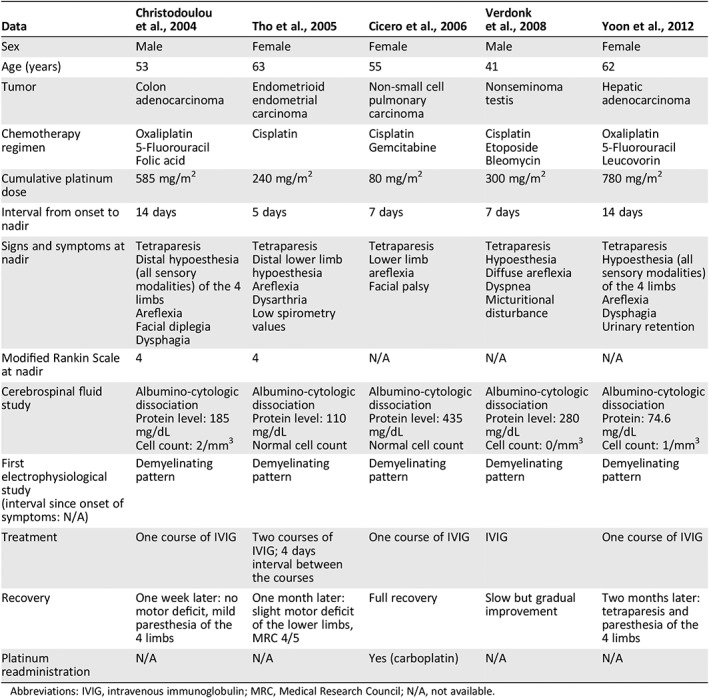
We identified five previously published cases of patients with GBS during chemotherapy for solid tumors, all of whom had received platinum compounds as part of their chemotherapy regimen (Table 2) 4, 5, 6, 7, 8. Three out of the five patients had received cumulative platinum doses below neurotoxic levels. All patients presented with moderate to severe sensorimotor impairment and reached symptom nadir after a median interval of 7 days (range, 5–14 days). Four patients out of the five eventually developed dysphagia and/or respiratory involvement. Electrophysiological and cerebrospinal fluid (CSF) studies were in all five cases typical of GBS, showing demyelinating features and albumino‐cytologic dissociation. Information about serum anti‐ganglioside antibodies level was not provided. All patients underwent platinum discontinuation and received IVIG treatment, with good clinical outcome. One patient was eventually retreated with carboplatin, but information on subsequent neurological toxicity was not reported.
Table 2.
Literature review
| Data | Christodoulou et al., 2004 | Tho et al., 2005 | Cicero et al., 2006 | Verdonk et al., 2008 | Yoon et al., 2012 |
|---|---|---|---|---|---|
| Sex | Male | Female | Female | Male | Female |
| Age (years) | 53 | 63 | 55 | 41 | 62 |
| Tumor | Colon adenocarcinoma | Endometrioid endometrial carcinoma | Non‐small cell pulmonary carcinoma | Nonseminoma testis | Hepatic adenocarcinoma |
| Chemotherapy regimen |
Oxaliplatin 5‐Fluorouracil Folic acid |
Cisplatin |
Cisplatin Gemcitabine |
Cisplatin Etoposide Bleomycin |
Oxaliplatin 5‐Fluorouracil Leucovorin |
| Cumulative platinum dose | 585 mg/m2 | 240 mg/m2 | 80 mg/m2 | 300 mg/m2 | 780 mg/m2 |
| Interval from onset to nadir | 14 days | 5 days | 7 days | 7 days | 14 days |
| Signs and symptoms at nadir |
Tetraparesis Distal hypoesthesia (all sensory modalities) of the 4 limbs Areflexia Facial diplegia Dysphagia |
Tetraparesis Distal lower limb hypoesthesia Areflexia Dysarthria Low spirometry values |
Tetraparesis Lower limb areflexia Facial palsy |
Tetraparesis Hypoesthesia Diffuse areflexia Dyspnea Micturitional disturbance |
Tetraparesis Hypoesthesia (all sensory modalities) of the 4 limbs Areflexia Dysphagia Urinary retention |
| Modified Rankin Scale at nadir | 4 | 4 | N/A | N/A | N/A |
| Cerebrospinal fluid study |
Albumino‐cytologic dissociation Protein level: 185 mg/dL Cell count: 2/mm3 |
Albumino‐cytologic dissociation Protein level: 110 mg/dL Normal cell count |
Albumino‐cytologic dissociation Protein level: 435 mg/dL Normal cell count |
Albumino‐cytologic dissociation Protein level: 280 mg/dL Cell count: 0/mm3 |
Albumino‐cytologic dissociation Protein: 74.6 mg/dL Cell count: 1/mm3 |
| First electrophysiological study (interval since onset of symptoms: N/A) | Demyelinating pattern | Demyelinating pattern | Demyelinating pattern | Demyelinating pattern | Demyelinating pattern |
| Treatment | One course of IVIG | Two courses of IVIG; 4 days interval between the courses | One course of IVIG | IVIG | One course of IVIG |
| Recovery | One week later: no motor deficit, mild paresthesia of the 4 limbs | One month later: slight motor deficit of the lower limbs, MRC 4/5 | Full recovery | Slow but gradual improvement | Two months later: tetraparesis and paresthesia of the 4 limbs |
| Platinum readministration | N/A | N/A | Yes (carboplatin) | N/A | N/A |
Abbreviations: IVIG, intravenous immunoglobulin; MRC, Medical Research Council; N/A, not available.
Discussion
Here we reported five cases of GBS during platinum‐based chemotherapy for solid tumors together with a review of the literature on this topic. Despite its intrinsic limitations related to small sample size and retrospective design, our work highlights the importance of distinguishing GBS from the classical toxic neuropathy associated with platinum administration, as early platinum discontinuation and IVIG treatment can potentially lead to full recovery.
The clinical features observed in GBS cases in our series differed from those associated with platinum‐related toxic peripheral neuropathy: patients with GBS presented with dominant motor symptoms, whereas patients with toxic sensory neuropathy usually present with sensory ataxia and distal painful paresthesias. Electroneuromyography (ENMG) and CSF analysis helped to circumstantiate the diagnosis of GBS and to start proper treatment. These assessments should promptly be performed when facing clinical features that are not classical for a toxic platinum‐related neuropathy.
Although patients in our series displayed one or more clinical‐paraclinical features typical of inflammatory polyradiculoneuropathy (e.g., prominent motor deficits, albumino‐cytologic dissociation, demyelinating features on ENMG), the exclusion of alternative causes has high relevance in this context. Concomitant conditions responsible for acquired peripheral neuropathies were excluded by specific testing. Neither could we consider our cases to be paraneoplastic: clinical onset did not precede tumor diagnosis nor coincide with tumor relapse, onconeural autoantibodies were negative, and motor deficits rapidly resolved following intravenous immunoglobulin administration (which is consistent with GBS but not with paraneoplastic disorders).
None of the patients in our series and in literature had received immune checkpoint inhibitors, which are known to be associated with acute polyradiculoneuropathy. None had a history of recent infection or vaccination, which are common antecedents in idiopathic GBS, but they all received platinum‐based chemotherapy. Although a formal link between GBS and platinum chemotherapy has not been established yet, among 449 patients with solid tumors in our database, GBS was diagnosed exclusively in those receiving platinum compounds. All five literature cases were also associated with platinum administration 4, 5, 6, 7, 8, making plausible the assumption that there is a pathophysiological link between platinum administration and GBS. Evidence suggests in fact that platinum compounds may act as triggers of autoimmunity by inducing an elevation of proinflammatory cytokines (tumor necrosis factor‐α and interleukin‐6) and by enhancing anticancer immune responses, which could in turn facilitate the development of an immune reaction towards myelin antigens 4, 9, 10. However, the mechanisms through which platinum agents could trigger GBS remain elusive, and larger prospective observations are needed to clarify whether patients with cancer have a higher risk to develop GBS, as suggested by some authors 11, and whether platinum‐based chemotherapy increases the risk of developing this complication.
Conclusion
Although GBS associated to platinum‐based chemotherapy is a rare event, prompt diagnosis of this condition is of utmost importance. Platinum discontinuation, together with IVIG administration, can reduce the risk of permanent neurological sequelae.
Disclosures
The authors indicated no financial relationships.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Ewertz M, Qvortrup C, Eckhoff L. Chemotherapy‐induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol 2015;54:587–591. [DOI] [PubMed] [Google Scholar]
- 2. Park SB, Lin CS, Krishnan AV et al. Oxaliplatin‐induced neurotoxicity: Changes in axonal excitability precede development of neuropathy. Brain 2009;132:2712–2723. [DOI] [PubMed] [Google Scholar]
- 3. Fokke C, van den Berg B, Drenthen J et al. Diagnosis of Guillain‐Barré syndrome and validation of Brighton criteria. Brain 2014;137(pt 1):33–43 [DOI] [PubMed] [Google Scholar]
- 4. Christodoulou C, Anastasopoulos D, Visvikis A et al. Guillain‐Barré syndrome in a patient with metastatic colon cancer receiving oxaliplatin‐based chemotherapy. Anticancer Drugs 2004;15:997–999. [DOI] [PubMed] [Google Scholar]
- 5. Yoon JY, Nam TS, Kim MK et al. Acute inflammatory demyelinating polyradiculoneuropathy in a patient receiving oxaliplatin‐based chemotherapy. Asia Pac J Clin Oncol 2012;8:201–204. [DOI] [PubMed] [Google Scholar]
- 6. Cicero G, Fulfaro F, Caraceni A et al. A case of Guillain‐Barré syndrome in a patient with non small cell lung cancer treated with chemotherapy. J Chemother 2006;18:325–327. [DOI] [PubMed] [Google Scholar]
- 7. Verdonk RC, Enting RH, Janmaat M et al. Weakness and numbness after chemotherapy for metastatic non‐seminoma testis: A new neurological complication. Acta Oncol 2008;47:1596–1598. [DOI] [PubMed] [Google Scholar]
- 8. Tho LM, O'Leary CP, Horrocks I et al. Guillain‐Barre syndrome occurring after adjuvant chemo‐radiotherapy for endometrial cancer. Gynecol Oncol 2006;100:615–617. [DOI] [PubMed] [Google Scholar]
- 9. Terenzi A, Pirker C, Keppler BK et al. Anticancer metal drugs and immunogenic cell death. J Inorg Biochem 2016;165:71–79. [DOI] [PubMed] [Google Scholar]
- 10. Bencardino K, Mauri G, Amatu A et al. Oxaliplatin immune‐induced syndrome occurs with cumulative administration and rechallenge: Single institution series and systematic review study. Clin Colorectal Cancer 2016;15:213–221. [DOI] [PubMed] [Google Scholar]
- 11. Vigliani MC, Magistrello M, Polo P et al.; Piemonte and Valle d'Aosta Register for Guillain‐Barré Syndrome. Risk of cancer in patients with Guillain‐Barré syndrome (GBS). A population‐based study. J Neurol 2004;251:321–326. [DOI] [PubMed] [Google Scholar]


